Abstract
Purpose
Differences in definitions of the condition, relevant triggers, and the geographical locations of study centers, cause estimates of the prevalence of anaphylaxis to vary. Recent epidemiological data indicate that the incidence of anaphylaxis is rising.
Methods
To investigate the causes and clinical features of anaphylaxis in Korean adults, factors associated with the severity of the condition, and serious outcomes, a retrospective medical record review was performed on adult patients diagnosed with anaphylaxis between 2007 and 2011 in 15 University Hospitals of South Korea.
Results
A total of 1,806 cases (52% male, age 16-86 years) were reported. Cutaneous symptoms (84.0%), combined with respiratory (53.9%) and/or cardiovascular (55.4%) symptoms, were the most frequent presentations. Using a recognized grading system, 1,776 cases could be classified as either mild, 340; moderate, 690; or severe, 746. Although eliciting factors varied significantly by age, gender, and regional and seasonal factors, drugs (46.5%; including nonsteroidal anti-inflammatory drugs, antibiotics, and radiocontrast media) were the most common cause of anaphylaxis, followed by foods (24.2%), insect stings (16.4%), exercise (5.9%), and unknown etiology (7.0%). All of age, multi-organ involvement, a history of allergic disease, and drug-induced anaphylaxis, were significant predictors of serious outcomes requiring hospital admission or prolongation of hospital stay. Epinephrine auto-injectors were prescribed for 7.4% of reported cases.
Conclusions
The principal causes of anaphylaxis in Korean adults were drugs, food, and insect stings. Drug-associated anaphylaxis, a history of allergic disease, multi-organ involvement, and older age, were identified as predictors of serious outcomes.
Keywords: Anaphylaxis, adult, epidemiology, multicenter study, severity, serious outcomes
INTRODUCTION
Anaphylaxis is a severe and life-threatening systemic hypersensitivity reaction.1,2 Some attacks are fatal even if immediate treatment is given and the rates of unexpected late responses and recurrence may be high. An episode of anaphylaxis, the incidence of which has been continuously rising worldwide over the past 20 years,3 can have a profound effect on the quality of life of the patient and his/her family. Identifying those at higher risk of serious outcomes4,5 of anaphylaxis would reduce the socioeconomic burden of the condition. It is important to understand the epidemiology of anaphylaxis both to manage patients and to prevent recurrence. However, estimates of the prevalence and causes of anaphylaxis vary worldwide.3 A recent nationwide cross-sectional survey in the United States found that 7.7% of the general population had experienced a prior episode of anaphylaxis, and that medications, food, and insect stings were the most common causes.6 Two retrospective studies performed in hospitals in Korea7 and Thailand8 found that the prevalence of anaphylaxis in Asian countries was less than 0.02%. Differences in the causes of anaphylaxis exist even within Korea. Drugs were the most common cause of anaphylaxis in patients treated in 2 University hospitals located in the capital area,7,9 but a Korean hospital located in a coastal region reported that food was the most common cause.10 No nationwide report has yet investigated the epidemiology of anaphylaxis in either Korean adults or other Asian populations. Therefore, we sought to understand the clinical features of anaphylaxis in Korean adults, and to identify predictors of severity and serious outcomes.
MATERIALS AND METHODS
Data were collected retrospectively on patients aged over 16 years of age who were diagnosed with anaphylaxis between January 2007 and December 2011 in 15 University hospitals covering most major cities of South Korea. According to International Statistical Classification of Diseases, 10th Revision (ICD-10), T78.0 (anaphylactic shock due to adverse food reaction), T78.2 (anaphylactic shock), T80.5 (anaphylactic shock due to serum), T63.4 (insect sting anaphylaxis), and T88.6 (anaphylactic shock due to adverse effect of correct medication) were selected as an anaphylaxis-associated codes. Demographic data including age, gender, smoking status, atopy status, and personal and family histories of allergic diseases, were collected. The causes of anaphylaxis were classified as drugs, radiocontrast media (RCM), exercise, food, insect stings, and idiopathic factors. Laboratory results were examined (when available) in efforts to determine the causes of anaphylaxis. Laboratory data included the levels of serum tryptase, total IgE, specific IgE directed against causative agents or component allergens, skin prick test results, and the results of oral provocation tests. In patients who had been simultaneously exposed to a variety of possible causes, and for whom no laboratory data were available, the causes of anaphylaxis were considered to be unknown.
Clinical manifestations of anaphylaxis were classified into 5 groups: cutaneous, respiratory, cardiovascular, gastrointestinal, and general symptoms. Generalized itching, urticaria, and angioedema were regarded as cutaneous symptoms. Respiratory symptoms were dyspnea, cough, stridor, wheezing, and cyanosis. Cardiovascular symptoms included dizziness, pallor, collapse, diaphoresis, syncope, and hypotension. Gastrointestinal symptoms were nausea, vomiting, diarrhea, and abdominal pain. General symptoms were anxiety, paresthesia, and weakness. All symptoms were documented and investigated.
The severity of anaphylaxis was graded as mild, moderate, or severe, using a previously established grading system for generalized hypersensitivity reactions.11 Mild reactions were defined by presentation of only cutaneous symptoms. Moderate symptoms were exhibited when respiratory, cardiovascular, or gastrointestinal involvement was apparent; whereas hypotension, hypoxia, loss of consciousness, or confusion was considered to reflect severe anaphylaxis. With respect to treatment, we collected data on the time intervals between exposure to anaphylactic causes and symptom development, and between symptom development and arrival at hospital. We also noted whether a patient had experienced a prior episode of anaphylaxis, and whether he or she had been treated before arrival at the hospital. Such treatment included epinephrine injection, treatment with systemic steroids and bronchodilators, and prescription of an epinephrine auto-injector to prevent re-occurrence of anaphylaxis.
The clinical courses of the patients were categorized by classifying outcomes as serious or non-serious. Serious outcomes were defined by new admission, or prolongation of hospitalization, caused by anaphylaxis. Patients who were discharged within 1 day of urgent treatment in the emergency department (ED), or who did not require hospitalization, were considered to exhibit non-serious outcomes. All data were recorded in case report form and entered into a customized Microsoft Access database by 2 trained research nurses. The study was approved by the Institutional Review Board of each participating hospital.
Statistical analyses were performed using SPSS for Windows version 19.0 (SPSS, Chicago, IL, USA). We used multiple logistic regression analysis to identify predictors of the severity, and serious outcomes, of anaphylaxis. To compare the numbers of anaphylaxis cases/total ED patients yearly, the proportion test using by R 3.0.2 (R Foundation for Statistical Computing, http://www.R-project.org/) was performed. Statistical significance was considered present when a P value was less than 0.05.
RESULTS
Demographics
During the 5 years of the study period, a total of 1,806 patients were diagnosed with anaphylaxis-related ICD-10 code in a total of 15 University hospitals in South Korea. The number of patients diagnosed countrywide gradually increased every year from 2007 to 2011 in all hospitals. Thus, 244 patients (13.6% of the total) were diagnosed in 2007, and 328 (18.3%), 334 (18.7%), 390 (21.8%), and 493 (27.6%) during the 4 years commencing in 2008. Data on all patients were collected and analyzed.
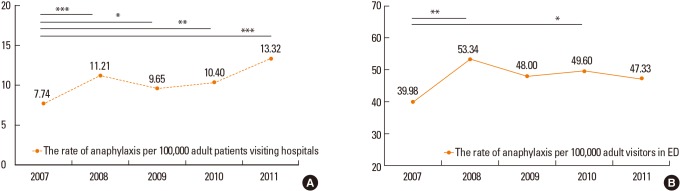
In addition, we made approximate estimations of the frequencies of anaphylaxis by considering the total numbers of patients visiting outpatient clinics, admitted as inpatients, and visiting EDs, during the study period, using data from 13 of the 15 participating hospitals. The Korean Health Statistics published by the Ministry of Health & Welfare12 showed that about 15% of patients are under 15 years of age in 2011. Therefore, we assumed that the total numbers of adult patients visiting the study hospitals constituted 85% of all such patients. The rates of anaphylaxis per 100,000 of all and ED adult patients were 10.46 and 47.65 per year, respectively, over the 5-year period (Fig. 1). The numbers of anaphylaxis cases/all adult patients/year were as follows: 200/2,583,635/2007, 248/2,211,980/2008, 237/2,456,046/2009, 293/2,818,370/2010, and 376/2,821,981/2011. The numbers of anaphylaxis cases/ED adult patients/year were as follows: 155/387,665/2007, 199/373,101/2008, 219/456,246/2009, 219/441,508/2010, and 209/411,590/2011. The anaphylaxis rate in adult patients significantly increased from 7.74 per 100,000 in 2007 to 11.21 in 2008 (P<0.001), to 9.65 in 2009 (P=0.024), to 10.40 in 2010 (P=0.001), and to13.32 in 2011 (P<0.001).
Fig. 1. The prevalence of anaphylaxis among the adult patients visiting 12 of the 15 hospitals studied over a 5-year period. (A) The rate of anaphylaxis per 100,000 adult patients visiting hospitals. (B) The rate of anaphylaxis per 100,000 adult visitors in emergency departments. P values were examined by proportion test using R 3.0.2 from 2007 to 2008, 2009, 2010, and 2011. *P<0.05; **P<0.01; ***P<0.001.
The mean age of all study subjects was 46.0 years (range, 16-89 years); 47.8% were female and 52.1% male; 29.1% were atopy-positive; 44.6% had a history of allergic disease; and 14.6% had experienced a prior episode of anaphylaxis (Table 1). Allergic rhinitis (19.1%), food allergy (17.4%), and asthma (10.2%), were common comorbid allergic diseases of patients with anaphylaxis. A family history of any allergic disease was identified in 16.8% of patients for whom medical records were available. We obtained records on prior exposure to allergens triggering the instant anaphylactic episodes, and symptoms developing after such exposure, from a total of 671 patients. Of these, 195 (10.8% of all subjects) experienced no adverse reaction, 213 (11.8%) developed allergic symptoms but not anaphylaxis, whereas 263 (14.6%) had already experienced anaphylaxis upon prior exposure.
Table 1. Clinical characteristics of the study subjects.
| Characteristic | n (%) |
|---|---|
| Gender, Male | 941 (52.1) |
| Age (year) | 46.0±16.0 (16-89) |
| 16-30 | 363 (20.1) |
| 31-50 | 694 (38.4) |
| ≥ 51 | 749 (41.5) |
| Atopy | 228/783 (29.1) |
| Past history of | |
| Allergic disease | 547/1,403 (44.6) |
| Asthma | 137/1,411 (10.2) |
| Allergic rhinitis | 271/1,457 (19.1) |
| Atopic dermatitis | 28/1,375 (2.5) |
| Chronic urticaria | 59/1,381 (4.3) |
| Food allergy | 248/1,422 (17.4) |
| Family history of allergic disease | 114/680 (16.8) |
| Symptoms at the time of prior exposure | |
| None | 195 (10.8) |
| Non-anaphylactic allergic reactions | 213 (11.8) |
| Anaphylaxis | 263 (14.6) |
Clinical features
The most common clinical symptoms of anaphylaxis were cutaneous in nature (84.0%), followed by cardiovascular (55.4%), respiratory (53.9%), general (21.0%), and gastrointestinal (18.5%) symptoms (Table 2). Of all patients, 76.9% exhibited more than two types of clinical manifestation. Cutaneous symptoms, combined with respiratory or cardiovascular symptoms (61.4%), were the most frequently observed. The mean latency period, defined as the mean time interval between exposure to an anaphylactic trigger and development of anaphylactic symptoms, was 181.6 minutes. The mean time to arrival at hospital after development of symptoms was 279.8 minutes. Of the 1,776 patients for whom symptoms and signs were recorded in medical charts, 340 (19.1%) with skin manifestations alone were allocated to the mild anaphylaxis group; 690 (38.9%) with respiratory, cardiovascular, or gastrointestinal involvement (also with cutaneous symptoms) to the moderate group; and 746 (42.0%) with shock, hypoxia, loss of consciousness, or confusion, to the severe group (Table 3). The grade of anaphylactic severity was well-correlated with the frequency of use of epinephrine and systemic steroids. The numbers of patients given epinephrine injections were significantly higher in those with moderate and severe anaphylactic grades, being 157 (22.8%; odds ratio [OR] 4.555, 95% confidence interval [CI] 2.843-7.322, P<0.001) and 364 (48.8%; 15.820, 9.935-25.193, P<0.001), respectively, compared with patients of mild anaphylactic grade, of whom only 22 (6.5%) were recorded as having been given epinephrine injections. In terms of the frequency of systemic steroid use, 147 (43.2%) mildly anaphylactic patients received steroids, whereas the figures for those with moderate and severe anaphylaxis were 426 (61.7%; OR 2.920, 95% CI 2.152-3.962, P<0.001) and 537 (72.0%; 4.908, 3.572-6.743, P<0.001), respectively.
Table 2. Assessment and management of anaphylaxis.
| Characteristic | n (%) |
|---|---|
| Clinical manifestations | |
| Cutaneous | 1,495 (84.0) |
| Respiratory | 971 (53.9) |
| Cardiovascular | 997 (55.4) |
| Gastrointestinal | 334 (18.5) |
| General | 378 (21.0) |
| Latency period (minute) | 181.6±1,210.1 |
| Time to arrival at hospital after developing symptoms (minute) | 279.8±1,065.8 |
| Management | |
| OPD/ED/Ward/ICU | 35.8/44.4/16.4/3.4 |
| Epinephrine use (Yes/No/Unknown) | 543 (30.1%)/879/384 |
| Total treatment period (days) | 9.5±37.8 |
| Positive on confirmatory testing | 415/536 (77.4) |
| Prescription of EpiPEN for secondary prevention | 126 (7.0) |
OPD, outpatient department; ED, Emergency Department; ICU, intensive care unit; EpiPEN, epinephrine auto-injector.
Table 3. Grading of severity according to the cause of anaphylaxis.
| Total n=1,776 | Mild n=340 | Moderate n=690 | Severe n=746 | |
|---|---|---|---|---|
| Drugs & RCM | 827 (46.6%) | 99 (29.1%) | 334 (48.4%) | 394 (52.8%) |
| NSAIDs | 236 (13.3%) | 32 (9.4%) | 126 (18.3%) | 78 (10.5%) |
| Antibiotics | 186 (10.5%) | 20 (5.9%) | 77 (11.2%) | 89 (12.0%) |
| RCM | 214 (12.0%) | 27 (7.9%) | 58 (8.4%) | 129 (17.3%) |
| Insect stings | 291 (16.4%) | 121 (35.6%) | 90 (13.0%) | 80 (10.7%) |
| Food | 430 (24.2%) | 72 (21.2%) | 176 (25.5%) | 182 (24.4%) |
| Seafood | 145 (8.2%) | 28 (8.2%) | 61 (8.9%) | 56 (7.5%) |
| Wheat | 147 (8.3%) | 16 (4.7%) | 60 (8.7%) | 71 (9.5%) |
| Nuts | 29 (1.6%) | 3 (0.9%) | 12 (1.7%) | 14 (1.9%) |
| Meats | 56 (3.2%) | 7 (2.1%) | 24 (3.5%) | 25 (3.4%) |
| Vegetable | 58 (3.3%) | 13 (3.8%) | 21 (3.0%) | 24 (3.2%) |
| Exercise with/without food | 104 (5.9%) | 8 (2.4%) | 46 (6.7%) | 50 (6.7%) |
| FDEIA | 73 (4.1%) | 6 (1.8%) | 35 (5.1%) | 32 (4.3%) |
| Exercise | 31 (1.7%) | 2 (0.6%) | 11 (1.6%) | 18 (2.4%) |
| Unknown | 124 (7.0%) | 40 (11.7%) | 44 (6.4%) | 40 (5.3%) |
RCM, radiocontrast media; NSAID, nonsteroidal anti-inflammatory drug; FDEIA, food-dependent exercise-induced anaphylaxis.
Of patients with mild anaphylaxis, 60/316 (19.0%) were recorded as presenting with angioedema in the absence of any other objective skin feature. In those with moderate and severe anaphylaxis, 64/658 (9.7%) and 46/716 (6.4%), respectively, did not exhibit any objective skin feature that was documented in medical records.
Causes of anaphylaxis
The causes of anaphylaxis were drugs (46.6%), food (24.2%), insect stings (16.4%), exercise (5.9%), and unknown (7.0%), in order of prevalence (Table 3). In terms of drugs, nonsteroidal anti-inflammatory drugs (NSAIDs, 13.3% of all cases), antibiotics (10.5%), and RCM (here considered a drug) (12.0%), were the most common causes of anaphylaxis. As shown in Table 4, anaphylaxis caused by drugs and exercise, thus not by food, was more prevalent in the moderate and severe anaphylaxis groups compared with the mild anaphylaxis group. Insect stings were the most common cause of mild anaphylaxis. Wheat and seafood were the most common causes of food-induced anaphylaxis. Wheat consumption was significantly associated with both moderate and severe anaphylaxis (4.7% of patients in the mild group vs 9.1% of those in the moderate and severe groups, P=0.011), but no difference in the prevalence of seafood-induced anaphylaxis was noted among the three groups.
Table 4. Predictors of the severity of anaphylaxis.
| Mild | Moderate | Severe | |||
|---|---|---|---|---|---|
| Reference | P value | OR (95% CI) | P value | OR (95% CI) | |
| Age | 1 | 0.137 | 0.991 (0.980-1.003) | 0.008 | 1.016 (1.004-1.028) |
| Male gender | 1 | 0.906 | 0.981 (0.713-1.349) | 0.342 | 1.170 (0.846-1.620) |
| Allergic disease | 1 | < 0.001 | 2.732 (1.898-3.932) | < 0.001 | 2.188 (1.499-3.193) |
| DM±HTN | 1 | 0.001 | 2.335 (1.432-3.809) | < 0.001 | 2.394 (1.486-3.858) |
| RCM | 1 | 0.006 | 2.379 (1.281-4.419) | < 0.001 | 3.215 (1.767-5.851) |
| NSAID use | 1 | 0.008 | 1.922 (1.189-3.105) | 0.661 | 1.122 (0.670-1.881) |
| Antibiotic use | 1 | 0.017 | 2.149 (1.144-4.036) | 0.002 | 2.761 (1.474-5.173) |
| Seafood | 1 | 0.469 | 1.230 (0.702-2.155) | 0.781 | 1.088 (0.602-1.967) |
| Wheat | 1 | 0.376 | 1.476 (0.623-3.499) | 0.037 | 2.425 (1.054-5.581) |
| Meat | 1 | 0.460 | 1.426 (0.556-3.658) | 0.887 | 1.076 (0.389-2.979) |
| Vegetable | 1 | 0.869 | 1.084 (0.416-2.828) | 0.407 | 1.382 (0.522-3.658) |
| Exercise (+FDEIA) | 1 | 0.484 | 1.633 (0.414-6.449) | 0.034 | 4.119 (1.112-15.248) |
OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; RCM, radiocontrast media; NSAID, nonsteroidal anti-inflammatory drug; FDEIA, food-dependent exercise-induced anaphylaxis.
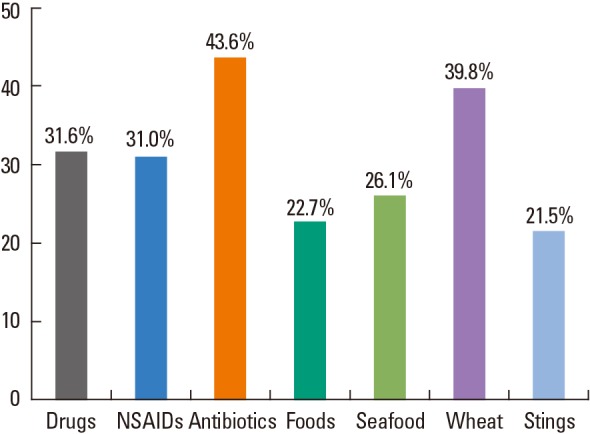
Multivariate logistic regression showed that older age (OR 1.016; 95% CI 1.004-1.028), a past history of allergic disease (2.188; 1.499-3.193), comorbidities of diabetes and/or hypertension (2.394; 1.486-3.858), drugs (especially RCM [3.215; 1.767-5.851] and antibiotics [2.761; 1.474-5.173]), exercise (4.119; 1.112-15.248), and wheat consumption (2.425; 1.054-5.581), were all significant predictors of severe, as distinct from mild, anaphylaxis (Table 4). In addition, all of allergic disease, comorbidities, exposure to RCM, and ingestion of NSAIDs and antibiotics, were useful to discriminate moderate from mild anaphylaxis. To determine the causes of anaphylaxis, a total of 536 patients (31.0%) had been subjected to allergen evaluation, including skin testing, measurement of specific IgE in serum, and provocation testing, after their anaphylactic events. Of these, 415 showed positive responses to immunological or provocation tests after recovery was complete. Fig. 2 shows the proportions of positive responses by causes. Of patients with drug-induced anaphylaxis, 31.6% were positive on oral or bronchial provocation tests (these were usually patients with NSAID-induced anaphylaxis [31.0%]), and 43.6% were positive to penicillins including ampicillin and amoxicillin, and several cephalosporins by skin testing or measurement of serum levels of specific IgE. In cases of food-associated anaphylaxis, the positive response rates on such testing were relatively lower, but wheat-induced anaphylaxis was often identified by detection of serum specific IgE antibodies to wheat, gluten, and/or gliadin; and a positive allergic skin test to wheat flour.
Fig. 2. The positivity rates upon post-recovery allergen testing by cause of anaphylaxis.
Predictors of serious outcomes
In terms of anaphylaxis treatment, 35.8% of all patients visited outpatient clinics; 44.4% were discharged within 24 hours after urgent management in EDs; and 19.8% were hospitalized in wards (16.4%) and intensive care units (3.4%) to manage and observe their conditions. The mean total treatment period was 9.5 days. About 30% of patients were given epinephrine injections, and epinephrine auto-injectors were prescribed for 7.4% of patients, to prevent secondary anaphylaxis.
Multivariate analysis confirmed that multi-organ involvement (OR 2.313; 95% CI 1.725-3.100, P<0.001), anaphylaxis caused by drugs (2.111; 1.299-3.430, P=0.003), and older age (1.018; 1.008-1.029, P<0.001), were independent predictors of serious outcomes (Table 5). Interestingly, a past history of allergic disease was a risk factor for development of severe anaphylaxis, but actually reduced the risk of a serious outcome. Of patients with a history of allergic disease, 37.1% had visited clinics near their homes (compared to 22.9% of patients without prior allergic disease, P<0.01), and 3.8% had received epinephrine injections before visiting our study institutions (compared to 2.2% of patients without allergic disease, P=0.121). In other words, a prior history of allergic disease was associated with patient insight into such disease, including anaphylaxis, and the ability of patients to deal with an anaphylactic episode. None of female gender (OR 0.912, P>0.05), exposure to RCM (OR 1.245, P>0.05), consumption of particular food (OR 0.978, P>0.05), exercise (OR 0.712, P>0.05), or being stung by insects (OR 0.674, P>0.05), was associated with admission to hospital or prolongation of hospital stay, despite the existence of positive associations between these factors and the severity of anaphylaxis.
Table 5. Predictors of serious outcomes, including prolongation of admission, or new admission, for anaphylaxis.
| P value | OR (95% CI) | |
|---|---|---|
| Age | < 0.001 | 1.018 (1.008-1.029) |
| Female gender | 0.519 | 0.912 (0.689-1.207) |
| Allergic disease | 0.036 | 0.721 (0.531-0.978) |
| Drug use | 0.003 | 2.111 (1.299-3.430) |
| RCM exposure | 0.469 | 1.245 (0.688-2.251) |
| Food | 0.937 | 0.978 (0.571-1.678) |
| Exercise | 0.667 | 0.712 (0.152-3.343) |
| Insect sting | 0.194 | 0.674 (0.371-1.223) |
| With skin, respiratory, and cardiovascular symptoms | < 0.001 | 2.313 (1.725-3.100) |
OR, odds ratio; CI, confidence interval; RCM, radiocontrast media.
DISCUSSION
To our knowledge, this is the first nationwide study to investigate the clinical features of adult patients with anaphylaxis not only in Korea, but in all of Asia. Although a few studies on the epidemiology of anaphylaxis in Asian populations have been conducted,7,13,14,15 the results are of limited utility, being simply reports on common causes and manifestations. Allergic disease has become a major public health problem in Asia because population densities are high and urbanization and economic development are rapid.16 Although the present study had several limitations, including the retrospective nature of medical record review, possibly inaccurate diagnostic coding, and inclusion of only patients who visited University hospitals, we suggest that the rates of anaphylaxis among adult patients were close to 10.46 per 100,000 of all hospital visitors (0.010%), and 47.65 per 100,000 ED visitors (0.048%), during 2007-2011. Our present results are consistent with other data on anaphylaxis rates in Asian populations. Yang et al.7 reported a 0.014% anaphylaxis incidence rate in 2006, in adult and pediatric patients treated at a University hospital in Korea. In a 4-year observational study conducted in the ED of a University hospital in Thailand, the anaphylaxis rate was 49 per 100,000 patient-years during 2004-2008.17 However, this may be compared with the incidence of 49.8 per 100,000 patient-years calculated in a recent retrospective population-based study conducted in Rochester, Minnesota, from 1990 through 2000,18 and suggests that the anaphylaxis rates in Asian countries remain underestimated. The number of patients diagnosed with anaphylaxis in the hospitals participating in the present study gradually increased every year from 2007 to 2011, as has also been noted in other countries.19 The anaphylaxis rate in adult patients significantly increased from 7.74 per 100,000 in 2007 to 13.32 per 100,000 in 2011. Therefore, further prospective investigations using common diagnostic criteria defined via nationwide or pan-Asian co-operation are necessary to estimate the exact anaphylaxis rates in Asian populations.
In general, a prior episode of anaphylaxis and/or a mild allergic reaction to a specific trigger can predict a subsequent anaphylactic event. It is well-recognized that epinephrine is the drug of choice for first-aid treatment of anaphylaxis. Of 671 patients whose medical records documented previous allergic reactions, 14.6% and 11.8% had experienced prior anaphylaxis and mild reactions, respectively, but only 13 had personal epinephrine auto-injectors at the time of the anaphylactic incident studied in the present report. Also, the prescription rate of epinephrine auto-injectors for treatment of any recurrence of anaphylaxis was only 7.0%, as in most other countries, and ownership of an epinephrine auto-injector for first-aid treatment remains out-of-reach for many subjects at risk of anaphylaxis in Korea.
The clinical manifestations of our study population were similar to those noted in previous studies. Mucocutaneous symptoms were the most common, and cardiovascular and respiratory symptoms were noted in more than half of all adult patients. We observed a relatively lower rate of gastrointestinal symptoms (18.5%) compared to what was noted in both a Central European cohort (40%)20 and in a retrospective study conducted in an Australian hospital (32%).11 The 2 cited studies enrolled large numbers of patients, both pediatric and adult. However, studies on adult patients in Asian countries found that only 10%21 and 16%14 had gastrointestinal symptoms. Moreover, the most common cause of anaphylaxis in the 2 cited studies on Asian adults was medication; insect stings and food, which were the leading causes of anaphylaxis in the European and Australian studies,11,20 were involved in only 2% of anaphylactic events.14,21 As our work was retrospective, it is possible that gastrointestinal manifestations went unnoticed. However, in contrast to previous suggestions that gastrointestinal11 or cutaneous7 symptoms were associated with hypotension, we could not find any significant association between the severity of the anaphylactic reaction and specific organ involvement. In terms of the severity of anaphylaxis, 42.0% of patients of the present study experienced severe reactions. Multiple logistic regression analysis revealed that the severity of anaphylactic reaction was significantly influenced by age; comorbidities including diabetes and hypertension; a past history of allergic disease; exposure to RCM, antibiotics, and wheat flour; and exercise, among specific causes. The severity of anaphylaxis had a significant impact on outcomes. Of patients with severe anaphylaxis, 31.4% required hospital admission, whereas only 5.1% of mild anaphylaxis patients were hospitalized, or needed prolonged admission, to manage their anaphylaxis. In addition, the severity grade was also associated with the frequency of use of epinephrine and systemic steroids. Patients with severe reactions more frequently took such drugs.
To date, drugs and insect stings have been shown to be the more common causes of anaphylaxis in adults, whereas food was the most common cause of anaphylaxis in childhood.15,22 In the present study, the main causes of anaphylaxis were drugs (46.6%; including 12.0% of patients exposed to RCM); foods (24.2%); insect stings (16.4%); and exercise (5.9%), in that order. These results are quite distinct from those of a recent study of anaphylaxis epidemiology in Korean children, wherein most cases were induced by unknown causes (61.7%), followed by food (24.9%) and medications (12.4%).23 Common triggers of anaphylaxis may differ by geographical area and study design within a country. Three retrospective studies with anaphylaxis conducted in a single center in Korea also found that drugs, food, and stings were the most common causes of anaphylaxis, but in an order of frequency different from that found in the present work.7,9,10 When we further analyzed the causes of anaphylaxis by patient age, we found that, in those less than 30 years of age, food (34.2%) was the most common trigger of anaphylaxis, whereas drugs were the prime cause in patients aged 31-50 years (34.0%) and also in those older than 51 years (36.3%). Among triggers of anaphylaxis, RCM and insect stings were rare in younger compared to middle-aged and older patients. In the present study, NSAIDs were the most common drugs triggering anaphylaxis, followed by RCM and antibiotics, consistent with findings of previous reports.21,24,25 One-third of our study subjects were subjected to post-recovery allergen tests, such as skin tests, measurement of specific IgE levels in serum, and/or oral or bronchial provocation tests. Of all tested patients, 77.4% showed positive responses to the anaphylactic triggers. Of course, the results were explained to the patients, and this would help to reduce anaphylaxis recurrence. It is thus important not only to discover the risk factors for anaphylaxis but also to identify the exact cause of any anaphylactic episode, to alleviate the socioeconomic burden of the disease.
Few published reports have yet identified potential factors predicting the severity and outcomes of anaphylaxis in Asia. The results of the present study suggest that multi-organ involvement, older age, associated allergic diseases, and drug use, were all independent predictors of serious anaphylactic outcomes. This is consistent with the findings of a 5-year retrospective study in a community-based hospital in the United States, where involvement of multiple organ systems and non-sting allergens including medications and food, were associated with increased rates of hospitalization for anaphylaxis.24 However, another study performed between 1991 and 1995 in the United Kingdom proposed that the risk of anaphylaxis-associated admission was substantially higher in females aged between 15 and 55 years.22 In the present study, drug use and older age, regardless of gender, were risk factors for anaphylaxis-associated admission, and these findings are comparable to those of other studies conducted in Florida26 and Australia.27 Although the epidemiology of anaphylaxis may vary by ethnicity, socioeconomic status, population demographics, and the diagnostic criteria used,3 the risk factors for anaphylaxis-associated hospitalization identified in the present work do not differ from those identified in Western countries.
In conclusion, the principal causes of anaphylaxis in Korean adults are drugs, food, and insect stings. The severity of anaphylactic reaction is dependent on age, the presence of comorbidities, and specific causes. Drug-associated anaphylaxis, multi-organ involvement, and older age, are independent predictors of serious outcomes.
ACKNOWLEDGMENTS
This research was supported by the Korean Academy of Asthma, Allergy and Clinical Immunology and partly by a grant from the Ministry of Food and Drug Safety to operation of the Regional Pharmacovigilance Center in 2012. The authors wish to acknowledge the Ajou Clinical Trial Center for their generous support of the development of database and the statistical analysis.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY World Allergy Organization. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy. 2011;66:1–14. doi: 10.1111/j.1398-9995.2010.02422.x. [DOI] [PubMed] [Google Scholar]
- 4.Jung JW, Jeon EJ, Kim JW, Choi JC, Shin JW, Kim JY, Park IW, Choi BW. A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res. 2012;4:107–109. doi: 10.4168/aair.2012.4.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Yoon SY, Park SY, Kwon HS, Cho YS, Moon HB, Kim TB. A case of idiopathic anaphylaxis followed by acute liver injury. Allergy Asthma Immunol Res. 2013;5:245–247. doi: 10.4168/aair.2013.5.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood RA, Camargo CA, Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, Collins C, Tringale M, Wilkinson M, Boyle J, Simons FE. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, Kwon HS, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008;100:31–36. doi: 10.1016/S1081-1206(10)60401-2. [DOI] [PubMed] [Google Scholar]
- 8.Techapornroong M, Akrawinthawong K, Cheungpasitporn W, Ruxrungtham K. Anaphylaxis: a ten years inpatient retrospective study. Asian Pac J Allergy Immunol. 2010;28:262–269. [PubMed] [Google Scholar]
- 9.Kim MJ, Choi GS, Um SJ, Sung JM, Shin YS, Park HJ, Ye YM, Nahm DH, Lee SY, Park HS. Anaphylaxis; 10 years' experience at a university hospital in Suwon. Korean J Asthma Allergy Clin Immunol. 2008;28:298–304. [Google Scholar]
- 10.Park HJ, Kim SH. Factors associated with shock in anaphylaxis. Am J Emerg Med. 2012;30:1674–1678. doi: 10.1016/j.ajem.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Son CK, Do SR, Jang YS, Kim EJ, Shin ES, Jin JH. 2011 patient survey in Korea. Seoul: Korea Institute for Health and Social Affairs; 2012. [Google Scholar]
- 13.Cetinkaya F, Incioglu A, Birinci S, Karaman BE, Dokucu AI, Sheikh A. Hospital admissions for anaphylaxis in Istanbul, Turkey. Allergy. 2013;68:128–130. doi: 10.1111/all.12069. [DOI] [PubMed] [Google Scholar]
- 14.Hsin YC, Hsin YC, Huang JL, Yeh KW. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac J Allergy Immunol. 2011;29:307–312. [PubMed] [Google Scholar]
- 15.Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in seoul. Allergy Asthma Immunol Res. 2014;6:131–136. doi: 10.4168/aair.2014.6.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong GW, Leung TF, Ko FW. Changing prevalence of allergic diseases in the Asia-pacific region. Allergy Asthma Immunol Res. 2013;5:251–257. doi: 10.4168/aair.2013.5.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lertnawapan R, Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011;60:283–289. doi: 10.2332/allergolint.10-OA-0256. [DOI] [PubMed] [Google Scholar]
- 18.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koplin JJ, Martin PE, Allen KJ. An update on epidemiology of anaphylaxis in children and adults. Curr Opin Allergy Clin Immunol. 2011;11:492–496. doi: 10.1097/ACI.0b013e32834a41a1. [DOI] [PubMed] [Google Scholar]
- 20.Worm M, Edenharter G, Ruëff F, Scherer K, Pföhler C, Mahler V, Treudler R, Lang R, Nemat K, Koehli A, Niggemann B, Hompes S. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67:691–698. doi: 10.1111/j.1398-9995.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelincik A, Demirtürk M, Yılmaz E, Ertek B, Erdogdu D, Çolakoğlu B, Büyüköztürk S. Anaphylaxis in a tertiary adult allergy clinic: a retrospective review of 516 patients. Ann Allergy Asthma Immunol. 2013;110:96–100. doi: 10.1016/j.anai.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Tang ML, Osborne N, Allen K. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2009;9:351–356. doi: 10.1097/ACI.0b013e32832db95a. [DOI] [PubMed] [Google Scholar]
- 23.Lim DH. Epidemiology of anaphylaxis in Korean children. Korean J Pediatr. 2008;51:351–354. [Google Scholar]
- 24.Steele R, Camacho-Halili M, Rosenthal B, Davis-Lorton M, Aquino M, Fonacier L. Anaphylaxis in the community setting: determining risk factors for admission. Ann Allergy Asthma Immunol. 2012;109:133–136. doi: 10.1016/j.anai.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh A, Alves B. Age, sex, geographical and socio-economic variations in admissions for anaphylaxis: analysis of four years of English hospital data. Clin Exp Allergy. 2001;31:1571–1576. doi: 10.1046/j.1365-2222.2001.01203.x. [DOI] [PubMed] [Google Scholar]
- 26.Mulla ZD, Simon MR. Hospitalizations for anaphylaxis in Florida: epidemiologic analysis of a population-based dataset. Int Arch Allergy Immunol. 2007;144:128–136. doi: 10.1159/000103224. [DOI] [PubMed] [Google Scholar]
- 27.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–442. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]


