Abstract
Coronary atherosclerosis and myocardial infarction in postmenopausal women have been linked to inflammation and reduced nitric oxide (NO) formation. Natural estrogen exerts protective effects on both processes, yet also displays uterotrophic activity. Here, we used genetic and pharmacologic approaches to investigate the role of the G protein-coupled estrogen receptor (GPER) in atherosclerosis. In ovary-intact mice, deletion of gper increased atherosclerosis progression, total and LDL cholesterol levels and inflammation while reducing vascular NO bioactivity, effects that were in some cases aggravated by surgical menopause. In human endothelial cells, GPER was expressed on intracellular membranes and mediated eNOS activation and NO formation, partially accounting for estrogen-mediated effects. Chronic treatment with G-1, a synthetic, highly selective small molecule agonist of GPER, reduced postmenopausal atherosclerosis and inflammation without uterotrophic effects. In summary, this study reveals an atheroprotective function of GPER and introduces selective GPER activation as a novel therapeutic approach to inhibit postmenopausal atherosclerosis and inflammation in the absence of uterotrophic activity.
Atherosclerosis is a chronic and systemic vascular inflammatory process that forms the pathological basis of coronary artery disease, myocardial infarction, and stroke1,2. Coronary artery disease represents the main cause of death in men and women alike, and shows a distinct gender difference with premenopausal women being largely protected1,2. Cessation of estrogen production due to natural or surgical menopause increases the risk of developing coronary atherosclerosis1,2,3. Thus, vascular protection in premenopausal1 but not postmenopausal4 women has been linked to the ovarian production of estrogens2,5, of which 17β-estradiol represents the physiologically relevant form3,5. Current estimates predict that by the year 2050 one billion women worldwide will be postmenopausal6, requiring preventive or therapeutic intervention to limit coronary artery disease and its associated health risks7,8. Attempts to reduce the increased risk of postmenopausal coronary atherosclerosis and its complications have included the use of estrogens as hormone therapy1,2,3; however, estrogen treatment is associated with adverse effects, such as blood clots and endometrial stimulation, increasing the risk of hyperplasia and carcinoma2,9.
Natural estrogens, such as 17β-estradiol, exert their vascular effects through soluble nuclear receptors and membrane-bound receptors5,10. In human coronary arteries, activation of estrogen receptors exerts both acute and chronic effects, including vasodilation11, reducing inflammation in atherosclerotic plaques12, and inhibiting proliferation of vascular smooth muscle cells (VSMC)13. Formation of endothelial nitric oxide (NO, a short-lived gas implicated in protection from atherosclerosis and inflammation14) and expression of the NO-synthesizing enzyme, eNOS, are also regulated in an estrogen-dependent fashion15. In vitro studies have shown that the “classical” estrogen receptors, ERα/esr1, and ERβ/esr2 activate eNOS16, and have identified ERα as one of the mediators of estrogen-dependent inhibition of atherogenesis17. However, since the inhibition brought about by estrogen is at least partially maintained in female esr1-deficient mice17,18, estrogen targets distinct from ERα must be involved in its atheroprotective effects.
In humans, exposure of vascular endothelial cells to laminar shear stress inhibits the progression of underlying atheroma19. Shear stress represents an important physiological stimulus of endothelial NO production14, which is centrally involved in protection from cardiovascular disease5,14,20. Exposing human endothelial cells to laminar shear stress also led to the detection and cloning of an orphan G protein-coupled receptor (GPR30)21. Studies have since established that this receptor binds and signals in response to estrogen22,23, which led to its designation as G protein-coupled estrogen receptor (GPER)24. Utilizing a transgenic gper-LacZ reporter mouse, its predominant expression in endothelial cells and VSMC has been reported25. The creation of gper-deficient mice26 and the identification of synthetic ligands that act as selective agonists or antagonists of GPER27,28,29 have facilitated studies of the role of GPER in physiology and disease, particularly in the context of ovarian sex steroid function24.
As inhibition of atherogenesis by estrogen must involve additional mechanisms distinct from ERα17,18 and because GPER shows a vasculotropic expression profile21,25,26 with its activation inducing vasodilation26,30,31 and inhibition of VSMC proliferation26, we hypothesized that GPER plays a role in atherosclerotic vascular disease26 and might represent a potential target for estrogen-mediated protection in women26. Additional support for this concept comes from the anti-inflammatory activity attributed to GPER24 as well as its involvement in the PI3K/Akt signaling pathway22, which regulates eNOS activation14. Thus, we set out to determine whether GPER expression and activity may contribute to the anti-inflammatory12 and NO-stimulating vascular effects of estrogen14. We also assessed whether treatment with a synthetic small molecule, GPER-selective agonist27 might be suitable as a new pharmacological strategy, distinct from classical hormone therapy, for the treatment of postmenopausal atherosclerosis.
Results
GPER is an intracellular estrogen receptor in endothelial and vascular smooth muscle cells
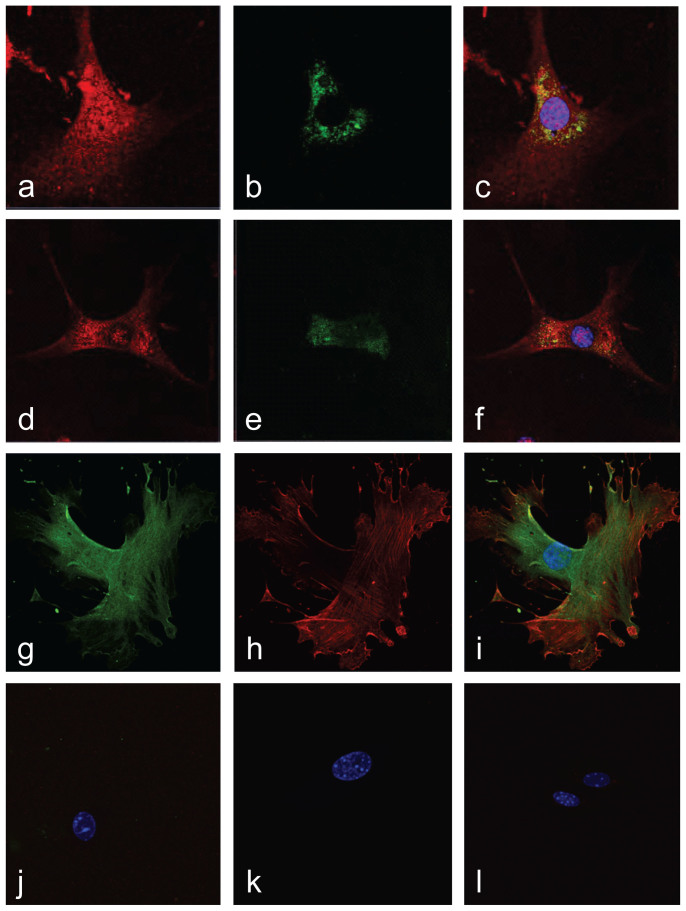
Both endothelial cells and VSMCs are essential for atherogenesis, with estrogen exhibiting anti-atherogenic effects in both cell types2,14. Thus, we first sought to determine the expression and subcellular localization of GPER in these cells. In endothelial cells, GPER staining revealed an intracellular expression pattern displaying colocalization with both endoplasmic reticulum and Golgi apparatus markers (Fig. 1a–f), similar to previous observations in other cell types22. VSMCs also exhibited an intracellular expression pattern. To confirm the predominant intracellular localization of GPER, we employed an anti-GPER antibody targeting the second “extracellular” loop, which would recognize a cell surface receptor in unpermeabilized cells. As this antibody only recognizes GPER under permeabilizing conditions (Fig. 1g–l), we conclude that, under steady state conditions, the great majority of GPER is also localized intracellularly in VSMCs, consistent with studies demonstrating constitutive internalization of surface-expressed GPER32.
Figure 1. GPER is an intracellular membrane receptor in vascular endothelial and smooth muscle cells.
(a)–(f), Endothelial cells were stained for GPER (red; a, d) and either endoplasmic reticulum (b) or Golgi apparatus (e), demonstrating colocalization of GPER with both markers in the merged images (c), (f), which include DAPI staining of the nucleus (blue). (g)–(j), Vascular smooth muscle cells were stained for GPER (green; g), employing an antibody targeting an “extracellular” epitope, and α-actin (red; h). merged with GPER staining in (i) under both permeabilizing conditions (g)–(i) and non-permeabilizing conditions (j). Pre-immune anti-GPER serum and negative control IgG were used to test the specificity of the GPER anti-serum and α-actin antibodies under permeabilizing (k) and non-permeabilizing (l) conditions. The cell nucleus is stained with DAPI (blue; i–l). The amino terminus of GPER, expected to be extracellular if the receptor is expressed on the cell surface (plasma membrane), is accessible only upon cell permeabilization, indicating that GPER is expressed predominantly on internal membranes.
Increased atherosclerosis in gper-deficient mice
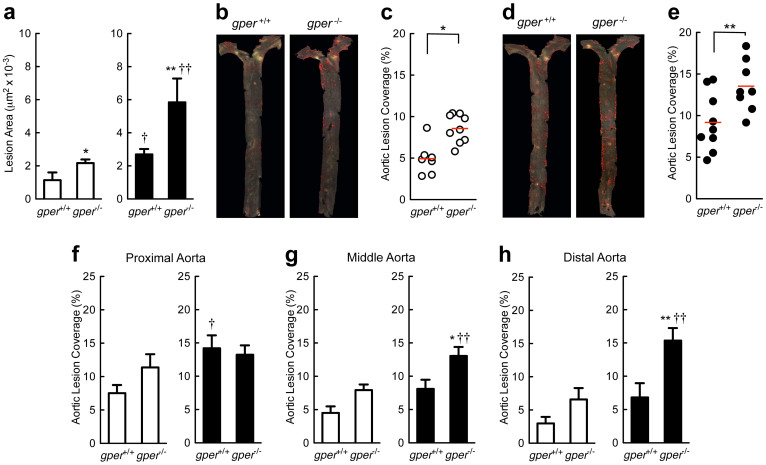
Wild type (gper+/+) and gper-deficient (gper−/−) mice were fed an atherogenic diet for 16 weeks and assessed for the development of early atherosclerosis in the aortic root and the aorta. Deletion of gper increased atherosclerosis in the aortic root (Fig. 2a) as well as macroscopic lesions in the aorta (Fig. 2b and 2c) and increased total cholesterol and LDL cholesterol plasma levels (Supplementary Table 1). Ovariectomy (surgical menopause33) accelerated development of aortic root atherosclerosis (Fig. 2a) as well as gross atherosclerotic lesions (Fig. 2d and e) to a similar extent. In ovariectomized mice, deletion of gper further aggravated atherosclerosis progression (Fig. 2a, d and e). In gper+/+ mice, the predilection site for atherosclerotic lesions was the proximal segment, with the lesion-covered aortic area decreasing distally, a pattern that was unaffected by gper deficiency or by ovariectomy (Fig. 2f–h). However, in mice exhibiting the most severe disease (gper deficiency and surgical menopause combined), the atherosclerotic distribution pattern changed, now showing similar levels of atherosclerosis in the proximal (Fig. 2f), middle (Fig. 2g), and distal segment (Fig. 2h) of the aorta. These findings (i) confirm atheroprotective effects of endogenous ovarian estrogens in female mice17,18 and (ii) provide evidence for a previously unrecognized atheroprotective function of GPER. The effects mediated by GPER may extend beyond its function as a receptor for ovarian estrogen, since its deletion surprisingly aggravated atherosclerosis even in ovariectomized mice. Thus GPER may exhibit significant basal ligand-independent activity, or being a vasculotropic receptor21,26, GPER may also be activated by estrogen produced locally in the vascular wall, which is increased in atherosclerosis34,35,36.
Figure 2. Increased atherosclerosis in mice lacking GPER.
Quantification of atherosclerosis in the aortic root (a) and macroscopic atherosclerosis on the aortic surface (b)–(h). Data are shown for ovary intact (open bars/circles) and ovariectomized (filled bars/circles) mice treated with an atherogenic diet. Deletion of gper increased both aortic root atherosclerosis (a) as well as macroscopic atherosclerosis (b)–(e). Both aortic root as well as macroscopic atherosclerosis development were also accelerated after ovariectomy (a), (d), (e). The effect of ovariectomy was further aggravated by deletion of gper (a), (d), (e). The predilection site for atherosclerotic lesions in gper+/+ animals was the proximal aortic segment (f), with lesions intensity decreasing from the middle (g) to the distal (h) segment. This distribution pattern was unaffected by gper deficiency or by ovariectomy alone (f)–(h); however, in ovariectomized gper−/− mice, the distribution pattern changed markedly, revealing equally extensive atherosclerosis in all three aortic segments (f)–(h). *P < 0.05 and **P < 0.01 compared with gper+/+ mice, †P < 0.05 and ††P < 0.01 compared with ovary intact genotype matched mice (ANOVA with Bonferroni post-hoc test). All data (n = 4–9 per group) represent mean ± s.e.m.
Vascular inflammation is enhanced in gper-deficient mice
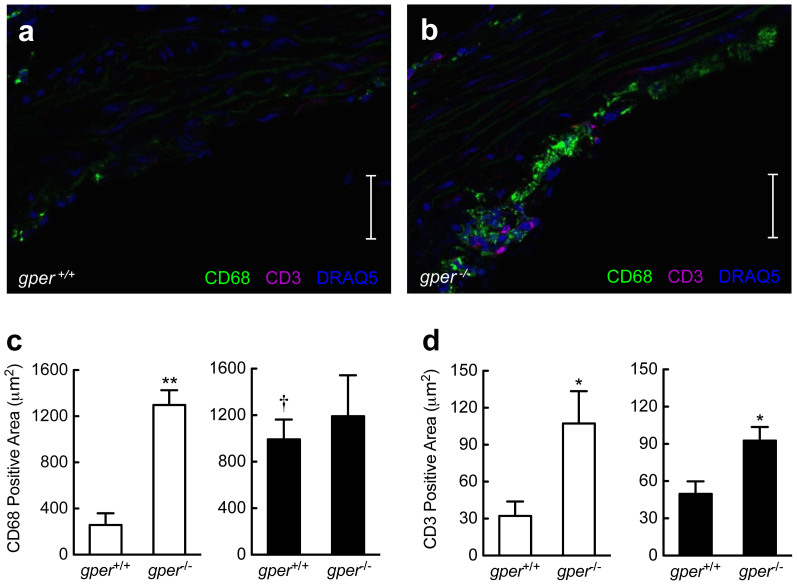
Macrophages and T cells play a central role in atherogenesis and are critical for disease initiation and progression37. We next determined whether endogenous GPER expression or surgical menopause affects macrophage and T cell accumulation in the aortic root using quantitative immunohistochemistry. Cell quantitation indicated ~10-fold fewer CD3+ cells (a T cell marker) than CD68+ cells (a macrophage marker) in all groups investigated (Fig. 3a–d). In ovary-intact mice, deletion of gper resulted in a striking increase in accumulation of CD68+ cells (Fig. 3a–c). Ovariectomy resulted in comparable effects to gper deficiency alone on CD68+ cell staining, which was not further aggravated by gper deficiency (Fig. 3c), suggesting that GPER was entirely responsible for the effect observed upon estrogen removal. With regard to CD3+ cell immunostaining, changes were less pronounced. In ovary-intact mice, gper deletion alone increased CD3+ cell staining (Fig. 3a, b and d), while ovariectomy had no significant effect (Fig. 3d). Deletion of gper also increased CD3+ cell staining in ovariectomized mice (Fig. 3d). These data are compatible with an important inhibitory role of endogenous GPER on vascular accumulation of macrophages and T cells and suggest that GPER-mediated atheroprotective effects likely involve inhibition of vascular inflammation.
Figure 3. Gper deficiency results in vascular accumulation of inflammatory cells.
Quantification of CD68+ cells (macrophages) and CD3+ cells (T cells) in the aortic root using quantitative immunohistochemistry. Data are shown for ovary intact (open bars) and ovariectomized mice (filled bars) treated with an atherogenic diet. Compared with ovary intact gper+/+ mice (a), (c), deletion of gper yielded a pronounced increase in CD68+ cells (b), (c). Ovariectomy alone also increased staining for CD68+ cells in gper+/+ mice, whereas deletion of gper had no further effect on this increase (c). Compared to either ovary intact or ovariectomized gper+/+ mice (a), (d), respectively, deletion of gper yielded a pronounced increase in CD3+ cells (b), (d). Ovariectomy did not further increase CD3+ staining in either gper+/+ or gper−/− mice (d). *P < 0.05 and **P < 0.01 compared with gper+/+ mice, †P < 0.05 compared to ovary intact genotype-matched mice (ANOVA with Bonferroni post-hoc test). All data (n = 3–6 per group) are mean ± s.e.m. Scale bar, 100 μm.
Vascular basal NO bioactivity is reduced in gper-deficient mice with atherosclerosis
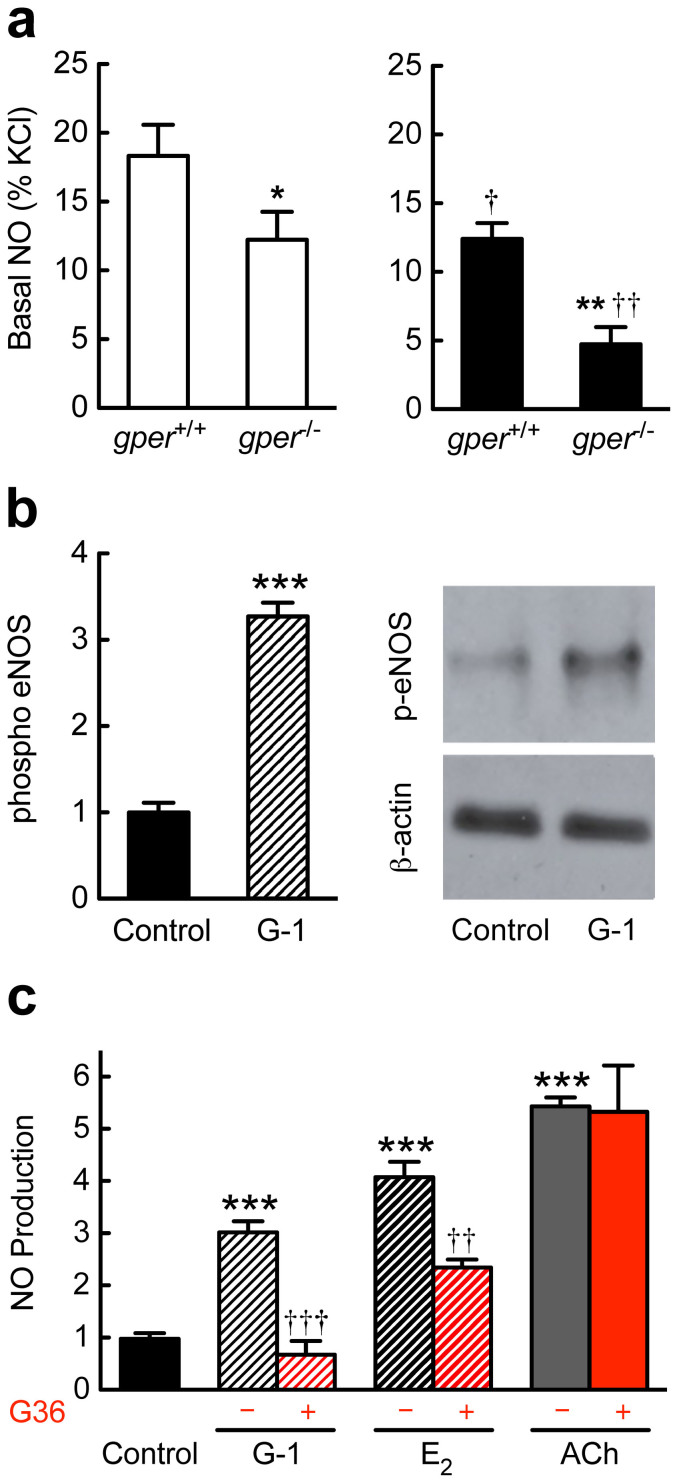
Vascular NO bioactivity regulates vascular tone and inhibits atherogenesis, and its production is critically dependent on intact ovarian steroid production14,38. Accordingly, a decrease in NO bioactivity has been implicated in the increased risk of cardiovascular disease in postmenopausal women14,38. Similar to surgically induced menopause in humans, ovariectomy in mice resulted in a significant reduction in vascular NO bioactivity measured ex vivo (Fig. 4a). Deletion of gper in ovary-intact animals reduced vascular NO bioactivity to a similar extent (Fig. 4a). However, in ovariectomized gper-deficient mice, vascular NO bioactivity was further reduced (Fig. 4a), indicating that endogenous GPER expression maintains NO bioactivity in both pre- and postmenopausal females in vivo in a milieu of vascular inflammation and atherosclerosis.
Figure 4. GPER regulates NO synthase function ex vivo and in vitro.
Basal vascular nitric oxide (NO) bioactivity was measured ex vivo (a) in mice treated with an atherogenic diet, with eNOS phosphorylation (b) and NO formation (c) determined in human endothelial cells. (a), Basal vascular NO bioactivity in ovary intact (open bars) and ovariectomized (filled bars) mice. Deletion of gper reduced vascular NO bioactivity to a similar extent (about 30%) as did ovariectomy in gper+/+ mice. However, in ovariectomized gper−/− mice, NO bioactivity was further reduced by more than 70%. *P < 0.05 and **P < 0.01 compared with gper+/+ mice, †P < 0.05 and ††P < 0.01 compared to ovary intact genotype-matched mice (ANOVA with Bonferroni post-hoc test). All data are mean ± s.e.m. (n = 5–7). (b), Stimulation of human endothelial cells with the GPER-selective agonist G-1 increased levels of activated eNOS-phosphorylated on serine1177. ***P < 0.001 compared with control (vehicle only, Student's t-test). Data are mean ± s.e.m. (n = 4). (c), Endothelial NO production was determined through the detection of stable NO metabolites NO2−/NO3−. Stimulation of GPER in human endothelial cells with the selective (G-1) or non-selective (17β-estradiol, E2) GPER agonist increased NO formation, as did the M3 muscarinic receptor agonist, acetylcholine (ACh). A selective GPER antagonist, G36, completely blocked G-1-stimulated endothelial NO formation, while E2-stimulated NO formation was only partly reduced. G36 had no effect on ACh-stimulated NO formation. ***P < 0.001 compared with control, ††P < 0.01 and †††P < 0.001 compared with no antagonist (Student's t-test). All data (n = 3–9 per group) are mean ± s.e.m.
GPER activation stimulates human endothelial nitric oxide synthase
Following the observation that GPER is critical to maintain vascular NO bioactivity ex vivo (Fig. 4a), and given that GPER was originally cloned from endothelial cells21, with vascular expression25 and function26, we next set out to determine whether and how GPER activation affects signaling of the L-arginine/NO pathway in human endothelial cells. For these experiments, acetylcholine was used as a known GPCR agonist to stimulate endothelial NO formation39, G-1 as selective GPER agonist27, 17β-estradiol (estrogen) as a non-selective agonist of ERα16, ERβ16, and GPER22, and G36 as a GPER-selective antagonist28,29. NO formation was determined by measuring the release of the stable NO metabolites NO2/NO340. Stimulation of human endothelial cells with G-1 resulted in a robust increase in eNOS protein phosphorylation at the Akt-mediated serine1177 activation site41,42 (Fig. 4b), and all three agonists induced robust, rapid NO formation (Fig. 4c). G36 completely abrogated G-1-stimulated NO production, and partly inhibited NO formation in response to estrogen, but had not effect on responses to M3 muscarinic receptor stimulation with acetylcholine (Fig. 4c), demonstrating the selectivity of G-1 and G36 in the modulation of GPER activity. In addition, stimulation of endothelial NO formation in response to G-1 was sensitive to PI3 kinase inhibition (data not shown).
A synthetic small molecule GPER agonist inhibits postmenopausal atherosclerosis and inflammation in vivo
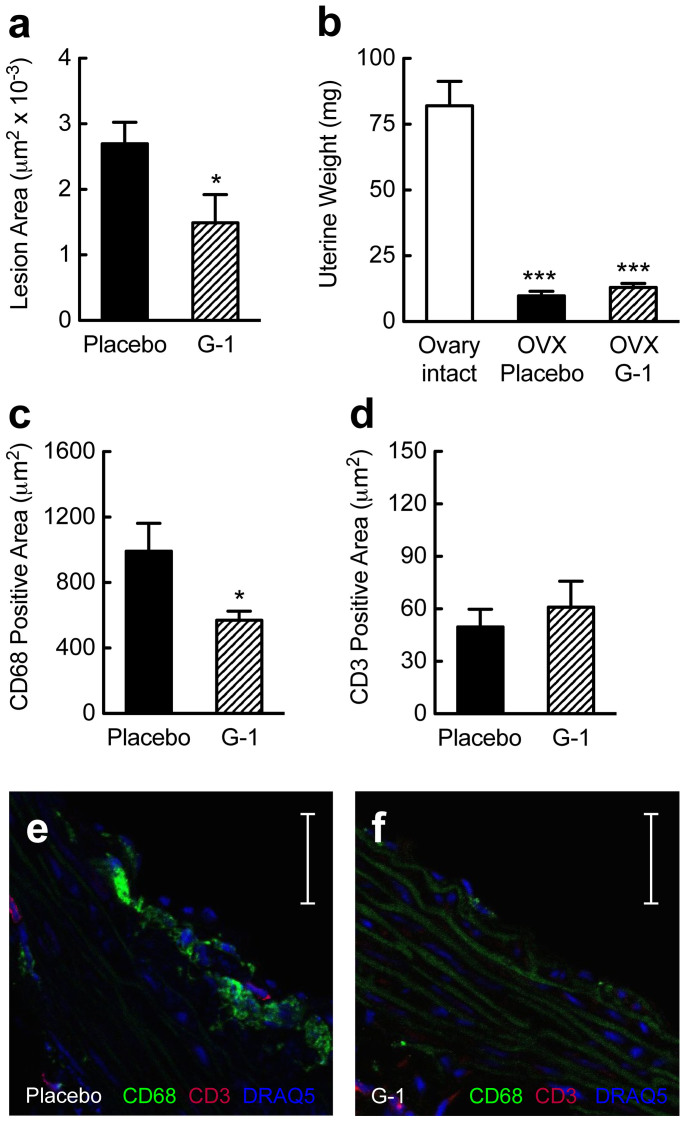
Treatment of postmenopausal women and experimental animals with natural estrogen is generally associated with a reduction in atherosclerosis and inflammation, yet increases the risks of endometrial hyperplasia and cancer due to uterotrophic stimulation9. This has been one of the key concerns of postmenopausal hormone therapy and requires additional preventive measures9. In the present study, using a model of postmenopausal atherosclerosis, we identified an endogenous inhibitory function of GPER on atherosclerosis progression (Fig. 2), the vascular inflammation associated with it (Fig. 3), and its protective effect on vascular NO bioactivity (Fig. 4a). We next set out to test whether treatment of postmenopausal mice with the synthetic small molecule GPER-selective agonist G-127 translates into inhibition of atherosclerosis progression and vascular inflammation. Treatment with G-1 for 16 weeks resulted in a marked reduction in aortic root atherosclerosis (Fig. 5a), yet was devoid of feminizing effects on the uterus (Fig. 5b). The reduction in atherosclerosis was accompanied by an inhibition of CD68+ cell infiltration, compatible with the notion that G-1 reduces vascular macrophage accumulation in vivo (Fig. 5c, e and f). Immunostaining for CD3+ cells (which was an order of magnitude lower than that for CD68+ cells) was however unaffected by G-1 treatment (Fig. 5d–f). These findings indicate that selective GPER activation by the synthetic small molecule G-1 may serve as a novel therapeutic approach to inhibit postmenopausal atherosclerosis and its associated vascular inflammation without undesirable estrogenic effects on the uterus.
Figure 5. A small molecule GPER-selective agonist inhibits atherosclerosis and vascular inflammation.
Treatment effects on atherosclerosis (a), uterine wet weight (b) or quantification of CD68+ (c), (e), (f) and CD3+ (d)–(f) staining of macrophages and T cells, respectively, in the aortic root. Data were obtained in ovariectomized (surgically postmenopausal) gper+/+ mice, which display accelerated atherogenesis (cf. Fig 1a–d). Effects are shown in response to treatment with placebo (filled bars) or G-1 (hatched bars), a selective small molecule agonist of GPER. G-1 treatment reduced atherosclerosis by 45% (a). Ovariectomy reduced uterine weight by about 90% compared with ovary-intact animals (b), in which estrogen levels are high. G-1 treatment had no feminizing effect on the uterus similar to placebo treatment (b). G-1 treatment reduced staining for CD68+ cells by 43% (c), (e), (f), but had no effect on CD3+ immunostaining (d)–(f). *P < 0.05 compared with placebo, ***P < 0.001 compared with ovary intact (Student's t-test). All data (n = 5–11 per group) are mean ± s.e.m. Scale bar, 100 μm.
Discussion
In the present study, we demonstrated that the in vivo activity of the intracellular, transmembrane G protein-coupled estrogen receptor (GPER) plays an essential, previously unrecognized role in atherogenesis, dyslipidemia and the associated inflammation. Treatment with G-1, a highly selective small molecule agonist of GPER27, was effective in reducing postmenopausal atherosclerosis and vascular inflammation without uterotrophic activity. Consistent with the vasculoprotective effect of its activation, genetic loss of gper worsened atherogenesis and inflammation.
Clinical studies have demonstrated that atherosclerotic vascular disease in women, the most prevalent cause of death, is strongly dependent on ovarian estrogen production2,33, and that natural estrogen(s) may effectively interfere with atherogenesis with treatment early but not late after menopause3,43. We thus chose to employ a therapeutically relevant model that resembles incipient atherogenesis in females, characterized by fatty streaks and early atheroma formation18. Our study revealed three principal new findings with regard to the role of GPER in atherosclerosis. First, gper deletion increases atherogenesis and plasma cholesterol, whereas pharmacological GPER activation following menopause attenuates disease progression. Second, the effect of gper deletion is further aggravated following surgical menopause, which itself accelerates atherogenesis to a similar extent as gper deficiency. Third, with regard to the topography of lesion development, the typical distribution pattern known for this model is dramatically altered in animals that are both postmenopausal and lack gper, wherein atherosclerosis increases in the otherwise largely lesion-resistant middle and abdominal portions of the aorta18. The findings reported here suggest that GPER plays an important role in mediating part of the atheroprotective effects of estrogen in premenopausal (ovary-intact) mice. Our results further indicate that even after menopause GPER retains a beneficial function and that its expression determines atherosclerotic lesion topography.
Currently known natural ligands of GPER include 17β-estradiol22 and 2-methoxy-estradiol44. Both steroids are produced within the arterial vascular wall36,45, and production has been shown to increase in human atherosclerosis35,36, suggesting an alternative and localized source of estrogen following menopause, particularly under disease conditions. Moreover, adrenal hormones such as dehydroepiandrosterone are converted to estrogen in the vascular wall46, and inhibit VSMC proliferation via a mechanism involving a yet unidentified membrane G protein-coupled receptor47. In addition, numerous GPCRs exhibit limited constitutive activity, thus contributing to signal transduction even in the absence of ligand activation48. Thus, an increased local production of ligands in atherosclerotic tissue and/or constitutive activity of GPER could well explain the effect of gper deletion that we observed in postmenopausal mice. Interestingly, a similar aggravation of atherosclerosis following ovariectomy has been shown in esr1-deficient mice18.
In postmenopausal women with an intact uterus, hormone therapy with estrogens requires additional pharmacological interventions to limit endometrial stimulation and the resulting risk of hyperplasia and malignancies. With this in mind, we set out to test the potential therapeutic efficacy of selective GPER activation with the synthetic, small molecule compound G-127 in postmenopausal atherosclerosis. We observed a robust inhibition of atherosclerosis, and importantly, G-1 treatment was without any uterotrophic effect28. This indicates that treatment with G-1, unlike currently used hormone formulations, affords vascular protection in postmenopausal female mice but is devoid of classical uterotrophic effects, which may have important therapeutic implications. Moreover, neither gper deficiency nor G-1 treatment had any effects on arterial blood pressure, further supporting a specific effect of this receptor on the vascular disease process.
Estrogens exert immunomodulatory functions that play numerous roles in physiology and disease49. The present study identifies GPER and ovarian function (i.e. estrogen production) as suppressors of vascular macrophage and T cell accumulation in atherogenesis and demonstrates that treatment with the GPER-selective agonist G-1 reduces macrophage accumulation in mice with postmenopausal atherosclerosis. These findings recapitulate observations in women with coronary artery disease, where inflammation and the severity of atherosclerotic lesions are much lower in premenopausal than in postmenopausal women12, and can be reduced in postmenopausal women by estrogen treatment12. Similarly, ovariectomy has been shown to exert proinflammatory effects in experimental atherosclerosis18, including macrophage infiltration and the recruitment of T cells50. The targets previously thought to be solely involved in these responses are the “classical” estrogen receptors, ERα and ERβ2, which are expressed in macrophages50. Recently, GPER expression has been demonstrated in leukocytes, and its activation exerts anti-inflammatory effects by inhibiting IL-1β and increasing IL-10 production51. In addition, GPER activation reduces TLR-4 expression52 and the CRP-induced upregulation of IL-8, ICAM-1, P-selectin, and several chemokines in murine macrophages50. The present study now demonstrates that GPER exerts inhibitory effects on vascular macrophage and T cell recruitment in vivo. Although we cannot exclude changes in the inflammatory environment secondary to altered atheroma formation, the extent of atherosclerosis did not entirely correlate with immune cell accumulation, which was disproportionally high in premenopausal gper-deficient mice. This argues in favor of direct GPER-mediated inhibition of vascular inflammation by endogenous estrogens, which was recapitulated with G-1 treatment in postmenopausal animals. As we have recently shown that GPER regulates Foxp3 expression and induces IL-10 expression under TH17-polarizing conditions53,54 it is intriguing to speculate that the TH17/IL-17A pathway, which plays an important role in atherogenesis and the associated inflammation55, is also involved in the anti-inflammatory and atheroprotective effects of GPER.
In women with coronary atherosclerosis, reduced NO bioactivity increases the risk of adverse cardiovascular events and death20. Endothelium-derived NO, which has anti-inflammatory properties14,50, is produced in response to laminar shear stress14,18 and in an estrogen-dependent fashion16. Previously, estrogen-induced NO release from human endothelial cells was thought to be mediated solely by ERα and ERβ14,16. Of note, GPER was cloned from human endothelial cells exposed to laminar shear stress21, and was shown here to be expressed as an intracellular membrane receptor in human endothelial cells. Furthermore, since we previously observed NO- and endothelium-dependent relaxation of epicardial coronary arteries in response to GPER agonists ex vivo31, we speculated that deletion of gper and/or menopause might affect NO bioactivity in the setting of atherosclerosis and vascular inflammation. In the present study, we identified GPER as an important determinant that contributes to vascular NO bioactivity in ovary-intact animals. Of note, much of the NO bioactivity in wild type mice is unaffected by ovariectomy, suggesting additional regulatory mechanisms. However, in surgically postmenopausal mice lacking gper, which also showed the most severe progression of atherosclerosis, NO bioactivity was dramatically reduced. These findings prompted us to further examine whether and how GPER might be involved in the regulation of endothelial NO synthase activity and the formation of NO. In human endothelial cells, we observed G-1-mediated phosphorylation of eNOS at serine1177, an Akt-mediated event that results in increased eNOS activity and NO production41,42. To recapitulate our ex vivo observations, the role of GPER was determined in the absence and presence of G3629, a GPER-selective antagonist, by measuring stimulated cellular NO release. The results demonstrate not only that NO is released from human endothelial cells in response to GPER activation, but also that a considerable portion of 17β-estradiol-mediated NO formation is GPER-dependent, whereas M3 muscarinic receptor-stimulated NO release (using the prototypic agonist acetylcholine39) is completely insensitive to GPER inhibition. Thus, both the ex vivo as well as the in vitro experiments identify GPER as a novel and important regulator of NO bioactivity, which likely contributes to GPER-mediated inhibitory effects on vascular inflammation and ultimately atherogenesis.
In summary, using both loss and gain of function approaches in female mice with atherosclerosis, dyslipidemia and vascular inflammation, we have identified a previously unrecognized role for GPER in regulating vascular disease progression. We further found that activating this receptor with a synthetic small molecule GPER-selective agonist is effective in reducing atherosclerosis in the absence of uterotrophic effects. In view of the limitations of current hormone therapies3,9 and the reported vasoprotective effects of G-1 in isolated human arteries26,30, the concept of selective GPER activation introduced in the present study could be considered as a new therapeutic strategy for the treatment and secondary prevention of coronary artery disease in postmenopausal women.
Methods
Expression and subcellular localization of GPER
Expression and localization of GPER were determined in telomerase-immortalized human umbilical vein endothelial cells and primary aortic vascular smooth muscle cells (VSMC) isolated from gper+/+ mice. VSMC and endothelial cells were seeded on coverslips and fixed in PBS containing 4% paraformaldehyde for 12 min at room temperature. For staining, cells were treated with either permeabilizing (PBS containing 3% BSA and 0.1% Triton X-100) or non-permeabilizing (PBS containing 3% BSA) blocking buffer for 1 h at room temperature, and incubated with the corresponding antibodies overnight at 4°C. Slides were then washed, incubated with secondary antibody for 1 h at room temperature, washed, mounted in Vectashield supplemented with DAPI, and visualized using a Zeiss LSM510 Meta confocal fluorescent microscope (Zeiss, Oberkochen, Germany). See Supplementary Information for details.
Mice
Female wild-type C57BL/6J mice (gper+/+, Harlan Laboratories, Indianapolis, IN, USA) and GPER-deficient (gper−/−) mice (Proctor & Gamble, Cincinnati, OH, USA, provided by Jan S. Rosenbaum) were housed at the Animal Resource Facility of the University of New Mexico Health Sciences Center. Animals were maintained under controlled temperature of 22–23°C on a 12 h light, 12 h dark cycle and had access to chow and water ad libitum. All procedures were approved by and carried out in accordance with institutional policies and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. See Supplementary Information for details.
Treatment protocols
At six weeks of age, female gper+/+ and gper−/− mice were changed from normal chow to an atherogenic, phytoestrogen-free, high-fat, high-cholesterol diet (15.8% fat, 1.25% cholesterol, 0.5% sodium cholate; Teklad TD.90221, Harlan Laboratories, Madison, WI, USA). Prior to changing to the atherogenic diet, all animals had undergone either sham surgery or ovariectomy (to induce surgical menopause). In a subset of menopausal mice, pellets releasing the GPER-selective agonist G-127,56 (33 μg/day) or placebo (Innovative Research, Sarasota, FL, USA) were implanted subcutaneously prior to changing to the atherogenic diet. Successful ovariectomy was confirmed post mortem by uterine atrophy (wet weight).
Quantification of atherosclerosis
Quantification of atherosclerosis was performed microscopically (in aortic root sections) and macroscopically (utilizing en face staining of the aorta). Briefly, alternate 10 μm-thick sections of the aortic root were stained with Oil Red O (ORO) for neutral lipid, followed by hematoxylin and light green for counter-staining. Atherosclerotic lesion area was quantified using a computer-assisted imaging system (Image J, version 1.46r, National Institutes of Health, USA) by an investigator blinded to treatment and genotype, and values are expressed as average per section. For the en face quantification of atherosclerosis, the aorta from the ascending part to the iliac bifurcation was carefully cleaned from adherent fat and connective tissue, cut open longitudinally, and fixed in paraformaldehyde (4%). Aortas were mounted en face and stained with ORO, and lesion area relative to total aortic surface was quantified. See Supplementary Information for details.
Quantification of CD68+ and CD3+ cells
Aortic root sections were stained for macrophages using rat anti-mouse CD68 antibody (clone FA11, AbD Serotec, Raleigh, NC, USA), and T cells using Armenian hamster anti-mouse CD3ε antibody (clone 145-2C11, BioLegend, San Diego, CA, USA), detected by Alexa Fluor 488 goat anti-rat (Life Technologies, Grand Island, NY, USA) and Cy3 goat anti-Armenian hamster (Jackson ImmunoResearch, West Grove, PA, USA) secondary antibodies. Nuclei were stained with DRAQ5 (Cell Signaling Technology, Danvers, MA, USA). Slides were analyzed utilizing a Leica SP5 confocal microscope (Wetzlar, Germany). Immunostained cells were quantified by computer-assisted histomorphometry (ImageJ).
Vascular NO bioactivity
Basal NO bioactivity was determined ex vivo in isolated carotid arteries. Briefly, contraction to phenylephrine (30 nmol/L) was recorded in the absence and presence of the NO synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME, 300 μmol/L for 30 min), and NO bioactivity was calculated as the difference between both contractions. See Supplementary Information for details.
eNOS activation and NO formation in human endothelial cells
To determine whether GPER affects eNOS ser1177 phosphorylation, endothelial cells were treated with the GPER-selective agonist G-127 (100 nmol/L) or solvent (DMSO 0.01%) for 20 min, lysed, electrophoresed by SDS PAGE and Western blotted for phosphorylation of eNOS residue ser1177 (antibody 9571, Cell Signaling, Danvers, MA, USA). Receptor-stimulated NO formation was determined by colorimetric detection of the stable NO metabolites NO2/NO3 (Abcam, Boston, MA, USA) in cells starved overnight in M199 medium, incubated with HEPES-PSS (composition in mmol/L: 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 0.026 EDTA, 10 glucose, and 10 HEPES; pH 7.4), and exposed either to the GPER-selective agonist G-1 (1–100 nmol/L)27, 17β-estradiol (1–100 nmol/L), acetylcholine (100 nmol/L), or solvent (DMSO 0.01%) for 10 min. A subset of cells was exposed to the GPER-selective antagonist G3629 (100 nmol/L) for 30 min prior to stimulation. NO metabolite concentrations were normalized to total protein (Bradford protein assay, BioRad, Hercules, CA, USA). See Supplementary Information for details.
Statistical analyses
Data were tested for distribution normality and analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test, the unpaired Student's t-test (two-tailed), or the non-parametric Mann-Whitney U test, as appropriate (Prism version 5.0 for Macintosh, GraphPad Software, San Diego, CA, USA). Values are expressed as the mean ± s.e.m. of independent experiments; n equals the number of animals or number of independent experiments. Statistical significance was accepted at p values of <0.05.
Author Contributions
M.R.M., N.C.F., T.A.H., C.H. and C.D. performed experiments; R.C. and J.B.A. synthesized G-1 and G36; M.R.M., N.C.F., C.D., M.B. and E.R.P. analyzed data; M.R.M., K.A., M.B. and E.R.P. interpreted results of experiments; M.R.M., M.B. and E.R.P. prepared figures and wrote the manuscript; M.R.M., N.C.F., T.A.H., C.H., C.D., K.A., M.B. and E.R.P. approved the final version of manuscript; M.R.M., M.B. and E.R.P. were involved in conception and design of research.
Supplementary Material
Supplementary info
Acknowledgments
We thank Daniel F. Cimino and Stefan Söllner for expert technical assistance. This study was supported by the National Institutes of Health (R01 CA127731 to J.B.A. and E.R.P. & CA163890 to E.R.P.), Dedicated Health Research Funds from the University of New Mexico School of Medicine allocated to the Signature Program in Cardiovascular and Metabolic Diseases (to E.R.P.), the Swiss National Science Foundation (grants 135874 & 141501 to M.R.M. and grants 108258 & 122504 to M.B.), and the Interdisciplinary Centre for Clinical Research (IZKF) Erlangen, project F1 (to K.A.). N.C.F. was supported by NIH training grant HL07736.
References
- Barrett-Connor E. Menopause, atherosclerosis, and coronary artery disease. Curr Opin Pharmacol 13, 186–191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon J. L., McDonnell D. P., Martin K. A. & Wise P. M. Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304, 1269–1273 (2004). [DOI] [PubMed] [Google Scholar]
- Hodis H. N. & Mack W. J. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol 142, 68–75 (2014). [DOI] [PubMed] [Google Scholar]
- Phillips G. B., Pinkernell B. H. & Jing T. Y. Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol 17, 695–701 (1997). [DOI] [PubMed] [Google Scholar]
- Khalil R. A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol 86, 1627–1642 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. & Meyer M. R. Postmenopausal hypertension: mechanisms and therapy. Hypertension 54, 11–18 (2009). [DOI] [PubMed] [Google Scholar]
- Gurney E. P., Nachtigall M. J., Nachtigall L. E. & Naftolin F. The Women's Health Initiative trial and related studies: 10 years later: a clinician's view. J Steroid Biochem Mol Biol 142, 4–11 (2014). [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., Melendez G. C. & Appt S. E. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20, 342–353 (2013). [DOI] [PubMed] [Google Scholar]
- Schenck-Gustafsson K. et al. EMAS position statement: Managing the menopause in the context of coronary heart disease. Maturitas 68, 94–97 (2011). [DOI] [PubMed] [Google Scholar]
- Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mügge A., Riedel M., Barton M., Kuhn M. & Lichtlen P. R. Endothelium independent relaxation of human coronary arteries by 17 β-oestradiol in vitro. Cardiovasc Res 27, 1939–1942 (1993). [DOI] [PubMed] [Google Scholar]
- Burke A. P., Farb A., Malcom G. & Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J 141, S58–62 (2001). [DOI] [PubMed] [Google Scholar]
- Seeger H., Wallwiener D. & Mueck A. O. Effect of medroxyprogesterone acetate and norethisterone on serum-stimulated and estradiol-inhibited proliferation of human coronary artery smooth muscle cells. Menopause 8, 5–9 (2001). [DOI] [PubMed] [Google Scholar]
- Xing D., Nozell S., Chen Y. F., Hage F. & Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29, 289–295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert H., Wallerath T., Euchenhofer C., Ihrig-Biedert I., Li H. & Forstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension 31, 582–588 (1998). [DOI] [PubMed] [Google Scholar]
- Klinge C. M. et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors α and β in endothelial cells. J Biol Chem 280, 7460–7468 (2005). [DOI] [PubMed] [Google Scholar]
- Hodgin J. B., Krege J. H., Reddick R. L., Korach K. S., Smithies O. & Maeda N. Estrogen receptor alpha is a major mediator of 17β-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 107, 333–340 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca A. C., Tenwolde A., Lee M., Huck M., Mumenthaler S. & Rutledge J. C. 17β-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res 2, 289–299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M. & Schroter R. C. Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1160 (1969). [DOI] [PubMed] [Google Scholar]
- Heitzer T., Schlinzig T., Krohn K., Meinertz T. & Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104, 2673–2678 (2001). [DOI] [PubMed] [Google Scholar]
- Takada Y., Kato C., Kondo S., Korenaga R. & Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun 240, 737–741 (1997). [DOI] [PubMed] [Google Scholar]
- Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B. & Prossnitz E. R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 (2005). [DOI] [PubMed] [Google Scholar]
- Thomas P., Pang Y., Filardo E. J. & Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 (2005). [DOI] [PubMed] [Google Scholar]
- Prossnitz E. R. & Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7, 715–726 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J. et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150, 1722–1730 (2009). [DOI] [PubMed] [Google Scholar]
- Haas E. et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104, 288–291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa C. G. et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2, 207–212 (2006). [DOI] [PubMed] [Google Scholar]
- Dennis M. K. et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol 5, 421–427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. K. et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol 127, 358–366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefin S. et al. Vasodilatory effects of the selective GPER agonist G-1 is maximal in arteries of postmenopausal women. Maturitas 78, 123–130 (2014). [DOI] [PubMed] [Google Scholar]
- Meyer M. R., Baretella O., Prossnitz E. R. & Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86, 58–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. B., Graeber C. T., Quinn J. A. & Filardo E. J. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids 76, 892–896 (2011). [DOI] [PubMed] [Google Scholar]
- Witteman J. C., Grobbee D. E., Kok F. J., Hofman A. & Valkenburg H. A. Increased risk of atherosclerosis in women after the menopause. BMJ 298, 642–644 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan L. et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A 98, 3589–3593 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. et al. Steroid sulfatase and estrogen sulfotransferase in the atherosclerotic human aorta. Am J Pathol 163, 1329–1339 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Suzuki T. & Sasano H. Estrogen actions and in situ synthesis in human vascular smooth muscle cells and their correlation with atherosclerosis. J Steroid Biochem Mol Biol 93, 263–268 (2005). [DOI] [PubMed] [Google Scholar]
- Hansson G. K. & Hermansson A. The immune system in atherosclerosis. Nat Immunol 12, 204–212 (2011). [DOI] [PubMed] [Google Scholar]
- Meyer M. R. & Barton M. ERα, ERβ, and gpER: novel aspects of oestrogen receptor signalling in atherosclerosis. Cardiovasc Res 83, 605–610 (2009). [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. & Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376 (1980). [DOI] [PubMed] [Google Scholar]
- Achan V., Tran C. T., Arrigoni F., Whitley G. S., Leiper J. M. & Vallance P. all-trans-Retinoic acid increases nitric oxide synthesis by endothelial cells: a role for the induction of dimethylarginine dimethylaminohydrolase. Circ Res 90, 764–769 (2002). [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R. & Zeiher A. M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (1999). [DOI] [PubMed] [Google Scholar]
- Fulton D. et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. P., Xing D., Feng W., Fintel M., Chen Y. F. & Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause 14, 251–260 (2007). [DOI] [PubMed] [Google Scholar]
- Koganti S., Snyder R., Gumaste U., Karamyan V. T. & Thekkumkara T. 2-methoxyestradiol binding of GPR30 down-regulates angiotensin AT(1) receptor. Eur J Pharmacol 723, 131–140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofer J. R. Estrogens and atherosclerosis: insights from animal models and cell systems. J Mol Endocrinol 48, R13–29 (2012). [DOI] [PubMed] [Google Scholar]
- Labrie F., Luu-The V., Labrie C. & Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 22, 185–212 (2001). [DOI] [PubMed] [Google Scholar]
- Bonnet S. et al. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT axis. Circulation 120, 1231–1240 (2009). [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. M., Rasmussen S. G. & Kobilka B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman C. J. Interactions between the gonadal steroids and the immune system. Science 227, 257–261 (1985). [DOI] [PubMed] [Google Scholar]
- Bowling M. R. et al. Estrogen effects on vascular inflammation are age dependent: role of estrogen receptors. Arterioscler Thromb Vasc Biol 34, 1477–1485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabas I., Rodenas M. C., Abellan E., Meseguer J., Mulero V. & Garcia-Ayala A. Estrogen signaling through the G protein-coupled estrogen receptor regulates granulocyte activation in fish. J Immunol 191, 4628–4639 (2013). [DOI] [PubMed] [Google Scholar]
- Rettew J. A., McCall S. H. t. & Marriott, I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol 328, 87–92 (2010). [DOI] [PubMed] [Google Scholar]
- Brunsing R. L., Owens K. S. & Prossnitz E. R. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J Immunother 36, 190–196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsing R. L. & Prossnitz E. R. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology 134, 93–106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40, 153–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burai R. et al. Highly efficient synthesis and characterization of the GPR30-selective agonist G-1 and related tetrahydroquinoline analogs. Org Biolmol Chem 8, 2252–2259 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary info