Abstract
Background:
Oral lichen planus (OLP) is one of the potentially malignant disorders (PMDs) with a malignancy rate of 0.2-2%. Aneuploidy is considered to be one of the important markers for malignant transformation and DNA-image cytometry (DIC) has been successfully employed in oral mucosal PMDs and also in tumors of the cervix, lung and biliary tract.
Aims:
In this study, we intend to assess the ploidy status of exfoliated cells in OLP using DIC.
Materials and Methods:
Exfoliated cells from 48 patients with different subtypes of OLP (reticular, plaque type, erosive and atrophic) and 10 controls were stained using Feulgen reaction and assessed for integrated optical density using image analysis software and the ploidy status was assessed.
Results:
All the patients in the control group and most of the patients (93.5%) who had reticular or plaque type of OLP (29 out of 31) exhibited diploid nuclei in the smears, whereas 11 patients who had erosive or atrophic types of OLP showed aneuploid nuclei.
Conclusions:
The patients with erosive or atrophic types of OLP are at more risk and assessment of ploidy status by exfoliative cytology can be used as an adjuvant for diagnosis.
Keywords: Cytology, DNA-image cytometry, integrated optical density, oral lichen planus
Introduction
Lichen planus was first described by a British physician named Erasmus Wilson. It is most common in the skin and mucous membrane of the oral cavity, esophagus and genitals.[1] The lesions in the oral mucous membrane are termed as oral lichen planus (OLP) and may occur with or without skin lesions. When compared with skin lesions OLP follows a more chronic course and can be exacerbated after periods of quiescence. Lesions involve multiple sites in the oral cavity and are often bilateral. In general, it is considered affecting about 1-2% of the general adult population. OLP has been described as a disease of the middle-aged, predominantly in adults over the age of 40, and more common in women than men in a ratio of 1.4:1.[2,3,4] Various clinical forms of OLP have been described by Andreasen such as papular, reticular, plaque type, atrophic, erosive and bullous.[5] OLP takes its clinical importance from the fact that it is considered a potentially malignant disorder (PMD).[6] Although OLP is considered a PMD, the subtype of OLP at most risk is still a matter of debate with most authors considering the atrophic and erosive types as being more susceptible and few believing all the subtypes are at equal risk.[7,8,9]
Recently, diagnostic cytopathology has attracted tremendous interest as new adjunctive diagnostic methods have been applied. One of them being DNA-image cytometry (DIC) on Feulgen stained smears and is considered the cytometric equivalent of chromosomal aneuploidy and is an internationally accepted marker for neoplastic transformation of cells in tumors of lung, cervix and also the oral cavity.[10,11,12,13] The aim of this study was to assess the ploidy status of 48 OLP cases using DIC. This study was carried out as there are numerous studies on other PMD lesions such as leukoplakia and erythroplakia, but there are very few previous studies specifically done on oral lichen using DIC and the first in an Indian population.[14,15]
Materials and Methods
This study was carried out after obtaining clearance from the ethical committee in the University. The study comprised of 48 patients (26 males and 22 females) with clinically and histologically proven OLP consisting of the reticular, plaque type, atrophic and erosive subtype and 10 controls (5 males and 5 females) [Table 1]. The 48 patients with OLP considered for the study were broadly divided into two groups. Group I (n = 31) consisted of patients with reticular and plaque type of OLP and Group II (n = 17) consisted of patients with erosive and atrophic type of OLP. All these patients had no history of consuming alcohol or smoking or any other tobacco related habits and were diagnosed for the first time. The control patients considered to be in Group III were systemically healthy and had no oral mucosal lesions.
Table 1.
Descriptive statistics of the samples in the study

After an informed consent, buccal keratinocytes were collected from the lesional areas of patients with OLP and controls using a slightly moistened sterilized wooden spatula (Vishvesh Pvt. Ltd., Chennai, India). The smears were then transferred and spread uniformly on a clean glass slide containing 0.02 M NaCl (Coral Marketing, India), which acts as a buffer and also helps in removing cell debris and bacteria. The slides containing smears were fixed in 95% alcohol for 30 min. The slides were then stained by using Feulgen reaction (Artisan Feulgen Kit, Dako). After reviewing, the smears were further subjected to DIC evaluation. For each slide, 200 representative cells with distinct cellular and nuclear outlines, avoiding overlapping cells were captured as high quality photomicrographs using a Nikon 4100 digital camera (Nikon, Japan) attached to an Olympus CK trinocular microscope (Olympus, Japan). Images of 20 lymphocytes from each slide were also captured for diploid (2c) reference. The captured images were transferred to a computer for image analysis. The final image captured on the monitor had a magnification of ×400.
The DIC evaluation was done using image analyzer software, ImageJ 1.48 v (National Institutes of Health, USA). Each image was first converted into 8-bit grayscale one. The nucleus of each exfoliated epithelial cell and lymphocyte was selected using the ‘free hand tool’ in the software and the following measurements were taken into account; integrated optical density (IOD), minimum, maximum and mean density [Figure 1]. These parameters were studied to evaluate the ploidy status. The recommendations contained in the European Society for Analytical Cellular Pathology consensus report on diagnostic DIC were followed.[16,17,18,19]
Figure 1.
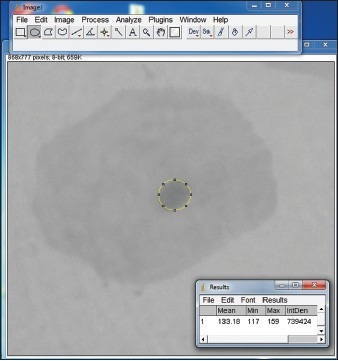
Screenshot of measurement of integrated optical density using Image J software
For each slide, the ploidy status was calculated as follows. The mean IOD of the nucleus of 20 lymphocytes was considered to be 2c. Then the mean IOD for nuclei of 200 keratinocytes was calculated. This value was divided by the IOD of lymphocytes to calculate DNA stemline (STL). The ploidy status of each slide depended on this STL. A slide was considered to be diploid if STL was between 1.80c and 2.20c and as tetraploid if the STL was between 3.60c and 4.40c. The lesion was characterized as DNA-aneuploid, if an abnormal STL was <1.80c and >2.20c or <3.60c and >4.40c and/or exceeding 9c.[20] Thus the ploidy status for each of the 48 samples was determined.
Statistical analysis of the data was performed using the SPSS software version 17.0 (IBM, USA) for Windows 7 (Microsoft, USA). The Chi-square test of analysis was done, and the significance level was set at 0.05.
Results
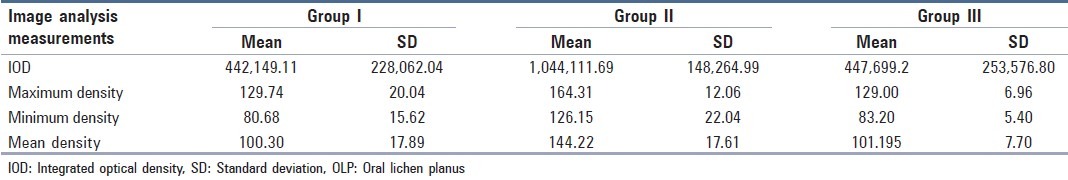
The mean IOD in Group I (reticular/plaque type OLP) was 442149.11 ± 228062.04. The maximum, minimum and mean intensity was 129.74 ± 20.04, 80.68 ± 15.62 and 100.30 ± 17.89, respectively. In Group II (erosive/atrophic type OLP) the IOD was observed to be 1044111.69 ± 148264.99 whereas the maximum, minimum and mean intensity was 164.31 ± 12.06, 126.15 ± 22.04 and 144.22 ± 17.61, respectively. In controls (Group III) the IOD was 447699.2 ± 253576.80. The maximum, minimum, and mean densities were 101.195 ± 7.70, 83.2 ± 5.40 and 129.00 ± 6.96 respectively [Table 2].
Table 2.
Image analysis of reticular/plaque type (Group I), erosive/atrophic type (Group II) of OLP and Group III (controls)
The ploidy status was calculated using the IOD of the nuclei of exfoliated cells. In the reticular and plaque type OLP group (Group I), 29 of the 31 were diploid, 2 were tetraploid and none was aneuploid. The number of cases with a diploid set of chromosomes showed a statistically significant value in Group I. A statistically significant number of cases showed aneuploidy (11 out of 17) in the erosive and atrophic types of OLP (Group II), whereas as only 1 was tetraploid and 6 were diploid [Table 3]. When the ploidy status was compared between the erosive OLP cases and atrophic OLP cases, no statistical difference was observed [Table 4].
Table 3.
Ploidy status in various clinical forms of OLP

Table 4.
Ploidy status in atrophic and erosive OLP

Discussion
The application of cytology as a noninvasive and an adjuvant for oral lesions is well established. Detection of ploidy status using DIC in exfoliated cells has proven to give satisfactory results with sensitivity and specificity close to the hematoxylin and eosin stained biopsies, which are considered to be the gold standard for diagnosis.[12,19,20,21] In our study, we have assessed the ploidy status using DIC. Aneuploidy is considered to be one of the most sensitive and effective indicators of malignant transformation in the oral cavity and has been used to assess PMD's such as leukoplakia, erythroplakia and oral submucous fibrosis. In this study, we have assessed the ploidy status in OLP which is also considered to be PMD.[22,23] In this study, we have used the Feulgen stain to quantitatively assess the ploidy status of the cells. Feulgen reaction ensures that staining intensity is in proportion to DNA content, and since it is a stoichiometric procedure and each fixed molecule of Schiff's reagent corresponds to a constant and equivalent portion of the DNA molecule. Another advantage of this procedure is that staining intensities can be automatically detected by image analysis software. To minimize the error caused by evaluation of cytological samples where the superficial degenerated cells may show an altered DNA content, a second cytological smear was taken as done by Souto et al.[24,25,26]
Oral lichen planus is considered a PMD and the percentage of them turning malignant has been debated over the years and authors have concluded that 0.2-2% of OLP can turn malignant, but the reason for this change is still not known. Authors have not been able to know whether OLP has the inherent potential to turn malignant or is it the altered epithelium that makes it more susceptible to carcinogens.[27,28,29] The subtype of OLP that is more prone for malignancy is also controversial. This has been investigated by few authors using cytology.[29,30,31] This study has been done as there are no exclusive studies done to assess the ploidy status of subtypes of OLP using exfoliative cytology and DNA-ICM.
In this study, we observed that 11 smears of patients with erosive and atrophic types of OLP were aneuploid. This is in accordance with the study done by Hosni et al.,[32] in tissue sections who observed that the majority of the aneuploid cases were in the erosive and atrophic subtypes of OLP when compared to reticular type of OLP. Our study also showed similar results. Some authors believe that aneuploidy may be the result of an aberrant nuclear replication or mitotic process that allows an altered quantity of DNA to be conserved within the nuclei or these are likely to represent nonproliferating cells with abnormal chromosomal aneuploidies.[17,33] Few believe that this might be the result of a multi-step process of accumulated genetic alterations. These alterations affect epithelial cell behavior by way of loss of the chromosomal heterozygosity that in turn leads to a series of events progressing to the ultimate stage of aneuploidy.[34] The clinical importance of aneuploidy is that the tumor cells with aneuploid nuclei tend to grow faster than normal diploid cells and tend to develop multiple clonal genetic alterations, which lends a clonal population of cells which finally might lead to malignancy. Thus, identification of the ploidy status of a PMD is very important to predict the prognosis.[35,36] If we take aneuploidy to be a marker of neoplasm, our study shows that the atrophic and erosive types of OLP has a greater propensity to malignant transformation than the reticular and plaque clinical forms of. In our study, we did not observe any statistically significant difference between the erosive and atrophic subtypes of OLP. This could have been due to the small sample size. However, a long-term follow-up study of the cases analyzed here is necessary to confirm these hypotheses.
Conclusions
Based on the results observed in our study, it can be summarized that the cells in reticular and plaque type of OLP mostly exhibits diploidy where the cells in erosive and atrophic types of OLP show aneuploidy. Thus, it is very important to follow-up the patients with OLP and exfoliative cytology and DIC can be employed to assess ploidy status. It should be noted that cytology can be a very important adjuvant for diagnosis, follow-up and for mass screening purposes of not only OLP but other PMD's such as oral submucous fibrosis, leukoplakia and erythroplakia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wilson E. On lichen planus. J Cutan Med Dis Skin. 1869;3:117–32. [Google Scholar]
- 2.Mollaoglu N. Oral lichen planus: A review. Br J Oral Maxillofac Surg. 2000;38:370–7. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 3.Chainani-Wu N, Silverman S, Jr, Lozada-Nur F, Mayer P, Watson JJ. Oral lichen planus: Patient profile, disease progression and treatment responses. J Am Dent Assoc. 2001;132:901–9. doi: 10.14219/jada.archive.2001.0302. [DOI] [PubMed] [Google Scholar]
- 4.Lozada-Nur F, Miranda C. Oral lichen planus: Epidemiology, clinical characteristics, and associated diseases. Semin Cutan Med Surg. 1997;16:273–7. doi: 10.1016/s1085-5629(97)80016-8. [DOI] [PubMed] [Google Scholar]
- 5.Andreasen JO. Oral lichen planus 1. A clinical evaluation of 115 cases. Oral Surg Oral Med Oral Pathol. 1968;25:31–42. doi: 10.1016/0030-4220(68)90194-1. [DOI] [PubMed] [Google Scholar]
- 6.George A, Sreenivasan BS, Sunil S, Varghese SS, Thomas J, Gopakumar D, et al. Potentially malignant disorders of oral cavity. Oral Maxillofac Pathol J. 2011;2:95–100. [Google Scholar]
- 7.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol. 2002;46:207–14. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- 8.Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, et al. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: A follow-up study in an Italian population. Oral Oncol. 2004;40:77–83. doi: 10.1016/s1368-8375(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 9.Lanfranchi-Tizeira HE, Aguas SC, Sano SM. Malignant transformation of atypical oral lichen planus: A review of 32 cases. Med Oral. 2003;8:2–9. [PubMed] [Google Scholar]
- 10.Motherby H, Marcy T, Hecker M, Ross B, Nadjari B, Auer H, et al. Static DNA cytometry as a diagnostic aid in effusion cytology: I. DNA aneuploidy for identification and differentiation of primary and secondary tumors of the serous membranes. Anal Quant Cytol Histol. 1998;20:153–61. [PubMed] [Google Scholar]
- 11.Motherby H, Nadjari B, Remmerbach T, Marcy T, Pomjanskaja N, Müller W, et al. Static DNA cytometry as a diagnostic aid in effusion cytology: II. DNA aneuploidy for identification of neoplastic cells in equivocal effusions. Anal Quant Cytol Histol. 1998;20:162–8. [PubMed] [Google Scholar]
- 12.Remmerbach TW, Weidenbach H, Pomjanski N, Knops K, Mathes S, Hemprich A, et al. Cytologic and DNA-cytometric early diagnosis of oral cancer. Anal Cell Pathol. 2001;22:211–21. doi: 10.1155/2001/807358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grote HJ, Nguyen HV, Leick AG, Böcking A. Identification of progressive cervical epithelial cell abnormalities using DNA image cytometry. Cancer. 2004;102:373–9. doi: 10.1002/cncr.20644. [DOI] [PubMed] [Google Scholar]
- 14.Kämmerer PW, Koch FP, Santoro M, Babaryka G, Biesterfeld S, Brieger J, et al. Prospective, blinded comparison of cytology and DNA-image cytometry of brush biopsies for early detection of oral malignancy. Oral Oncol. 2013;49:420–6. doi: 10.1016/j.oraloncology.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Acha-Sagredo A, Jiménez Y, Bagán JV, Echebarria-Goicouria MA, Aguirre-Urizar JM. Cytometric analysis of oral scrapings of patients with oral lichen planus. Cytopathology. 2011;22:106–10. doi: 10.1111/j.1365-2303.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 16.Haroske G, Giroud F, Reith A, Böcking A. 1997 ESACP consensus report on diagnostic DNA image cytometry. Part I: Basic considerations and recommendations for preparation, measurement and interpretation. European Society for Analytical Cellular Pathology. Anal Cell Pathol. 1998;17:189–200. doi: 10.1155/1998/390837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroske G, Baak JP, Danielsen H, Giroud F, Gschwendtner A, Oberholzer M, et al. Fourth updated ESACP consensus report on diagnostic DNA image cytometry. Anal Cell Pathol. 2001;23:89–95. doi: 10.1155/2001/657642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böcking A, Giroud F, Reith A. Consensus report of the ESACP task force on standardization of diagnostic DNA image cytometry. European Society for Analytical Cellular Pathology. Anal Cell Pathol. 1995;8:67–74. [PubMed] [Google Scholar]
- 19.Giroud F, Haroske G, Reith A, Böcking A. 1997 ESACP consensus report on diagnostic DNA image cytometry. Part II: Specific recommendations for quality assurance. European Society for Analytical Cellular Pathology. Anal Cell Pathol. 1998;17:201–8. doi: 10.1155/1998/237659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maraki D, Becker J, Boecking A. Cytologic and DNA-cytometric very early diagnosis of oral cancer. J Oral Pathol Med. 2004;33:398–404. doi: 10.1111/j.1600-0714.2004.0235.x. [DOI] [PubMed] [Google Scholar]
- 21.Bradley G, Odell EW, Raphael S, Ho J, Le LW, Benchimol S, et al. Abnormal DNA content in oral epithelial dysplasia is associated with increased risk of progression to carcinoma. Br J Cancer. 2010;103:1432–42. doi: 10.1038/sj.bjc.6605905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandizzi D, Lanfranchi HE, Cabrini RL. Ploidy study in oral carcinomas: Use of improved methodology to assess its clinical prognostic value. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:406–12. doi: 10.1016/j.tripleo.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Islam MN, Kornberg L, Veenker E, Cohen DM, Bhattacharyya I. Anatomic site based ploidy analysis of oral premalignant lesions. Head Neck Pathol. 2010;4:10–4. doi: 10.1007/s12105-009-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Del Moral R, Quesada MJ, Ruiz-Avila I. Histochemistry protein, biogenic amines and nucleic acids. In: García-Del Moral R, editor. Madrid Laboratory of Pathology. Madrid: McGraw-Hill; 1993. pp. 245–63. [Google Scholar]
- 25.Mehrotra R, Gupta A, Singh M, Ibrahim R. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Mol Cancer. 2006;5:11. doi: 10.1186/1476-4598-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Souto GR, Caliari MV, Lins CE, de Aguiar MC, de Abreu MH, Mesquita RA. Tobacco use increase the number of aneuploid nuclei in the clinically healthy oral epithelium. J Oral Pathol Med. 2010;39:605–10. doi: 10.1111/j.1600-0714.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmstrup P. The controversy of a premalignant potential of oral lichen planus is over. Oral Surg Oral Med Oral Pathol. 1992;73:704–6. doi: 10.1016/0030-4220(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 28.van der Meij EH, Schepman KP, Smeele LE, van der Wal JE, Bezemer PD, van der Waal I. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307–10. doi: 10.1016/s1079-2104(99)70033-8. [DOI] [PubMed] [Google Scholar]
- 29.Mattsson U, Jontell M, Holmstrup P. Oral lichen planus and malignant transformation: Is a recall of patients justified? Crit Rev Oral Biol Med. 2002;13:390–6. doi: 10.1177/154411130201300503. [DOI] [PubMed] [Google Scholar]
- 30.Sugerman PB, Savage NW, Williams SL, Joynson OB, Daley TJ, Cowpe JG. A quantitative cytological study of lesional and non-lesional mucosa in oral lichen planus. Arch Oral Biol. 1996;41:117–20. doi: 10.1016/0003-9969(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 31.Teja CS, Devy AS, Nirmal RM, Sunil PM, Deepasree M. Cytomorphometric analysis of exfoliated cells in oral lichen planus. Cytojournal. 2014;11:3. doi: 10.4103/1742-6413.127214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosni ES, Yurgel LS, Silva VD. DNA ploidy in oral lichen planus, determined by image cytometry. J Oral Pathol Med. 2010;39:206–11. doi: 10.1111/j.1600-0714.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Rendon A, Stewart R, Craig GT, Wells M, Speight PM. DNA ploidy analysis by image cytometry helps to identify oral epithelial dysplasias with a high risk of malignant progression. Oral Oncol. 2009;45:468–73. doi: 10.1016/j.oraloncology.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Elhamd KE, Habib TN. The flow cytometric analysis of premalignant and malignant lesions in head and neck squamous cell carcinoma. Oral Oncol. 2007;43:366–72. doi: 10.1016/j.oraloncology.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Morita M, Kuwano H, Tsutsui S, Ohno S, Matsuda H, Sugimachi K. Cytophotometric DNA content and argyrophilic nucleolar organiser regions of oesophageal carcinoma. Br J Cancer. 1993;67:480–5. doi: 10.1038/bjc.1993.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mithani SK, Mydlarz WK, Grumbine FL, Smith IM, Califano JA. Molecular genetics of premalignant oral lesions. Oral Dis. 2007;13:126–33. doi: 10.1111/j.1601-0825.2006.01349.x. [DOI] [PubMed] [Google Scholar]


