Abstract
Mechanisms by which the intestinal epithelium resists invasion by food-borne pathogens such as Listeria monocytogenes are an evolving area of research. Intestinal P glycoprotein is well known to limit the absorption of xenobiotics and is believed to act as a cytotoxic defense mechanism. The aim of this study was to determine if intestinal P glycoprotein is involved in host defense against L. monocytogenes. Caco-2 cells and a P-glycoprotein-overexpressing subclone (Caco-2/MDR) were employed in addition to mdr1a−/− mice and wild-type controls. In vitro invasion assays and in vivo experiments were employed to measure bacterial invasion and dissemination. In addition, L. monocytogenes proteins were labeled with [35S]methionine, and the transepithelial transport across Caco-2 monolayers was characterized in both directions. Overexpression of P glycoprotein in Caco-2/MDR cells led to increased resistance to L. monocytogenes invasion, whereas P-glycoprotein inhibition led to increased invasion. Flux of [35S]methionine-labeled L. monocytogenes proteins was significantly greater in the basolateral-to-apical direction than in the apical-to-basolateral direction, indicating dependence on an apically located efflux transporter. Moreover, inhibiting P glycoprotein reduced the basolateral-to-apical flux of the proteins. Early dissemination of L. monocytogenes from the gastrointestinal tract was significantly greater in the mdr1a−/− mice than in wild-type controls. Expression and function of intestinal P glycoprotein is an important determinant in resistance to early invasion of L. monocytogenes.
P glycoprotein is the 170-kDa product of the human MDR1 gene and is arguably one of the most extensively studied members of the ATP-binding cassette superfamily of transport proteins (28). P glycoprotein is best known for its ability to transport drug substrates out of cells in a variety of tissues, including the intestine (1, 12, 27). Both the expression and the function of P glycoprotein have been linked to considerable variability in oral drug absorption; however, the precise physiological role of intestinal P glycoprotein is unknown. Because P glycoprotein is well conserved throughout evolution and has a broad substrate affinity, it is widely believed to act as a cytotoxic protection mechanism. Given its apical distribution on the enterocyte, P glycoprotein is exquisitely positioned to limit the absorption of substances that the cell perceives as harmful. Thus, it is conceivable that P glycoprotein restricts the absorption of other, nondrug substances in the intestine. Proteins facilitating invasion of pathogenic bacteria would be ideal candidates given the purported mechanism of action of P glycoprotein.
Listeria monocytogenes is a food-borne pathogen responsible for considerable morbidity and mortality (11, 24). Although multiple sites of invasion have been proposed, the vanguard of the body's interaction with L. monocytogenes is the intestinal epithelial barrier. Intestinal epithelial cells come in contact with not only the bacterium but also numerous secreted and surface-attached L. monocytogenes proteins. Almost 5% of the coding capacity of the L. monocytogenes EGD genome is dedicated to surface proteins. These proteins can be characterized into three categories: (i) LPXTG proteins covalently linked to peptidoglycan by carboxy-terminal domains, (ii) noncovalently bound proteins, including GW proteins, hydrophobic tail proteins, and P60-like proteins, and (iii) lipoproteins attached to the surface by their amino-terminal regions (5). Although a number of epithelial receptors for L. monocytogenes proteins important for invasion have been identified, little is known regarding mechanisms by which the enterocyte resists invasion at the apical membrane (2, 3, 14, 18). Given the intimate contact between the bacterium and intestinal epithelial cells, it is conceivable that some bacterial proteins, either secreted or loosely attached, come in contact with enterocyte proteins such as P glycoprotein. Whether P glycoprotein can prevent the attachment or entry of L. monocytogenes proteins that promote invasion has not previously been determined. The aim of the present study was to test the hypothesis that intestinal P glycoprotein influences the extent of L. monocytogenes invasion. We report that the expression and function of P glycoprotein in vitro and in vivo influence the extent of L. monocytogenes invasion.
MATERIALS AND METHODS
Cell culture.
The human colonic adenocarcinoma cell line Caco-2 was obtained from the American Type Culture Collection (Manassas, Va.). Growth medium consisted of Dulbecco's modified Eagle's medium without antibiotics supplemented with 25 mM glucose (Mediatech, Herndon, Va.), 10% fetal bovine serum, 1% nonessential amino acids, 2 mM l-glutamine, and 1 mM sodium pyruvate (Sigma, St. Louis, Mo.). Invasion assays and digoxin uptake experiments were conducted with cells grown in 24-well cell culture plates (Becton Dickinson, Franklin Lakes, N.J.). For bidirectional transport studies, cells were seeded on porous (0.4-μm) Transwell inserts (Costar, Cambridge, Mass.) in 24-well plates. Fully differentiated cells were employed for all studies (18 to 21 days postseeding).
Bacterial cultures.
L. monocytogenes EGD (serotype 1/2a) was employed for all studies. Seven hundred fifty microliters of an overnight culture of L. monocytogenes EGD was inoculated into 50 ml of prewarmed brain heart infusion medium and shaken at 37°C until mid-log-phase growth was reached. A 20-ml aliquot of the culture was centrifuged, the supernatant was discarded, and the pellet was resuspended in Caco-2 growth medium. Using the optical density at 600 nm and a growth curve, serial dilutions were made to obtain the desired concentration of bacteria in Caco-2 medium.
Caco-2 invasion assay.
Caco-2 medium was aspirated from the 24-well plate and replaced with 1 ml of medium containing 105, 106, 107, or 108 L. monocytogenes EGD organisms/ml (n = 6 per condition). Following a 1-h incubation period, the bacterium-containing medium was drawn off, and the wells were washed five times with Hanks balanced salt solution (HBSS). Subsequently, cells were incubated for 2.5 h with Caco-2 medium containing 5 μg of gentamicin sulfate per ml to kill remaining extracellular bacteria. After the gentamicin phase, cells were again washed five times with HBSS and lysed with 1% Triton X-100 in phosphate-buffered saline (PBS). Viable intracellular L. monocytogenes organisms were quantified by serial dilutions of the cell lysates plated in duplicate on blood agar (Difco Laboratories, Detroit, Mich.). Colonies were counted after 48 h. Data are expressed as the log10 CFU of L. monocytogenes organisms per ml of lysate (means ± standard deviations [SD]).
Modulation of P-glycoprotein expression and function in Caco-2 cells.
A P-glycoprotein-overexpressing subclone of Caco-2 cells (designated Caco-2/MDR) was derived by stepwise selection with vinblastine (final concentration, 1.5 ng/ml). Vinblastine was removed from the medium 24 h before the addition of bacteria so that intracellular concentrations would be negligible. For P-glycoprotein functional inhibition studies, growth medium was aspirated 1 h prior to bacterial infection and replaced with 0.5 ml of medium containing the monoclonal antibody MRK16 (20 μg/ml) (Kamiya Biomedical, Seattle, Wash.). Unlike other P-glycoprotein inhibitors, MRK16 is nonpermeative and therefore does not have the ability to accumulate in the cytosol and subsequently interfere with intracellular growth of the organism. The isotype-matched control, mouse monoclonal immunoglobulin G2a (clone NCG2A.01) antibody, was used to confirm the absence of nonspecific antibody effects on invasion (Kamiya). After the 1-h preincubation, 0.5 ml of growth medium containing 2 × 105, 2 × 106, 2 × 107, and 2 × 108 L. monocytogenes EGD organisms/ml was added to yield bacterial concentrations (105 to 108/ml) similar to those used in other invasion assays.
L. monocytogenes protein transport studies.
Bacterial cultures were grown to the mid-log phase and resuspended in Caco-2 medium without methionine. Two-milliliter aliquots were placed in glass tubes containing 100 μCi of [35S]methionine (ICN Biomedicals, Irvine, Calif.). Radioactive methionine was incorporated into newly synthesized proteins at 37°C over a period of 2 h with shaking. L. monocytogenes proteins in the supernatant were removed with a 0.22-μm calcium acetate filter, and the fraction containing whole bacteria was discarded. Separated radioactive proteins were placed in either the apical or basolateral chamber for 150 min, and the bidirectional flux across the Caco-2 cell monolayer was measured. Aliquots (10 μl) were taken from the opposite chamber at 0, 30, 60, 90, 120, and 150 min, and 35S activities were determined by liquid scintillation counting. Flux in the apical-to-basolateral and basolateral-to-apical directions (Ja-b and Jb-a) was determined from the slope of the linear regression line of flux versus time (in micrograms per square centimeter per minute). In selected wells, cells were preincubated with Dulbecco's modified Eagle's medium containing MRK16 or the isotype control as described above to confirm P-glycoprotein specificity. In addition to MRK16, cyclosporine (10 μM) or verapamil (100 μM) was also employed to inhibit P glycoprotein. For each condition, the assay was performed in triplicate. L. monocytogenes protein concentrations in the experiments were similar to those seen when the bacteria were present. Epithelial integrity was confirmed prior to and following each experiment by determining the transepithelial resistance (EVOM Epithelial Voltohmmeter; WPI, Sarasota, Fla.).
[3H]digoxin competition experiments.
To further confirm that L. monocytogenes proteins exhibited high affinity for P glycoprotein, a digoxin competition assay was performed. Substrates with higher affinities for P glycoprotein will compete with other known substrates such as digoxin for transport, resulting in an increased intracellular accumulation of digoxin. Therefore, competition assays can serve as a tool to corroborate P-glycoprotein substrate specificity of unknown substances. Caco-2 cells were washed three times with uptake buffer (HBSS with 0.1% fetal bovine serum; pH 7.0) and allowed to equilibrate for 15 min. After equilibration, 0.5 ml of uptake buffer containing [3H]digoxin and cold digoxin (0.5 μCi/well) at a final concentration of 30 nM was placed in each well, with and without various concentrations of L. monocytogenes proteins. Proteins were isolated as described above with the exception that proteins were not radioactive. After 1 h, uptake buffer was removed, and cells were washed three times with ice-cold uptake buffer and treated with 0.5 ml of 1% Triton X-100 in PBS prior to being scraped from the wells. The entire mixture was transferred to glass vials containing 5 ml of liquid scintillation cocktail (Scintisafe Econo 1; Fisher Scientific, Pittsburgh, Pa.). Albumin, in concentrations similar to those of the L. monocytogenes proteins, was used as a control for amount of protein in each well. Each condition was tested in triplicate.
Inoculation of mdr1a−/− and FVB mice.
Animal experiments were performed according the NIH Guide for Care and Use of Laboratory Animals and the animal protocol was approved by the Research Animal Resource Center at the University of Wisconsin—Madison. Male 8-week-old mdr1a−/− mice and the wild-type controls, FVB mice, were obtained from Taconic Laboratories (Germantown, N.Y.) and housed in microisolator cages throughout the study. Mice acclimated to the environment with access to food and water ad libitum for 7 days prior to study. Five hours prior to inoculation, food was removed from the cage. For intragastric challenge, mice were mildly sedated with intraperitoneal pentobarbital (0.75 to 1 mg per 25 g of body weight), and 107 organisms in 0.2 ml of PBS were delivered into the stomach by using a 24-gauge pediatric feeding needle. For intravenous challenge, mice were administered 104 organisms in 0.2 ml of PBS via tail vein. To measure the dissemination of L. monocytogenes from the gastrointestinal tract, mice were euthanized by CO2 asphyxiation at days 1 and 3 postinfection, and the livers and spleens were aseptically harvested and weighed (five to six per strain per study day). The tissues were homogenized in 1 ml of sterile saline, and serial dilutions were plated in duplicate on blood agar to allow the enumeration of CFU. The plates were allowed to dry and then incubated at 37°C for 48 h. The colonies were counted after 48 h, and data were expressed as the log10 CFU of L. monocytogenes per gram (wet weight) of tissue (mean ± SD).
Statistics.
Data were analyzed by one-way analysis of variance using SigmaStat Statistical Software 2.03 (SPSS, Chicago, Ill.). If significant differences were detected, pairwise comparisons were made using a Tukey post-hoc test. Significance was defined as a P value of <0.05.
RESULTS
Effect of P-glycoprotein modulation on L. monocytogenes invasion in Caco-2 cells.
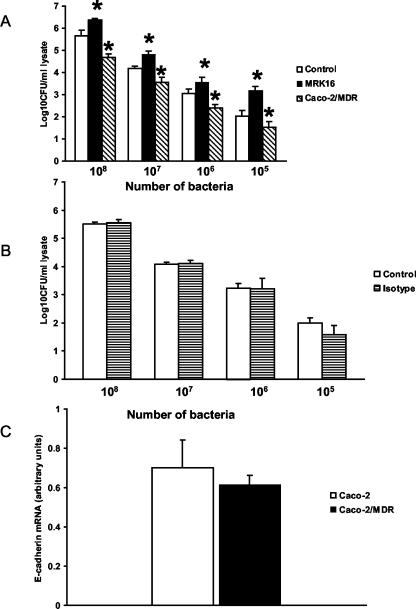
To evaluate whether the expression level of P glycoprotein affected L. monocytogenes invasion, a subclone of Caco-2 cells that overexpressed P glycoprotein (Caco-2/MDR) was derived and an in vitro invasion assay conducted (Fig. 1A). Quantitative real-time PCR confirmed that expression of E-cadherin, a key epithelial receptor for L. monocytogenes, was similar between the two cell lines (Fig. 1C). Compared to the control Caco-2 cells, Caco-2/MDR cells were more resistant than the parent line to L. monocytogenes invasion for all concentrations of bacteria employed (P < 0.05). We next determined if inhibition of P glycoprotein with MRK16 led to increased infection. Preincubation of Caco-2 cells with MRK16 led to significantly increased invasion of the cells (Fig. 1A). An isotype control antibody had no effect on invasion, confirming that increased invasion was due to P-glycoprotein inhibition (Fig. 1B). Thus, increased P-glycoprotein expression on the apical membrane of Caco-2 cells led to decreased infection, and, conversely, functional inhibition resulted in increased infection.
FIG. 1.
L. monocytogenes invasion of Caco-2 monolayers. (A) Caco-2 cells, Caco-2/MDR cells, or Caco-2 cells treated with MRK16 (final concentration, 10 μg/ml) were incubated with 108, 107, 106, and 105 L. monocytogenes EGD organisms, and invasion was measured after 1 h (n = 6 per condition). Caco-2/MDR cells were less susceptible and MRK16-treated cells were more susceptible to L. monocytogenes invasion. *, P < 0.05 compared to untreated Caco-2 cells. (B) An isotype control antibody had no effect on invasion (n = 3 per condition). Data are means plus SD. (C) Real-time PCR results confirming no difference in E-cadherin mRNA expression between Caco-2 and Caco-2/MDR cell lines (P > 0.05). The relative concentration of E-cadherin mRNA was normalized to GAPDH mRNA levels. Values are means plus SD from duplicate experiments.
Bidirectional flux of 35S-labeled L. monocytogenes proteins.
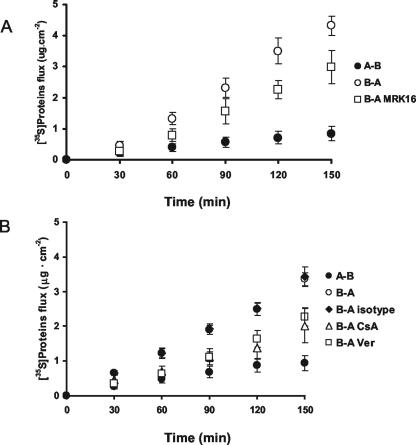
To further delineate the mechanism of P-glycoprotein involvement, newly synthesized L. monocytogenes EGD proteins were labeled with [35S]methionine, and nonbacterial bound proteins were isolated. The bidirectional flux of the 35S-proteins across Caco-2 cells grown on permeable inserts was calculated. P glycoprotein was inhibited by using MRK16, cyclosporine, and verapamil. As depicted in Fig. 2A, the transepithelial fluxes of the 35S-labeled L. monocytogenes proteins showed a striking asymmetry. Jb-a was markedly greater than Ja-b, indicating that at least some of the L. monocytogenes proteins are substrates for an apically located efflux transporter. Addition of MRK16 lowered the Jb-a, confirming P-glycoprotein affinity for at least one of the L. monocytogenes proteins. The isotype control antibody had no effect on transport, indicating that the decrease was due to P-glycoprotein inhibition (Fig. 2B). Further data suggesting that some of the L. monocytogenes proteins are substrates for P glycoprotein were provided by results obtained with the P-glycoprotein inhibitors cyclosporine and verapamil, which also reduced Jb-a (Fig. 2B).
FIG. 2.
Bidirectional transport of 35S-labeled L. monocytogenes proteins across Caco-2 cells grown on permeable inserts. 35S-labeled proteins were placed into either the apical or basolateral compartment, and their flux into the other compartment was measured. A-B, apical-to-basolateral transport; B-A, basolateral-to-apical transport. (A) MRK16 was used to inhibit P-glycoprotein-mediated B-A transport (n = 3 per condition). (B) Additional P-glycoprotein inhibitors, cyclosporine (CsA) and verapamil (Ver), decrease the B-A transport of 35S-labeled proteins (n = 3 per condition). An isotype control to MRK16 did not alter B-A flux of 35S-proteins. Data are means ± SD.
Competition of L. monocytogenes proteins with digoxin for P glycoprotein.
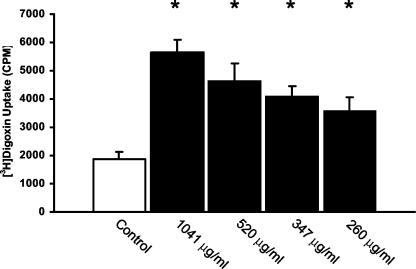
High affinity of the L. monocytogenes proteins for P glycoprotein was supported by competitive uptake experiments with the classic P-glycoprotein substrate, digoxin. Nonradioactive L. monocytogenes protein(s) was able to preferentially compete with [3H]digoxin for P-glycoprotein binding in a concentration-dependent manner, as demonstrated by increased [3H]digoxin accumulation (Fig. 3). Albumin, at concentrations similar to those used with the L. monocytogenes proteins, did not affect [3H]digoxin uptake (data not shown). Based on these experiments, we believe that at least one of the nonattached surface or secreted proteins of L. monocytogenes is a substrate of P glycoprotein.
FIG. 3.
Effect of L. monocytogenes proteins on the intracellular accumulation of the P-glycoprotein substrate digoxin. Cells were treated with [3H]digoxin (30 nM); its uptake into Caco-2 cells was measured after 1 h (n = 3 per condition) in the presence and absence of increasing amounts of L. monocytogenes proteins (x axis). L. monocytogenes proteins preferentially compete for P-glycoprotein binding, resulting in increased intracellular accumulation of digoxin. Data are means plus SD. *, P < 0.05 compared to digoxin alone.
L. monocytogenes dissemination in FVB and mdr1a−/− mice.
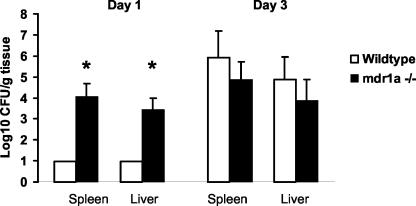
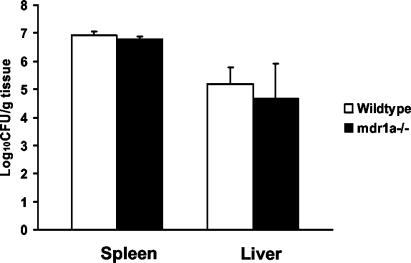
Based on the in vitro experiments, expression levels of P glycoprotein influenced L. monocytogenes invasion. Therefore, we next tested whether expression levels of intestinal P glycoprotein alter L. monocytogenes invasion in vivo. mdr1a−/− mice were challenged with 107 L. monocytogenes EGD organisms via gavage, and dissemination from the gastrointestinal tract to the liver and spleen was quantified on days 1 and 3 postinfection. Compared to wild-type animals, the mdr1a−/− mice had significantly higher bacterial counts in both the liver and spleen at day 1 postinfection (Fig. 4). No dissemination differences were observed at day 3, indicating that any protective effect of P glycoprotein is limited to early dissemination of the organism from the gastrointestinal tract. Lastly, to exclude the possibility that the absence of P glycoprotein adversely affected the systemic innate resistance to L. monocytogenes, the above experiment was repeated with an intravenous challenge with the organism. No difference in the number of CFU per organ between mdr1a−/− and wild-type mice was detected when L. monocytogenes was administered intravenously, further supporting the importance of intestinal P glycoprotein (Fig. 5).
FIG. 4.
Dissemination of L. monocytogenes from the gastrointestinal tract to the spleen and liver is greater in P-glycoprotein knockout (mdr1a−/−) mice than wild-type mice after oral inoculation (five per group per study day). Mice were given 107 L. monocytogenes organisms in the stomach, and dissemination was measured on days 1 and 3 postinoculation. Data are means plus SD. *, P < 0.05.
FIG. 5.
Dissemination of L. monocytogenes to the spleen and liver does not differ in P-glycoprotein knockout (mdr1a−/−) and wild-type mice after intravenous inoculation. Mice were given 104 L. monocytogenes EGD organisms via tail vein injection, and dissemination was measured on day 1 postinoculation. No differences in numbers of CFU per organ were detected (P > 0.05). Data are means plus SD (n = 5 for wild-type mice and 6 for mdr1a−/− mice).
DISCUSSION
Considerable research is directed towards elucidating the role of intestinal P glycoprotein in diminished absorption of drug substrates. Little is known regarding other physiological roles of P glycoprotein in the intestine. In the present study, both the expression and function of P glycoprotein influenced the degree of L. monocytogenes invasion. These findings were consistent for both in vitro and in vivo investigations. The proposed mechanism of protection may be the efflux of a bacterial protein(s) necessary for invasion, as 35S-labeled L. monocytogenes proteins were preferentially transported in the basolateral-to-apical direction.
Undoubtedly, there are numerous host defense mechanisms in place which interfere with the invasion of pathogenic bacteria such as L. monocytogenes (4, 6, 9, 20). P glycoprotein appears to be one important protein at the epithelial level, most likely working in concert with these other factors to protect the host. However, since inhibition and overexpression of P glycoprotein in Caco-2 cells did lead to significant changes in invasion, the role of P glycoprotein cannot be discounted. Although the dissemination differences at day 1 in the mdr1a−/− mice were almost fivefold greater than differences in wild-type mice, one may argue that these are not representative data, since gene deletion is not a realistic occurrence in humans. However, use of the mdr1a−/− mice does allow the assessment of maximal changes in invasion and assists with the elucidation of the role of P glycoprotein. Furthermore, differences in dissemination potentially may have been due to disparities in gut flora between the mdr1a−/− and wild-type mice. Unfortunately, there is no way to confirm if there were differences in flora, since fecal testing was not performed immediately prior to shipping (as confirmed by Taconic). However, all animals were housed in the same facility under identical conditions, and therefore we believe it is unlikely that flora were different, since environmental exposures were similar once the animals arrived at our facility.
One recent investigation elegantly determined that mdr1a−/− mice have altered intestinal intraepithelial lymphocyte (IEL) populations which are linked to changes in proliferation and cytokine secretion (8). Although one might suggest that altered IELs may have influenced our findings with L. monocytogenes dissemination, we believe that it is unlikely that these differences in IEL populations explain the results that we observed. First, in the previous study the total number of IEL were not significantly different between mdr1a−/− and wild-type mice. Second, the proportions of CD8 αβ and T-cell receptor αβ+ IELs were significantly increased, whereas CD8 αα and T-cell receptor γδ+ IELs were diminished. Given the overall makeup of the IEL imbalance, one would not predict significant changes in host response to an enteric pathogen. Third, cell proliferation and phorbol myristate acetate- and ionomycin-stimulated gamma interferon secretion by IELs from mdr1a−/− mice were actually greater (P < 0.05) than those by normal IELs. Because gamma interferon is essential for host response to L. monocytogenes, any cytokine changes in the present study would not be in a direction detrimental to host defense (19, 20, 23). Lastly, peripheral-T-cell proliferation, cytokine secretion, and cytotoxic function are unaffected by a lack of the mdr1a gene in mice (7). Thus, we believe it to be unlikely that any IEL differences in the mdr1a−/− mice negatively affected resistance to the L. monocytogenes challenge.
Several lines of evidence support the concept of a role of intestinal P glycoprotein in host defense against bacteria. Decreased P glycoprotein or the lack of P glycoprotein on the apical surface of the enterocyte may constitute a “functional defect” in the intestinal epithelial barrier, rendering the host susceptible to bacteria. Bacteria have long been hypothesized to play a central role in the development of inflammatory bowel disease (IBD) (30). Knockout mice demonstrating cytokine imbalances or T-cell abnormalities do not develop IBD in germ-free environments; however, when placed in conventional housing, they develop severe colitis, presumably due to exposure to commensal organisms (26, 29). Panwala and colleagues were the first to report that mdr1a−/− mice spontaneously develop colitis when housed under conventional housing conditions over a long period of time (21). Because mdr1a−/− mice are immunologically normal, it has been suggested that lack of P glycoprotein led to diminished barrier function against colitis-inducing luminal bacteria. These authors conducted a follow-up study which demonstrated that inoculation of mdr1a−/− mice with Helicobacter bilis, an organism known to cause inflammation in other murine IBD models, accelerated the development of colitis (16). Helicobacter-induced colitis in mdr1a−/− mice and other models may be due to a family of cytolethal distending toxins (31). Thus, P glycoprotein may protect the enterocyte against these toxins, and the lack of the efflux transporter may explain the accelerated inflammation in the mdr1a−/− mice.
Altered intestinal P-glycoprotein levels due to polymorphic variations and ulcerative colitis (UC) in humans has also been detected (25). Polymorphisms are relatively common in the MDR1 gene, with some mutations being linked to altered transport of P-glycoprotein substrates. Specifically, individuals homozygous for the T allele of the mutation on exon 26 (C3435T) have considerably lower intestinal P-glycoprotein levels than those with the 3435CC genotype. This correlates with decreased oral absorption of the P-glycoprotein substrate digoxin (10). Because of these altered expression levels with the C3435T mutation, Schwab and colleagues hypothesized that lower expression levels of intestinal P glycoprotein may result in altered barrier function and decreased resistance to inflammation-inducing bacteria (25). In their case-control study involving 145 patients with UC and sex-matched healthy controls, UC patients were found to have significantly higher frequencies of the TT genotype than the sex-matched controls, supporting the concept that intestinal P glycoprotein is important for host defense against bacteria and inflammation-inducing toxins.
Our data are not the first to suggest a protective role for efflux transporters in protecting the host against pathogenic bacteria. Schultz and colleagues investigated the efflux transporter, mrp1, in protection against Streptococcus pneumoniae-induced pneumonia (25). In addition to the excretion of anticancer drugs and glutathione-S conjugates of drugs, mrp1 transports cysteinyl leukotrienes (LT) which are important for defense against bacterial pathogens. mrp1−/− mice challenged with S. pneumoniae were more resistant to infection than wild-type controls. The mechanism for increased protection is believed to involve increased production of LTB4, which is important for resistance to S. pneumoniae and occurs as a result of decreased secretion of LTC4 by mrp1. Thus, efflux transporters may play a role in host defense not only by the secretion of virulence proteins, as in our study, but also by alterations of metabolic pathways which subsequently lead to increased production of antibacterial mediators.
It is interesting that P glycoprotein is also highly expressed in other tissues serving as primary barriers to L. monocytogenes invasion: the blood brain barrier and the placenta (13, 22). Furthermore, populations at high risk for listeriosis (immunocompromised persons, human immunodeficiency virus-seropositive individuals, and those with renal insufficiency) may take drugs known to affect P-glycoprotein function (cyclosporine, steroids, human immunodeficiency virus protease inhibitors, calcium channel blockers, etc.) or have baseline P-glycoprotein dysfunction (15, 17). Granted, these patients also have decreased cellular immunity, but modulation of P glycoprotein may also affect early resistance to L. monocytogenes infection and therefore cannot be ruled out.
In summary, the present work indicates that the expression and function of intestinal P glycoprotein is important for host defense against L. monocytogenes. These results, combined with those from the mdr1a−/− IBD studies, suggest that the current research paradigm of P glycoprotein in the intestine must be expanded to include host defense against pathogenic bacteria.
Acknowledgments
This work was supported by USDA grant NRICGP 2002-02287 and the Wisconsin Pharmacy Practice Initiative.
Editor: J. B. Bliska
REFERENCES
- 1.Ambudkar, S. V., S. Dey, C. A. Hrycyna, M. Ramachandra, I. Pastan, and M. M. Gottesman. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol Toxicol. 39:361-398. [DOI] [PubMed] [Google Scholar]
- 2.Braun, L., B. Ghebrehiwet, and P. Cossart. 2000. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, L., H. Ohayon, and P. Cossart. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 4.Brombacher, F., and M. Kopf. 1996. Innate versus acquired immunity in listeriosis. Res. Immunol. 147:505-511. [DOI] [PubMed] [Google Scholar]
- 5.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 6.Daniels, J. J., I. B. Autenrieth, and W. Goebel. 2000. Interaction of Listeria monocytogenes with the intestinal epithelium. FEMS Microbiol. Lett. 190:323-328. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbraun, M. D., and R. A. Miller. 1999. mdr1a-encoded P-glycoprotein is not required for peripheral T cell proliferation, cytokine release, or cytotoxic effector function in mice. J. Immunol. 163:2621-2627. [PubMed] [Google Scholar]
- 8.Eisenbraun, M. D., R. L. Mosley, D. H. Teitelbaum, and R. A. Miller. 2000. Altered development of intestinal intraepithelial lymphocytes in P-glycoprotein-deficient mice. Dev. Comp. Immunol. 24:783-795. [DOI] [PubMed] [Google Scholar]
- 9.Emoto, M., O. Neuhaus, Y. Emoto, and S. H. Kaufmann. 1996. Influence of beta 2-microglobulin expression on gamma interferon secretion and target cell lysis by intraepithelial lymphocytes during intestinal Listeria monocytogenes infection. Infect. Immun. 64:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmeyer, S., O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. 2000. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd, S., Q. Phan, J. Hadler, B. Mackenzie, S. Lance-Parker, P. Blake, M. Deasy, J. Rankin, et al. 2000. Multistate outbreak of listeriosis—United States 2000. Morbid. Mortal. Wkly. Rep. 50:1129-1130. [PubMed] [Google Scholar]
- 12.Kartner, N., J. R. Riordan, and V. Ling. 1983. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science 221:1285-1288. [DOI] [PubMed] [Google Scholar]
- 13.Kim, R. B., M. F. Fromm, C. Wandel, B. Leake, A. J. Wood, D. M. Roden, and G. R. Wilkinson. 1998. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 101:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 15.Lin, J. H. 2003. Drug-drug interaction mediated by inhibition and induction of P-glycoprotein. Adv. Drug Deliv. Rev. 55:53-81. [DOI] [PubMed] [Google Scholar]
- 16.Maggio-Price, L., D. Shows, K. Waggie, A. Burich, W. Zeng, S. Escobar, P. Morrissey, and J. L. Viney. 2002. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am. J. Pathol. 160:739-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meaden, E. R., P. G. Hoggard, B. Maher, S. H. Khoo, and D. J. Back. 2001. Expression of P-glycoprotein and multidrug resistance-associated protein in healthy volunteers and HIV-infected patients. AIDS Res. Hum. Retrovir. 17:1329-1332. [DOI] [PubMed] [Google Scholar]
- 18.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 19.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa, S., T. Miura, S. Sasaki, and A. Nakane. 1996. The protective role of endogenous cytokines in host resistance against an intragastric infection with Listeria monocytogenes in mice. FEMS Immunol. Med. Microbiol. 16:291-298. [DOI] [PubMed] [Google Scholar]
- 21.Panwala, C. M., J. C. Jones, and J. L. Viney. 1998. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 161:5733-5744. [PubMed] [Google Scholar]
- 22.Pavek, P., Z. Fendrich, F. Staud, J. Malakova, H. Brozmanova, M. Laznicek, V. Semecky, M. Grundmann, and V. Palicka. 2001. Influence of P-glycoprotein on the transplacental passage of cyclosporine. J. Pharm. Sci. 90:1583-1592. [DOI] [PubMed] [Google Scholar]
- 23.Prada-Delgado, A., E. Carrasco-Marin, G. M. Bokoch, and C. Alvarez-Dominguez. 2001. Interferon-gamma listericidal action is mediated by novel Rab5a functions at the phagosomal environment. J. Biol. Chem. 276:19059-19065. [DOI] [PubMed] [Google Scholar]
- 24.Schlech, W. F., III, P. M. Lavigne, R. A. Bortolussi, A. C. Allen, E. V. Haldane, A. J. Wort, A. W. Hightower, S. E. Johnson, S. H. King, E. S. Nicholls, and C. V. Broome. 1983. Epidemic listeriosis—evidence for transmission by food. N. Engl. J. Med. 308:203-206. [DOI] [PubMed] [Google Scholar]
- 25.Schultz, M. J., J. Wijnholds, M. P. Peppelenbosch, M. J. Vervoordeldonk, P. Speelman, S. J. van Deventer, P. Borst, and T. van der Poll. 2001. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J. Immunol. 166:4059-4064. [DOI] [PubMed] [Google Scholar]
- 26.Schwab, M., E. Schaeffeler, C. Marx, M. F. Fromm, B. Kaskas, J. Metzler, E. Stange, H. Herfarth, J. Schoelmerich, M. Gregor, S. Walker, I. Cascorbi, I. Roots, U. Brinkmann, U. M. Zanger, and M. Eichelbaum. 2003. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology 124:26-33. [DOI] [PubMed] [Google Scholar]
- 27.Sellon, R. K., S. Tonkonogy, M. Schultz, L. A. Dieleman, W. Grenther, E. Balish, D. M. Rennick, and R. B. Sartor. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparreboom, A., J. van Asperen, U. Mayer, A. H. Schinkel, J. W. Smit, D. K. Meijer, P. Borst, W. J. Nooijen, J. H. Beijnen, and O. van Tellingen. 1997. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 94:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda, K., I. Pastan, and M. M. Gottesman. 1987. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J. Biol. Chem. 262:17432-17436. [PubMed] [Google Scholar]
- 30.Veltkamp, C., S. L. Tonkonogy, Y. P. De Jong, C. Albright, W. B. Grenther, E. Balish, C. Terhorst, and R. B. Sartor. 2001. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology 120:900-913. [DOI] [PubMed] [Google Scholar]
- 31.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]