Abstract
Mice primed with Mycobacterium bovis bacillus Calmette-Guérin (BCG) are highly sensitive to lipopolysaccharide (LPS)-induced liver injury and lethality. We found that interleukin-15 (IL-15) transgenic (Tg) mice primed with BCG were more susceptible to LPS-induced liver injury than non-Tg mice. The numbers of CD44+ CD8+ T cells expressing intracellular gamma interferon (IFN-γ) significantly increased in the livers of BCG-primed IL-15 Tg mice after LPS injection, and the depletion of CD8+ T cells from BCG-primed IL-15 Tg mice completely abolished the susceptibility to LPS-induced lethality. Liver T cells from BCG-primed IL-15 Tg mice produced IFN-γ in vitro in response to LPS, which was inhibited by the addition of anti-IL-12 monoclonal antibody (MAb). In vivo treatment with anti-IL-12 MAb inhibited the appearance of CD44+ CD8+ T cells expressing intracellular IFN-γ after LPS injection. These results suggest that the overexpression of IL-15 increases susceptibility to LPS-induced liver injury in BCG-primed mice via bystander activation of CD8+ T cells.
The incidence of infection with gram-negative bacteria such as Escherichia coli in patients undergoing abdominal surgery has increased in recent years. These infections frequently result in liver injury caused by lipopolysaccharide (LPS) derived from gram-negative bacteria (2, 4, 27). LPS-induced acute liver injury in mice pretreated with heat-killed Propionibacterium acnes or viable Mycobacterium bovis bacillus Calmette-Guérin (BCG) has been widely used as a model of hepatic failure associated with endotoxin. Tumor necrosis factor alpha (TNF-α) and Fas ligand have been shown to be effector molecules in these models (14, 19, 32, 33). Interleukin-12 (IL-12), IL-18, and gamma interferon (IFN-γ) have been reported to be intimately associated with this experimental liver injury, suggesting that IL-12- or IL-18-activated Th1-dominant immune responses might be involved in the pathogenesis of liver tissue injury (32, 33). In fact, Th1 cells capable of producing IFN-γ have been shown to be responsible for triggering lethal shock (13, 23, 26, 29). Although CD8+ T cells are known to be involved in liver injury in several hepatitis models (1, 30, 39), the contribution of CD8+ T cells to LPS-induced liver injury and lethality has not been elucidated.
IL-15 uses the β and γ chains of IL-2 receptor for signal transduction and thus shares many properties of IL-2 in spite of having no sequence homology with IL-2 (28, 37, 38). IL-15 has been reported to have potential roles in the development and maintenance of significant fractions of lymphocytes, including NK cells, T-cell receptor γδ (TCRγδ) intestinal intraepithelial lymphocytes, and memory phenotype CD8+ T cells (3, 10, 12, 42). It has been reported that IL-15 transgenic (Tg) mice contain a large number of central-memory-phenotype CD8+ T cells expressing CD44high CD62L+ Ly6C+ in peripheral lymphoid tissues (5, 16, 22) and show strong protection against microbial infection via the increased generation of antigen (Ag)-specific CD8+ T cells (34, 35, 40). It is also evident in IL-15 Tg mice that the overexpression of IL-15 can stimulate Ag-nonspecific memory phenotype CD8+ T cells in a bystander manner, providing protection at an early stage of bacterial infection (41). Thus, IL-15-dependent CD8+ T cells may play important roles in both innate immunity and acquired immunity against bacterial infection.
With the aim of determining the involvement of IL-15-dependent CD8+ T cells in LPS-induced liver injury in BCG-primed mice, we examined the susceptibility of IL-15 Tg mice inoculated with BCG to LPS-induced liver injury. We found that BCG-primed IL-15 Tg mice were highly susceptible to LPS-induced liver injury. The number of CD44+ CD8+ T cells expressing intracellular IFN-γ increased in BCG-primed IL-15 Tg mice after LPS injection. The T cells from BCG-primed IL-15 Tg mice produced IFN-γ in vitro in response to LPS, which was inhibited by the addition of anti-IL-12 monoclonal antibody (MAb). The depletion of CD8+ T cells in BCG-primed IL-15 Tg mice in vivo prevented LPS-induced lethal shock. IL-15-dependent CD8+ T cells activated in a bystander manner may be involved in LPS-induced lethal shock in BCG-primed mice.
MATERIALS AND METHODS
Mice.
C57BL/6-background IL-15 Tg mice, whose transgenicity was constructed using originally described IL-15 cDNA under the control of a major histocompatibility complex (MHC) class I promoter, have been described previously (22). Age- and sex-matched C57BL/6 mice obtained from Japan SLC (Hamamatsu, Japan) were used as control mice. All mice were 6 to 8 weeks of age. All experiments were done according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (20a).
Microorganisms and LPS.
M. bovis BCG strain Tokyo was grown in Middlebrook 7H9 medium (Difco, Detroit, Mich.) supplemented with albumin-dextrose-catalase enrichment (Difco) and Tween 80 (Difco) at 37°C. At the mid-log phase, the bacteria in the culture were stored in Middlebrook 7H9 medium supplemented with albumin-dextrose-catalase enrichment, Tween 80, and 20% (vol/vol) glycerol at −70°C until they were used. E. coli-derived LPS (E. coli serotype O55) was purchased from Sigma (St. Louis, Mo.). Mice were infected intravenously (i.v.) with 106 CFU of M. bovis BCG and challenged 7 days later with LPS.
Antibodies and reagents.
Fluorescein isothiocyanate (FITC)-conjugated anti-CD3ɛ (145-2C11), anti-CD44 (IM7), and anti-IFN-γ (XMG1.2); phycoerythrin (PE)-conjugated anti-CD8α (53-6.7), anti-CD44 (IM7), anti-TCRγδ (UC7-13D5), and anti-NK1.1 MAb (PK136); Cy-Chrome-conjugated anti-CD4 (RM4-5) and anti-TCRβ (H57-597); and allophycocyanin (APC)-conjugated CD44 (IM7) were purchased from Pharmingen (San Diego, Calif.). Cy-Chrome- and APC-conjugated streptavidin were also obtained from Pharmingen. The cells were incubated with saturating amounts of FITC-, PE-, Cy-Chrome-, APC-, and biotin-conjugated Abs for 30 min at 4°C. To detect biotin-conjugated MAbs, cells were stained with Cy-Chrome- or APC-conjugated streptavidin. For in vivo CD8+-T-cell depletion or IFN-γ neutralization, anti-CD8 or anti-IFN-γ MAb was obtained by growing hybridoma 2.43 or R4 6A2 cells in serum-free medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). Neutralizing rat anti-mouse IL-12 MAb (clone C15.6) was purchased from Biosource International. Neutralizing rat anti-mouse IL-15 MAb (G277-3588) was obtained from Pharmingen. Recombinant mouse IL-12 (rIL-12) and rIL-15 were purchased from Peprotech Corporation (San Diego, Calif.).
In vivo treatment with MAbs.
For in vivo cell depletion, mice were injected intraperitoneally (i.p.) with 400 μg of anti-CD8 MAb, 20 μg of anti-asialo GM1 Ab, or isotype control rat immunoglobulin G (IgG) at 24 h before LPS injection. In some experiments, mice were treated i.p. with neutralizing anti-IFN-γ MAb, anti-IL-12 MAb, or control rat IgG at 2 h before and after LPS injection.
Cell preparation.
Liver mononuclear cells were prepared as described previously (25). Briefly, fresh livers were immediately perfused with sterile Hanks balanced salt solution (HBSS) through the portal vein to wash out all remaining peripheral blood and then pressed through stainless steel mesh. After the coarse pieces had been removed by centrifugation at 50 × g for 1 min, the cell suspensions were again centrifuged, resuspended in 8 ml of 45% Percoll (Sigma Chemical Co.), and layered onto 5 ml of 67.5% Percoll. The gradients were centrifuged at 600 × g for 20 min at 20°C. Liver mononuclear cells at the interface were harvested and washed twice with HBSS. In some experiments, liver mononuclear cells were suspended in RPMI 1640 medium (Sigma Chemical Co.) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10 mM HEPES.
Cell culture.
Splenocytes or liver mononuclear cells were incubated without any stimulation or with purified protein derivative (PPD), LPS, or immobilized anti-CD3 MAb for 24 h at 37°C under 5% CO2 in 96-well flat-bottomed plates in a 0.2-ml volume of RPMI 1640 medium containing 10% fetal calf serum. After incubation for 24 h, the supernatant was collected to estimate cytokine production. The cytokine activity in the supernatants was assayed by enzyme-linked immunosorbent assay (ELISA).
Cytokine ELISA.
The cytokine activity in the supernatants or sera from the mice was assayed by using an ELISA development kit for mouse IFN-γ, TNF-α, IL-12, and IL-1β (Genzyme TECHNE). An ELISA for mouse IL-18 was performed using a mouse IL-18 ELISA kit (MBL).
Assay for serum alanine aminotransferase activity.
Liver injury was assayed by evaluating serum alanine aminotransferase (ALT) activity. This activity was determined by using a serum ALT test kit (DIA-Iatron, Tokyo, Japan). Briefly, 40 μl of the serum sample was incubated with a solution containing 200 μl each of l-alanine and l-ketoglutaric acid for 30 min at 37°C. Twenty minutes after the addition of 200 μl of 2,4-dinitrophenylhydrazine, 2 ml of 0.4 N NaOH was added, and visible light absorption was measured at 505 nm. ALT activities (in international units per milliliter) were calculated from the standard curve.
Intracellular cytokine staining.
BCG-primed mice were each injected i.v. with 10 μg of LPS. At 3 h after LPS injection, splenocytes or liver mononuclear cells were harvested, washed, and suspended at 106 cells/ml in complete culture medium, and then the cells were incubated for 4 h at 37°C in the presence of 10 μg of brefeldin A (Sigma Chemical Co.) per ml. After 4 h of incubation, the cells were harvested, washed once in HBSS containing 2.5% newborn horse serum and 0.1% NaN3 (staining buffer), and surface stained in staining buffer with either PE-conjugated anti-CD8 MAb, Cy-Chrome-conjugated anti-CD4 MAb, and APC-conjugated CD44 or Cy-Chrome-conjugated TCRβ and PE-conjugated NK1.1. After surface staining, cells were subjected to intracellular cytokine staining with a Fast Immune cytokine system (Becton Dickinson) according to the manufacturer's instructions. For intracellular cytokine staining, we used FITC-conjugated anti-IFN-γ or FITC-conjugated rat IgG1 or IgG2b as isotype controls. Samples were acquired in a FACSCalibur flow cytometer and analyzed with CELLQuest software.
Statistical analysis.
The statistical significance of the survival rate was determined by the generalized Wilcoxon test. Other data were determined by Student's t test. Differences with a P value of <0.05 were considered significant.
RESULTS
Susceptibility of IL-15 Tg mice primed with BCG to LPS-induced liver injury and lethal toxicity.
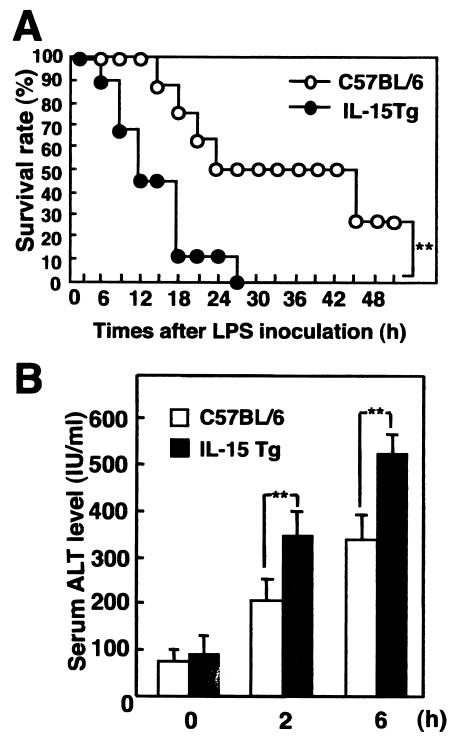
It is well known that pretreatment with viable BCG increases the susceptibility of mice to LPS-induced liver injury and lethal shock (14, 17, 24). We examined survival rate and liver injury following the injection of LPS into IL-15 Tg mice that had been inoculated with BCG 7 days previously. All of the IL-15 Tg mice died after LPS injection, whereas 30% of the C57BL/6 mice survived for more than 48 h after injection with the same dose of LPS (P < 0.01) (Fig. 1A). The ALT level in BCG-primed IL-15 Tg mice after LPS injection was significantly higher than that in BCG-primed control mice (P < 0.01) (Fig. 1B). Thus, the overexpression of IL-15 accelerated LPS-induced liver injury and lethal shock in BCG-primed mice.
FIG. 1.
Susceptibility to LPS-induced liver injury and lethal shock in BCG-primed IL-15 Tg mice. (A) Survival after LPS injection of IL-15 Tg and control C57BL/6 mice that had been inoculated with BCG 7 days previously. Mice were primed by i.v. injection of M. bovis BCG (1 mg ≈ 106 CFU) via tail veins and were challenged with an i.v. injection of LPS (10 μg) 7 days later. The survival rates after LPS challenge were calculated. **, significantly different from the value for control mice (P value of <0.01 by the generalized Wilcoxon test). Seven to 10 mice per group were used per experiment. The typical results of one of three independent experiments are shown. (B) Liver injuries in BCG-primed IL-15 Tg mice and control mice after LPS injection. Sera were collected at the indicated times after LPS challenge from IL-15 Tg mice and control C57BL/6 mice that had been inoculated i.v. with BCG 7 days previously. ALT levels in serum after LPS challenge in BCG-primed mice are shown. Data are presented as means + standard deviations (SDs) for five mice. **, P < 0.01.
Cytokine production in BCG-primed IL-15 Tg mice after LPS injection.
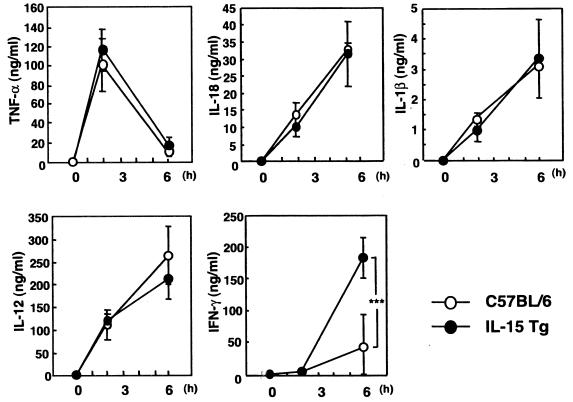
LPS-induced liver injury has been shown to be strongly correlated with increased production of several inflammatory cytokines such as TNF-α and IL-1β. Therefore, we next examined the levels of several inflammatory cytokines (TNF-α, IL-1β, IL-12, IL-18, and IFN-γ) in serum after i.v. inoculation with LPS. As shown in Fig. 2, serum TNF-α levels were maximal at 2 h after LPS injection, and IL-12 p40, IL-18, and IL-1β levels in serum were maximal at 6 h after LPS injection in both IL-15 Tg and non-Tg mice. There were no significant differences between the levels of production of these proinflammatory cytokines among IL-15 Tg mice and non-Tg mice. On the other hand, the level of IFN-γ in serum was significantly higher at 6 h after LPS injection in IL-15 Tg mice than in non-Tg mice (P < 0.001). Thus, these results suggest that the increased susceptibility of BCG-primed IL-15 Tg mice to LPS-induced liver injury is associated with increased production of IFN-γ.
FIG. 2.
Levels of inflammatory cytokines in serum after LPS injection in BCG-primed IL-15 Tg mice. IL-15 Tg and control C57BL/6 mice were injected i.v. with 1 mg of BCG and challenged with LPS 7 days after infection. Sera were collected at 0, 2, or 6 h after LPS injection, and levels of TNF-α, IL-1β, IL-12, IL-18, and IFN-γ in serum were determined by ELISA. The results shown are means ± SDs for five mice sera. ***, P < 0.001.
T-cell subset is responsible for increased production of IFN-γ and susceptibility after LPS injection.
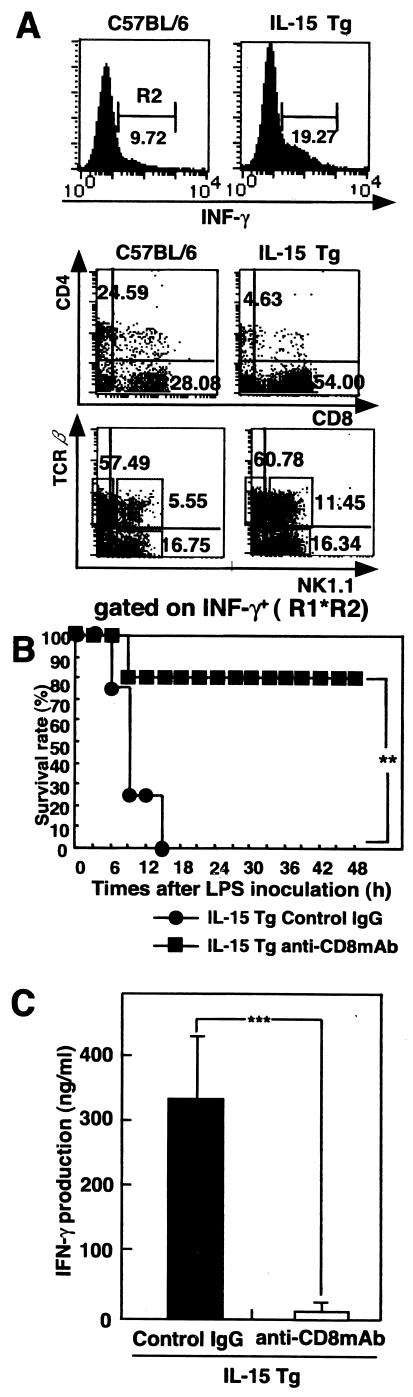
To determine which population of liver lymphocytes of BCG-primed IL-15 Tg mice was responsible for increased IFN-γ production after LPS injection, we utilized cytokine fluorescence-activated cell sorter (FACS) analysis for the expression of CD4, CD8, and intracellular IFN-γ in liver mononuclear cells harvested 3 h after LPS injection. As shown in Fig. 3A, the number of CD8+ T cells expressing intracellular IFN-γ in BCG-primed IL-15 Tg mice markedly increased after LPS injection. The number of NK T cells expressing intracellular IFN-γ also increased in BCG-primed IL-15 Tg mice after LPS injection, but the proportion of NK cells expressing IFN-γ in BCG-primed IL-15 Tg mice was not different from the proportion in BCG-primed control mice. These results indicated that liver CD8+ T cells are mainly responsible for increased IFN-γ production in BCG-primed IL-15 Tg mice after LPS injection.
FIG. 3.
CD8+ T cells are responsible for LPS-induced lethal shock in BCG-primed IL-15 Tg mice. (A) BCG-primed IL-15 Tg mice and control C57BL/6 mice were i.v. injected with LPS, and liver mononuclear cells were harvested 3 h later. Cytokine FACS analysis was carried out for the expression of CD4, CD8, and intracellular IFN-γ or for NK1.1, TCRαβ, and intracellular IFN-γ. The results of flow cytometry are presented as typical profiles after an analysis gate had beenset on lymphocytes using forward and side scatter (R1 data not shown) and on IFN-γ+ cells (R2). (B) Effect of depletion of CD8+ T cells on survival rate after LPS injection of BCG-primed IL-15 Tg mice. BCG-primed IL-15 Tg mice were i.p. injected with anti-CD8 MAb or control IgG 24 h before LPS inoculation. After CD8+ T cell depletion, mice were i.v. injected with 10 μg LPS. **, significantly different from the value for control mice (P value of <0.01 by the generalized Wilcoxon test). Seven to 10 mice per group were used per experiment. The typical results of one of three independent experiments are shown. (C) Effect of depletion of CD8+ T cells on IFN-γ production after LPS injection in BCG-primed IL-15 Tg mice. IL-15 Tg mice were treated i.p. with anti-CD8 MAb or control IgG 24 h before LPS injection. Sera were collected at 6 h after LPS challenge from Ab-treated mice. IFN-γ levels in serum after LPS challenge in BCG-primed IL-15 Tg mice are shown. Data are presented as the means + SDs for five mice. ***, P < 0.001.
To investigate the contribution of CD8+ T cells to the increased susceptibility to LPS-induced lethal shock in BCG-primed IL-15 Tg mice, anti-CD8 MAbs were administered i.p. 1 day before LPS injection. We confirmed by FACS analysis that CD8+ T cells were almost depleted in the spleens and livers of IL-15 Tg mice (data not shown). As shown in Fig. 3B, anti-CD8 MAb treatment improved the survival rate of IL-15 Tg mice primed with BCG after LPS injection (P < 0.01). Furthermore, we examined the level of IFN-γ in serum in CD8+ T cell-depleted IL-15 Tg mice after i.v. inoculation with LPS. The level of IFN-γ in serum was significantly lower at 6 h after LPS injection in CD8+ T cell-depleted IL-15 Tg mice than in control IL-15 Tg mice (Fig. 3C). These results suggest that CD8+ T cells contribute to the increased susceptibility to LPS-induced lethal shock in BCG-primed IL-15 Tg mice.
IFN-γ production by T cells in response to LPS in a bystander manner.
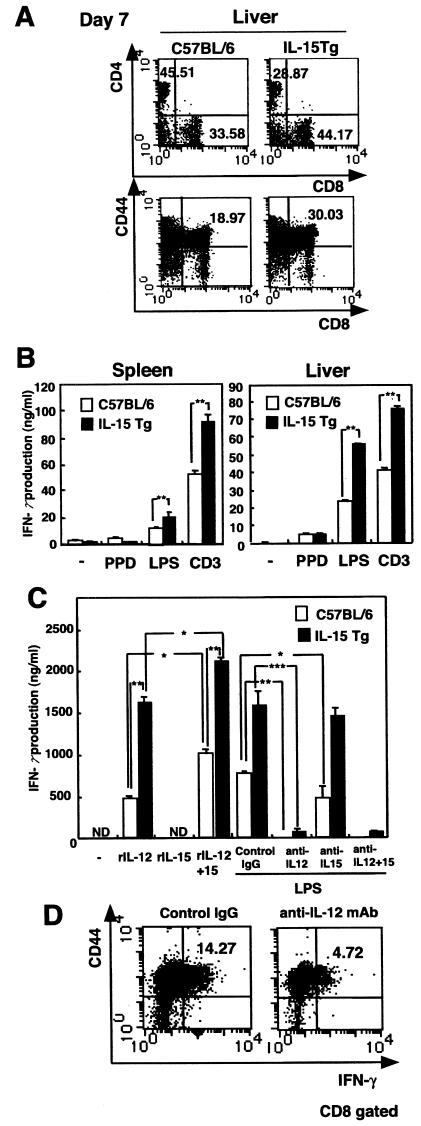
To determine how T cells produced IFN-γ in response to LPS in vivo, splenocytes or liver mononuclear cells isolated from IL-15 Tg mice that had been inoculated with BCG 7 days previously were cultured with PPD, LPS, or immobilized anti-CD3 MAb, and IFN-γ release in the culture supernatants was examined by ELISA. The number of CD44+ CD8+ cells had significantly increased in IL-15 Tg mice 7 days after BCG infection (Fig. 4A). Spleen and liver lymphocytes from BCG-primed IL-15 Tg mice produced higher levels of IFN-γ in response to CD3 triggering than did spleen and liver lymphocytes from non-Tg mice (Fig. 4B). On the other hand, IFN-γ production in response to PPD was only marginally detected at this stage in both IL-15 Tg mice and control mice. Surprisingly, lymphocytes produced a significant level of IFN-γ in response to LPS, and the level was higher in lymphocytes from BCG-primed IL-15 Tg mice than in lymphocytes from control mice (P < 0.01) (Fig. 4B).
FIG. 4.
Bystander stimulation of CD44+ CD8+ T cells for production of IFN-γ in the livers or spleens of BCG-primed IL-15 Tg mice. (A) Flow cytometry analysis of liver mononuclear cells from IL-15 Tg mice after BCG infection. Liver mononuclear cells from IL-15 Tg mice and control C57BL/6 mice infected with BCG 7 days previously werestained with FITC-CD3ɛ, PE-CD8α, and Cy-Chrome-CD4 MAb and then analyzed with a flow cytometer. The results of flow cytometry are presented as typical two-dimensional profiles after an analysis gate had been set on CD3+ cells. Cells were also stained with FITC-CD44 and PE-CD8α, and then the analysis gate was set on lymphocytes. (B) IFN-γ production by splenocytes and liver mononuclear cells from BCG-primed IL-15 Tg mice in response to LPS. Splenocytes and liver mononuclear cells from IL-15 Tg mice and control mice that had been infected with BCG 7 days previously were cultured with either PPD (5 μg/ml), LPS (1 μg/ml), or immobilized anti-CD3 MAb (20 μg/ml) for 24 h at 37°C. Thereafter, the supernatants were collected, and cytokine activity was determined by ELISA. The data are representative of three independent experiments using pooled cells from three IL-15 Tg or control mice and are shown as means of triplicate determinations ± SDs. **, statistically significant differences between IL-15 Tg mice and C57BL/6 mice (P < 0.01). (C) Cytokine production by splenocytes in response to rIL-15, rIL-12, or rIL-15 plus rIL-12 in IL-15 Tg and control mice was assessed by ELISA. The culture supernatants were collected, and IFN-γ activity was determined. The effect of anti-IL-12 MAb on the production of IFN-γ by splenocytes from BCG-primed IL-15 Tg mice in response to LPS is also shown. Splenocytes from BCG-primed IL-15 Tg and control mice were incubated with LPS in the presence or absence of 10 μg of anti-IL-12-, anti-IL-15-, or anti-IL-15- plus anti-IL-12-neutralizing MAb per ml. IFN-γ production in the culture supernatant was assessed as described above. The data are representative of two separate experiments and are expressed as means of triplicates ± SDs. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ND, not detectable. (D) Effect of in vivo treatment with anti-IL-12 MAb on IFN-γ production by CD44+ CD8+ T cells in IL-15 Tg mice. BCG-primed IL-15 Tg mice were i.p. injected with anti-IL-12 MAb or control IgG 2 h before and 2 h after LPS inoculation. Intracellular IFN-γ was detected in CD44+ CD8+ T cells from BCG-primed IL-15 Tg mice by flow cytometry 3 h after the LPS challenge.
Memory CD8+ T cells are characterized by bystander activation in response to cytokines (31, 42). It was previously reported that IL-15-dependent memory CD8+ T cells produced IFN-γ in response to IL-12 without any Ag stimulation (41). Therefore, we next examined the effect of exogenous IL-12 on IFN-γ production by splenocytes from BCG-primed IL-15 Tg mice. Splenocytes from IL-15 Tg mice and control mice infected with BCG 7 days previously were cultured with murine rIL-12 and/or rIL-15 for 24 h, and the culture supernatants were examined for IFN-γ release by ELISA. As shown in Fig. 4C, splenocytes produced IFN-γ in response to rIL-12 or rIL-12 plus rIL-15 at levels comparable to those seen in the case of LPS stimulation, and the level of IFN-γ production was significantly higher in IL-15 Tg mice than in control mice. To determine whether the observed IFN-γ production following LPS stimulation was dependent on endogenous IL-12, cultures were supplemented with anti-IL-12 MAb (10 μg/ml). Neutralizing IL-12 significantly suppressed IFN-γ secretion in LPS-stimulated spleen cells from both IL-15 Tg mice and control mice, indicating that endogenous IL-12 contributed to the IFN-γ production after LPS stimulation (Fig. 4C). These results suggest that splenocytes in BCG-primed IL-15 Tg mice produce a high level of IFN-γ in response to bystander stimulation with endogenous IL-12. It is notable that splenocytes from BCG-primed control mice responded more vigorously to rIL-12 and rIL-15 than to rIL-12 alone. Furthermore, anti-IL-15 MAb significantly inhibited IFN-γ production induced by LPS stimulation. These results suggest that endogenous IL-15 in BCG-primed mice, presumably induced by LPS, plays an important role in IFN-γ production by T cells in response to LPS.
To investigate the contribution of IL-12 to LPS-induced IFN-γ production by memory CD8+ T cells in BCG-primed IL-15 Tg mice, anti-IL-12 MAb was administered i.p. at 2 h before and 2 h after LPS injection. We confirmed by ELISA that the level of IL-12 in serum in IL-15 Tg mice treated with neutralizing anti-IL-12 MAb was significantly reduced at 3 h after LPS injection. Compared with control IgG-treated IL-15 Tg mice, IL-15 Tg mice treated with neutralizing anti-IL-12 MAb had significantly fewer IFN-γ+ CD44+ CD8+ cells after the LPS challenge (Fig. 4D). These results suggested that CD44+ CD8+ T cells in BCG-primed IL-15 Tg mice are at least partly involved in the production of a large amount of IFN-γ in response to bystander stimulation with IL-12.
Effects of neutralization of endogenous IFN-γ or depletion of CD8+-T-cell subset on susceptibility to LPS-induced liver injury and lethal shock in BCG-primed normal mice.
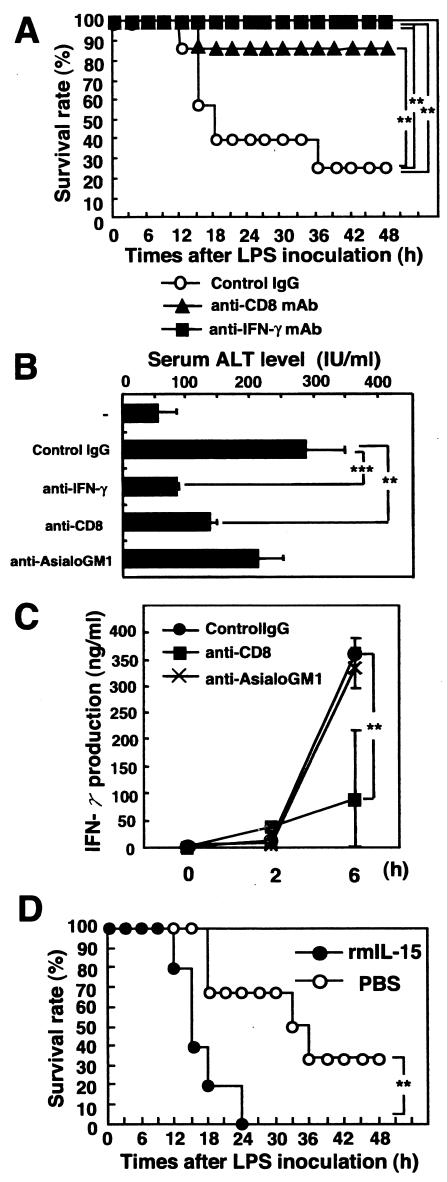
Our results suggest that liver CD8+ T cells in BCG-primed normal mice are involved in LPS-induced liver injury and lethal shock via IFN-γ production in response to LPS. To elucidate this possibility, we examined the effects of neutralization of endogenous IFN-γ or depletion of CD8+ T cells by in vivo administration of anti-IFN-γ MAb or anti-CD8 MAb on LPS-induced liver injury and lethal shock in C57BL/6 mice infected with BCG. Seventy percent of the control IgG-treated mice died within 48 h after LPS injection, whereas all of the mice given anti-IFN-γ MAb and 85% of the mice given anti-CD8 MAb survived after LPS injection (Fig. 5A). The level of ALT in serum was significantly lower in anti-IFN-γ MAb-treated mice and in anti-CD8 MAb-treated mice following LPS injection than in control IgG-treated mice (Fig. 5B). IFN-γ production in serum was impaired by the administration of anti-CD8 MAb (Fig. 5C). On the other hand, there were no significant differences between the levels of IFN-γ production in serum in anti-asialo GM1 Ab-treated mice and control IgG Ab-treated mice at 6 h after LPS injection. We confirmed by FACS analysis that NK cells were almost depleted in the spleens and livers of C57BL/6 mice (data not shown). These results suggest that IFN-γ and CD8 mainly contribute to the LPS-induced liver injury and lethal shock in BCG-primed mice not only in the situation of IL-15 overexpression but also under conditions of physiological stress.
FIG. 5.
IFN-γ production by T cells in BCG-primed C57BL/6 mice is responsible for LPS-induced lethal endotoxin shock. (A) Effect of neutralization of endogenous IFN-γ or depletion of CD8+ T cells on susceptibility to LPS-induced liver injury and lethal shock in BCG-primed mice. C57BL/6 mice were primed with an i.v. injection of M. bovis BCG (1 mg ≈ 106 CFU) via tail veins and were challenged 7 days later with an i.v. injection of LPS (10 μg). Mice were treated i.p. with neutralizing anti-IFN-γ MAb (R4 6A2), anti-CD8 MAb (2.43), or control rat IgG at 2 h before and 2 h after LPS injection. The survival of mice was checked every hour. **, significantly different from the value for control mice (P value of <0.01 by the generalized Wilcoxon test). (B) Effect of depletion of CD8 or NK cells on LPS-induced liver injury in BCG-primed C57BL/6 mice. C57BL/6 mice that had been infected with BCG 7 days previously were treated i.p. with anti-CD8, anti-asialo GM1 Ab, or isotype control IgG 24 h before LPS injection. Sera were collected at 6 h after LPS challenge from Ab-treated mice. ALT levels in serum after LPS challenge in the BCG-primed mice areshown. Data are presented as means + SDs for five mice. **, P < 0.01; ***, P < 0.001. (C) Effect of depletion of CD8+ T or NK cells on IFN-γ production in BCG-infected mice. C57BL/6 mice were treated i.p. with anti-CD8 MAb, anti-asialo GM1 Ab, or control IgG 24 h before LPS injection. Sera were collected at 2 and 6 h after LPS challenge from Ab-treated mice. IFN-γ levels in serum after LPS challenge in BCG-primed mice are shown. Data are presented as the means + SDs for five mice. **, P < 0.01. (D) Effects of in vivo administration of rIL-15 on LPS-induced lethal shock in C57BL/6 mice infected with BCG. rIL-15 (2 μg in 200 μl of PBS) or PBS (200 μl) for control was injected i.p. at 2 h before and 2 h after LPS injection, and survival was monitored for 48 h. Seven to 10 mice per group were used per experiment. The typical results of one of three independent experiments are shown. **, significantly different from the value for control mice (P value of <0.01 by the generalized Wilcoxon test).
Furthermore, we next examined the effects of the in vivo administration of rIL-15 on LPS-induced lethal shock in C57BL/6 mice primed with BCG. rIL-15 or phosphate-buffered saline (PBS) for control was injected i.p. at both 2 h before and 2 h after LPS injection. As shown in Fig. 5D, 30% of PBS-treated C57BL/6 mice survived for more than 48 h after LPS injection, whereas all of the rIL-15-treated mice died within 24 h after injection with the same dose of LPS (P < 0.01). Thus, the in vivo administration of rIL-15 accelerated LPS-induced lethal shock in BCG-primed normal mice.
DISCUSSION
We found that IL-15 Tg mice primed with BCG were highly susceptible to LPS-induced liver injury and lethal shock, accompanied by an increased number of CD44+ CD8+ T cells expressing intracellular IFN-γ in the liver. Depletion of CD8+ T cells from BCG-primed IL-15 Tg mice completely abolished the susceptibility to LPS-induced lethal shock. It was previously reported that memory CD8+ T cells produce IFN-γ in response to IL-15 and IL-12 without any relevant Ag stimulation and play a protective role at an early stage during the course of Listeria monocytogenes infection (41). In the present study, we also found that the T cells from BCG-primed IL-15 Tg mice produced a high level of IFN-γ in response to LPS and that the production was inhibited by the addition of anti-IL-12 MAb in vitro and in vivo. Furthermore, neutralizing IL-15 significantly suppressed IFN-γ secretion in LPS-stimulated spleen cells from normal mice in vitro, indicating that endogenous IL-15 also contributed to IFN-γ production after LPS stimulation. The failure of anti-IL-15 to inhibit LPS-induced IFN-γ production in IL-15 Tg splenocytes (Fig. 4C) may be explained by autocrine stimulation by cells that express the transgene. Alternatively, anti-IL-15 MAb may not be sufficient for the neutralization of IL-15 overproduction. Taken together, these results suggest that CD8+ T cells are activated to produce IFN-γ in a bystander manner by IL-12 released from LPS-stimulated macrophages and/or dendritic cells and contribute to increased susceptibility to LPS-induced liver injury in BCG-primed IL-15 Tg mice.
The specificity of memory-type CD8+ T cells appearing in the mouse livers and spleens 7 days after BCG infection remains unknown. Since IL-15 is known to play an important role in the homeostatic proliferation of polyclonal memory CD8+ T cells, it is most likely that polyclonal memory CD8+ T cells are subjected to non-Ag-specific bystander stimulation through contact with IL-12 released by LPS injection. In fact, cytokine production in in vitro culture and results of cytokine FACS analysis revealed that splenocytes from mice infected with BCG 7 days previously did not significantly respond to PPD, suggesting that the CD8+ T cells appearing after BCG infection are not specific for the mycobacterial Ag. However, most CD8+ T cells recognize endogenous Ag in the context of MHC class I molecules, and we assayed the Ag-specific CD8+ response by using exogenous PPD. Our assay system may not be appropriate for the detection of Ag specificity for CD8+ T cells. It has recently been reported that CD8+ T cells specific for bacterial peptide composed of formyl methionine in the context of MHC class Ib molecules H2-M3 appear at an earlier stage after bacterial infection than do conventional CD8+ T cells recognizing Ag in the context of classical MHC class I molecules (8, 15). Although a sufficient number of mycobacterial Ag-specific T cells may not be generated by 7 days after BCG infection, such unique CD8+ T cells presented by MHC class Ib molecules may be involved in expansion in a bystander manner with LPS stimulation. Further experiments are needed to clarify these possibilities.
A notable finding in the present study was that T cells even from BCG-primed non-Tg mice produced a significant level of IFN-γ in response to LPS or rIL-12 plus rIL-15. The depletion of CD8+ T cells just before LPS injection or neutralization of IFN-γ protected the mice from LPS-induced liver injury and lethal shock. This result suggests that not only in the specific situation of IL-15 overexpression in IL-15 Tg mice but also under conditions of physiological stress in non-Tg mice, IL-15-dependent CD8+ T cells play an important role in the pathogenesis of LPS-induced liver injury and lethality in BCG-primed mice.
IFN-γ is known to increase the susceptibility of various cells, including hepatocytes, to apoptosis induced by various stimuli (20, 21, 32). Cytotoxic activities via TNF family proteins, including Fas ligand, tumor necrosis factor-related apoptosis-inducing ligand, and serine proteases (among others, perforin and granzymes), are known to be involved in LPS-induced liver injury (6, 18, 36). At present, we do not know whether CD8+ T cells have cytotoxic activity against hepatocytes and other host cells. However, we found that the number of CD44+ CD8+ T cells expressing granzyme B significantly increased in BCG-primed IL-15 Tg mice after LPS injection (data not shown). Cytotoxic activity via granzymes from CD8+ T cells in IL-15 Tg mice may be involved in LPS-induced liver injury in addition to IFN-γ production. There are many lines of evidence indicating the involvement of TNF-α in LPS-associated liver injury and lethal shock (6, 18, 36). The present study showed that the levels of TNF-α in serum after LPS injection in BCG-primed IL-15 Tg mice and non-Tg mice were not different. IFN-γ also promotes TNF-α-mediated signaling via the inhibition of survival signaling via tumor necrosis factor receptor I (19). Therefore, it is possible that the susceptibility of hepatocytes to TNF-α-induced apoptosis may be increased in BCG-primed IL-15 Tg mice that produce a higher level of IFN-γ after LPS injection. However, it was recently reported that IL-15 protected cells from TNF-α-induced apoptosis via upregulation of antiapoptotic molecules (9). TNF family death receptors are known to result in only low levels of activation of the upstream initiator protease, caspase 8, and the successful execution of apoptosis requires the recruitment of the mitochondrial pathway via activation of the proapoptotic Bcl-2 family protein, bid (11). On the other hand, granzyme B is reported to induce apoptosis via direct caspase activation (7). Therefore, granzyme B-mediated apoptosis may be more important than TNF-α-mediated apoptosis in the pathogenesis of LPS-induced liver injury and lethal shock in BCG-primed mice. Further investigation is required to clarify this possibility.
In conclusion, the overexpression of IL-15 increased susceptibility to LPS-induced liver injury and lethal shock. CD8+ T cells and IFN-γ are critical for LPS-induced lethal shock in BCG-primed mice. IL-15 may play a pivotal role in LPS-induced lethal shock in BCG-primed models through bystander stimulation of CD8+ T cells to produce IFN-γ.
Acknowledgments
We thank Keiko Itano, Ayumi Nishikawa, and Eriko Nagasawa for their excellent technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas and Young Scientists (B) from the Japanese Society for the Promotion of Science and the Nakamura Foundation.
Editor: J. N. Weiser
REFERENCES
- 1.Ando, K., T. Moriyama, L. G. Guidotti, S. Wirth, R. D. Schreiber, H. J. Schlicht, S.-N. Huang, and F. V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg, P. 1996. Significance and pathogenesis of septic shock. Curr. Top. Microbiol. Immunol. 216:15-37. [DOI] [PubMed] [Google Scholar]
- 3.Carson, W. E., M. E. Ross, R. A. Baiocchi, M. J. Marien, N. Boiani, K. Grabstein, and M. A. Caligiuri. 1995. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 96:2578-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, L. C. 2000. Immunologic response to infection and its role in septic shock. Crit. Care Clin. 16:193-213. [DOI] [PubMed] [Google Scholar]
- 5.Fehringer, T. A., K. Suzuki, A. Ponnappen, J. B. VanDeusen, M. A. Cooper, S. M. Florea, A. G. Freund, M. L. Robinson, J. Durbin, and M. A. Caligiuri. 2001. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193:219-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantner, F., M. Leist, A. W. Lohse, P. G. Germann, and G. Tiegs. 1995. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21:190-198. [DOI] [PubMed] [Google Scholar]
- 7.Goping, I. S., M. Barry, P. Liston, T. Sawchuk, G. Constantinescu, K. M. Michalak, I. Shostak, D. L. Roberts, A. M. Hunter, R. Korneluk, and R. C. Bleackley. 2003. Granzyme B-induced apoptosis requires both direct caspase activation and relief of caspase inhibition. Immunity 18:355-365. [DOI] [PubMed] [Google Scholar]
- 8.Gulden, P. H., P. Fischer III, N. E. Sherman, W. Wang, V. H. Engelhard, J. Shabanowitz, D. F. Hunt, and E. G. Pamer. 1996. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity 5:73-79. [DOI] [PubMed] [Google Scholar]
- 9.Hiromatsu, T., T. Yajima, T. Matsuguchi, H. Nishimura, W. Wajjwalku, T. Arai, Y. Nimura, and Y. Yoshikai. 2003. Overexpression of IL-15 protects against Escherichia coli-induced shock accompanied by inhibition of TNF-α-induced apoptosis. J. Infect. Dis. 187:1442-1451. [DOI] [PubMed] [Google Scholar]
- 10.Hirose, K., H. Suzuki, H. Nishimura, A. Mitani, J. Washizu, T. Matsuguchi, and Y. Yoshikai. 1998. Interleukin-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect. Immun. 66:5677-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, D. C. S., and A. Strasser. 2000. BH3-only proteins—essential initiators of apoptotic cell death. Cell 103:839-842. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki-Ohara, K., H. Nishimura, A. Mitani, and Y. Yoshikai. 1997. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing gamma delta T cell receptor in mice. Eur. J. Immunol. 27:2885-2891. [DOI] [PubMed] [Google Scholar]
- 13.Katschinski, T., C. Galanos, A. Coumbos, and M. A. Freudenberg. 1992. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect. Immun. 60:1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi, S., J. Nishihira, S. Watanabe, and S. Todo. 1999. Prevention of lethal acute hepatic failure by antimacrophage migration inhibitory factor antibody in mice treated with bacille Calmette-Guerin and lipopolysaccharide. Hepatology 29:1752-1759. [DOI] [PubMed] [Google Scholar]
- 15.Lenz, L. L., B. Dere, and M. J. Bevan. 1996. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity 5:63-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks-Konczalik, J., S. Dubois, J. M. Losi, H. Sabzevari, N. Yamada, L. Feigenbaum, T. A. Waldmann, and Y. Tagaya. 2000. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA 97:11445-11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizoguchi, Y., Y. Sakagami, H. Kuboi, K. Kobayashi, and I. Yano. 1988. Effects of the polysaccharide chain of lipopolysaccharide in an experimental massive hepatic cell necrosis model. Biochem. Biophys. Res. Commun. 155:1205-1310. [DOI] [PubMed] [Google Scholar]
- 18.Mizuhara, H., E. O'Neill, N. Seki, T. Ogawa, C. Kusunoki, K. Otsuka, S. Satoh, M. Niwa, H. Senoh, and H. Fujiwara. 1994. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 179:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagakawa, J., I. Hishinuma, K. Hirota, K. Miyamoto, T. Yamanaka, K. Tsukidate, K. Katayama, and I. Yamatsu. 1990. Involvement of tumor necrosis factor-α in the pathogenesis of activated macrophage-mediated hepatitis in mice. Gastroenterology 99:758-765. [DOI] [PubMed] [Google Scholar]
- 20.Nagaki, M., M. Tanaka, A. Sugiyama, H. Ohnishi, and H. Moriwaki. 1999. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J. Hepatol. 31:815-824. [DOI] [PubMed] [Google Scholar]
- 20a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 21.Nicoletti, F., R. Di Marco, P. Zaccone, A. Salvaggio, G. Magro, K. Bendtzen, and P. Meroni. 2000. Murine concanavalin A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology 32:728-733. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura, H., T. Yajima, Y. Naiki, H. Tsunobuchi, M. Umemura, K. Itano, T. Matsuguchi, M. Suzuki, P. S. Ohashi, and Y. Yoshikai. 2000. Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo. J. Exp. Med. 191:157-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura, T., and A. Ohta. 1999. A critical role for antigen-specific Th1 cells in acute liver injury in mice. J. Immunol. 162:6503-6509. [PubMed] [Google Scholar]
- 24.Shands, J. W., Jr., and V. C. Senterfitt. 1972. Endotoxin-induced hepatic damage in BCG-infected mice. Am. J. Pathol. 67:23-40. [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu, H., T. Matsuguchi, Y. Fukuda, I. Nakano, T. Hayakawa, O. Takeuchi, S. Akira, and Y. Yoshikai. 2002. Toll-like receptor 2 contributes to liver injury induced by Salmonella infection through Fas ligand expression on NKT cells in mice. Gastroenterology 123:1265-1277. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu, Y., J. A. Margenthaler, K. Landeros, N. Otomo, G. Doherty, and M. W. Flye. 2002. The resistance of P. acnes-primed interferon gamma-deficient mice to low-dose lipopolysaccharide-induced acute liver injury. Hepatology 35:805-814. [DOI] [PubMed] [Google Scholar]
- 27.Stone, R. 1994. Search for sepsis drugs goes on despite past failures. Science 264:365-367. [DOI] [PubMed] [Google Scholar]
- 28.Tagaya, Y., R. N. Bamford, A. P. DeFilippis, and T. A. Waldmann. 1996. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4:329-336. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka, Y., A. Takahashi, K. Watanabe, K. Takayama, T. Yahata, S. Habu, and T. Nishimura. 1996. A pivotal role of IL-12 in Th1-dependent mouse liver injury. Int. Immunol. 8:569-576. [DOI] [PubMed] [Google Scholar]
- 30.Tiegs, G., J. Hentschel, and A. Wendel. 1992. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 90:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji, H., N. Mukaida, A. Harada, S. Kaneko, E. Matsushita, Y. Nakanuma, H. Tsutsui, H. Okamura, K. Nakanishi, Y. Tagawa, Y. Iwakura, K. Kobayashi, and K. Matsushima. 1999. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-gamma-deficient mice by a concomitant reduction of TNF-alpha, IL-12, and IL-18 production. J. Immunol. 162:1049-1055. [PubMed] [Google Scholar]
- 33.Tsutsui, H., K. Matsui, N. Kawada, Y. Hyodo, N. Hayashi, H. Okamura, K. Higashino, and K. Nakanishi. 1997. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 159:3961-3967. [PubMed] [Google Scholar]
- 34.Umemura, M., H. Nishimura, K. Hirose, T. Matsuguchi, and Y. Yoshikai. 2001. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette-Guerin infection via augmentation of NK and T cytotoxic 1 responses. J. Immunol. 167:946-956. [DOI] [PubMed] [Google Scholar]
- 35.Umemura, M., H. Nishimura, T. Yajima, W. Wajjwalku, T. Matsuguchi, M. Takahashi, Y. Nishiyama, M. Makino, Y. Nagai, and Y. Yoshikai. 2002. Overexpression of interleukin 15 prevents the development of murine retrovirus-induced acquired immunodeficiency syndrome. FASEB J. 16:1755-1763. [DOI] [PubMed] [Google Scholar]
- 36.Vassalli, P. 1992. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10:411-452. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19-49. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann, T. A., Y. Tagaya, and R. N. Bamford. 1998. Interleukin-2, interleukin-15, and their receptors. Int. Rev. Immunol. 16:205-226. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe, Y., M. Morita, and T. Akaike. 1996. Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology 24:702-710. [DOI] [PubMed] [Google Scholar]
- 40.Yajima, T., H. Nishimura, R. Ishimitsu, T. Watase, D. H. Busch, E. G. Pamer, H. Kuwano, and Y. Yoshikai. 2002. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J. Immunol. 168:1198-1203. [DOI] [PubMed] [Google Scholar]
- 41.Yajima, T., H. Nishimura, R. Ishimitsu, K. Yamamura, T. Watase, D. H. Busch, E. G. Pamer, H. Kuwano, and Y. Yoshikai. 2001. Memory phenotype CD8(+) T cells in IL-15 transgenic mice are involved in early protection against a primary infection with Listeria monocytogenes. Eur. J. Immunol. 31:757-766. [DOI] [PubMed] [Google Scholar]
- 42.Zang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]