Abstract
Background:
Gentamicin (GM) is used as antibiotic for Gram-negative infections, but its administration is limited due to a side-effect of nephrotoxicity. It was attempted to investigate the effect of Althaea officinalis flower extract (AOFE) against nephrotoxicity induced by GM in male rats.
Methods:
30-year-old male Wistar rats were divided into five groups. Group 1 as a negative control group received AOFE 250 mg/kg/day. Groups 2-5 received saline, AOFE 50 mg/kg/day, AOFE 250 mg/kg/day, and AOFE 500 mg/kg/day for 9 days, respectively, and GM (100 mg/kg/day) was added from the 3rd day on. At the end of the experiment, blood samples were obtained, animals were sacrificed, and the kidneys were removed immediately.
Results:
Gentamicin (in group 2) significantly increased serum levels of blood urea nitrogen and creatinine as well as the pathological damage score (P < 0.05) when compared with group 1. Low dose of AOFE did not decrease the nephrotoxicity induced by GM while the high dose of AOFE aggravated renal toxicity (P < 0.05).
Conclusions:
Although AOFE acts as an antioxidant, at the doses used in the current study did not ameliorate nephrotoxicity induced by GM.
Keywords: Gentamicin, Althaea officinalis, nephrotoxicity, rat
INTRODUCTION
Gentamicin (GM) is one of the amino glycoside drugs used in Gram-negative infections.[1,2,3] Nephrotoxicity is specified by renal dysfunction, which is distinguished by increasing serum levels of blood urea nitrogen (BUN) and creatinine (Cr).[4,5] Researchers have tested different compounds for preventing or treating damages induced by GM. Compounds such as lycopene,[6] metformin, garlic,[7,8] Vitamin E, probucol,[9] and erythropoietin[10] could prevent renal damage induced by GM. Furthermore, several studies have suggested that supplementations of herbal extracts such as Ginkgo biloba,[11] Bauhinia variegata,[12] Pongamia pinnata flower,[13] and grape seed[14] may attenuate GM-induced nephrotoxicity. Althaea officinalis (marshmallow, marshmallow, or a common marshmallow), the member of Malvaceae family, is well-known for its medicinal properties.[15,16] It is demonstrated that A. officinalis has potential therapeutic benefits in lipomia, inflammation, gastric ulcer, and platelet aggregation.[17] The pharmacological and antioxidant activities of A. officinalis refer to various compounds such as polysaccharides and flavonoids present in the plant.[16,18] In the present study, we attempted to investigate the effect of A. officinalis flower extract (AOFE) as an antioxidant against nephrotoxicity-induced by GM in male rats.
METHODS
Adult male Wistar rats (Animal Centre, Isfahan University of Medical Sciences) were used in this study. Animals were housed in standard conditions with free access to food and water. This research was approved in advance by the Isfahan University of Medical Sciences Ethics Committee.
Preparation of extract
Dried violet flowers of A. officinalis were selected and powdered. Preparation of the extract was fulfilled in two steps; first, 600 ml ethanol 70% was added to 150 g prepared powder and the total mixture was shaken for 24 h at the temperature of 23-25°C. Then, it was filtrated by Whatman paper (70 mm). After filtration, the removed extract was incubated at the temperature of 4°C. Then, 600 ml ethanol 96% was added to the material remained from the first step and again the total mixture was shaken for 24 h at the temperature of 23-25°C. The extract obtained after filtration in this step was mixed with the yield of the first step. Then, the total extract was incubated at the temperature of 50°C for 48 h and finally 100% dried extract was obtained.
Study design
Thirty animals (192.4 ± 4.6 g) were divided into five groups.
Group 1 (n = 6) as negative control group received AOFE 250 mg/kg/day for 9 days, and saline was added from day 3 on
Groups 2 (n = 5) as positive control group received saline during the study and GM (100 mg/kg/day) was added from day 3 on
Group 3 (n = 6) received AOFE 50 mg/kg/day for 9 days, and GM (100 mg/kg/day) was added from day 3 on. Groups 4 (n = 7) and 5 (n = 6) had the same regimen of group 3 except AOFE dose which were 250 mg/kg/day and 500 mg/kg/day, respectively. All administrations were done intraperitoneally. At the end of the experiment, animals were anesthetized by ketamine (75 mg/kg). Blood samples were obtained via heart puncture, and the serum was kept at −20ºC to measure the serum levels of BUN and Cr. Finally, the animals were killed. The kidneys were removed and weighed immediately. Left kidney was fixed in formalin and staining was performed to detect the tissue damage.
Pathological investigation
The left kidney was fixed in 10% neutral formalin and embedded in paraffin. After slicing, hematoxylin and eosin staining was performed to examine tissue damage including tubular atrophy, cast, debris, and necrotic materials in the tubular lumen. Intensity of tubular lesion was scored from 1 to 4, while zero score was assigned to normal tissue without damage.
Statistical analysis
Data were reported as mean ± standard error of the mean. The two groups were compared with regard to the serum levels of BUN and Cr, and kidney weight (KW) by independent Student's t-test. The parameters were analyzed by one-way ANOVA followed by least significant difference test among the groups. The kidney tissue damage score (KTDS) was compared using Kruskal–Wallis or Mann–Whitney tests. P <0.05 were considered as significant.
RESULTS
Gentamicin itself induced nephrotoxicity, which was confirmed by increasing in the serum levels of BUN and Cr as well as elevating in KTDS and KW (P < 0.05) [Table 1]. Administration of various doses of AOFE accompanied with GM did not attenuate the serum levels of BUN and Cr; rather it increased the values (P < 0.05). High dose of AOFE aggravated renal damage induced by GM in comparison with other groups (P < 0.05) [Figure 1]. Sample images from group 1 treated with AOFE alone and group 5 treated with GM plus high dose of AOFE are demonstrated in Figure 2.
Table 1.
BUN and Cr serum levels, KTDS, and KW g/100 g body weight in the experiment groups
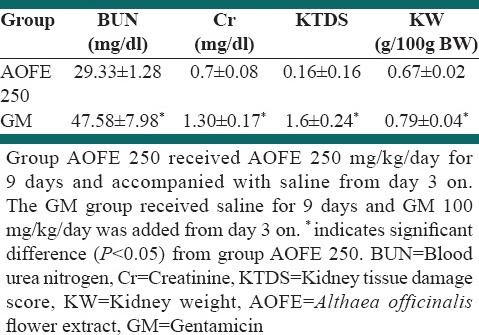
Figure 1.
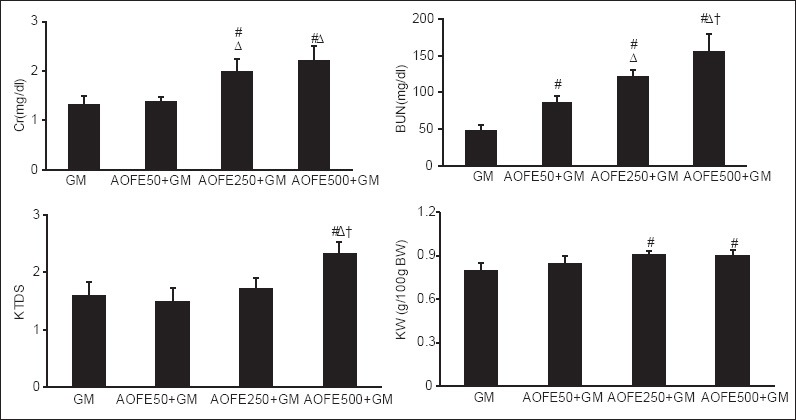
Blood urea nitrogen (BUN) and creatinine (Cr) serum levels, kidney tissue damage score (KTDS), and kidney weight g/100g of body weight in the experiment groups. Gentamicin (GM), Althaea officinalis flower extract (AOFE) 50 + GM, AOFE 250 + GM, and AOFE 500 + GM groups received saline, AOFE 50 mg/kg/day, AOFE 250 mg/kg/day, and AOFE 500 mg/kg/day for 9 days, respectively, and GM (GM; 100 mg/kg/day) was added from day 3 on. #indicates significant difference from GM group. Δindicates significant difference from AOFE 50 + GM group. †indicates significant difference from AOFE 250 + GM group
Figure 2.
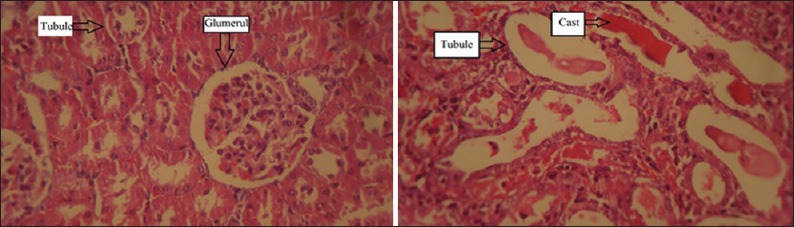
Sample images from group 1 treated with Althaea officinalis flower extract (AOFE) alone (left) and group 5 treated with GM plus high dose of AOFE (right). The tissue damage in group 5 is higher than other groups
DISCUSSION
The aim of this study was to investigate whether AOFE could ameliorate nephrotoxicity induced by GM in the male rat. We observed that AOFE administration did not ameliorate nephrotoxicity induced by GM; rather it intensified renal failure. GM induces renal dysfunction, which is characterized by increase in levels of Cr, uric acid, and BUN.[19,20,21] In addition, it is accompanied with tissue alterations such as glomerular congestion, disruption of glomerular capillaries, vacuolar degeneration of tubular epithelial cells, and hyaline cast formation.[20,21] Our findings are in agreement with the results of these studies. Furthermore, the present study indicated that GM enhanced normalized KW probably due to edema caused by tubular necrosis.[22] Useful properties of A. officinalis flower were documented in the literature,[17] but we did not obtain positive results in the administered doses. It is demonstrated that administration of 50 mg/kg dose of A. officinalis flower result in a significant increase in serum HDL cholesterol level.[17] Also, antiinflammatory and antiulcerogenic effects of the extract were observed at doses of 50, 100, and 250 mg/kg.[17] In contrast, we observed that doses of 50 and 250 mg/kg of AOFE aggravated the increased levels of BUN and Cr induced by GM. In addition, AOFE at the dose of 500 mg/kg aggravated both renal dysfunction and tissue damage. It has reported that increasing the dose of AOFE to 500 mg/kg significantly decreased stool water content.[17] It is also reported that high doses of some antioxidants provide a harmful effect on survival of the subjects.[23,24] Therefore, it is possible that AOFE at the doses lower than 50 mg/kg may ameliorate nephrotoxicity induced by GM.
CONCLUSIONS
Although AOFE acts as an antioxidant, doses of AOFE used in the current study did not ameliorate nephrotoxicity induced by GM, and it is necessary to test doses lower than 50 mg/kg.
ACKNOWLEDGMENTS
This research was supported by the Isfahan University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kagan BM. Gentamicin in the treatment of gram-negative infections. Calif Med. 1970;112:60–1. [PMC free article] [PubMed] [Google Scholar]
- 2.Noone P, Parsons TM, Pattison JR, Slack RC, Garfield-Davies D, Hughes K. Experience in monitoring gentamicin therapy during treatment of serious gram-negative sepsis. Br Med J. 1974;1:477–81. doi: 10.1136/bmj.1.5906.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavafi M. Protection of renal tubules against gentamicin induced nephrotoxicity. J Ren Inj Prev. 2013;2:5–6. doi: 10.12861/jrip.2013.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker PD, Shah SV. Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest. 1988;81:334–41. doi: 10.1172/JCI113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton DC, Plamp CE, 3rd, DeFehr JM, Bennett WM, Porter G, Gilbert D. Gentamicin and tobramycin nephrotoxicity. A morphologic and functional comparison in the rat. Am J Pathol. 1978;93:137–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Karahan I, Atessahin A, Yilmaz S, Ceribasi AO, Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215:198–204. doi: 10.1016/j.tox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Pedraza-Chaverrí J, Maldonado PD, Medina-Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, Hernández-Pando R, et al. Garlic ameliorates gentamicin nephrotoxicity: Relation to antioxidant enzymes. Free Radic Biol Med. 2000;29:602–11. doi: 10.1016/s0891-5849(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 8.Rafieian-Kopaei M, Baradaran A, Merrikhi A, Nematbakhsh M, Madihi Y, Nasri H. Efficacy of co-administration of garlic extract and metformin for prevention of gentamicin-renal toxicity in wistar rats: A biochemical study. Int J Prev Med. 2013;4:258–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Naim AB, Abdel-Wahab MH, Attia FF. Protective effects of vitamin e and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 1999;40:183–7. doi: 10.1006/phrs.1999.0494. [DOI] [PubMed] [Google Scholar]
- 10.Rafieian-Kopaei M, Nasri H, Nematbakhsh M, Baradaran A, Gheissari A, Rouhi H, et al. Erythropoietin ameliorates genetamicin-induced renal toxicity: A biochemical and histopathological study. J Nephropathol. 2012;1:109–16. doi: 10.5812/nephropathol.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naidu MU, Shifow AA, Kumar KV, Ratnakar KS. Ginkgo biloba extract ameliorates gentamicin-induced nephrotoxicity in rats. Phytomedicine. 2000;7:191–7. doi: 10.1016/s0944-7113(00)80003-3. [DOI] [PubMed] [Google Scholar]
- 12.Sharma RK, Rajani G, Sharma V, Komala N. Effect of ethanolic and aqueous extracts of Bauhinia variegata Linn. On gentamicin-induced nephrotoxicity in rats. Indian J Pharm Educ Res. 2011;45:192–8. [Google Scholar]
- 13.Shirwaikar A, Malini S, Kumari SC. Protective effect of Pongamia pinnata flowers against cisplatin and gentamicin induced nephrotoxicity in rats. Indian J Exp Biol. 2003;41:58–62. [PubMed] [Google Scholar]
- 14.El-Ashmawy IM, El-Nahas AF, Salama OM. Grape seed extract prevents gentamicin-induced nephrotoxicity and genotoxicity in bone marrow cells of mice. Basic Clin Pharmacol Toxicol. 2006;99:230–6. doi: 10.1111/j.1742-7843.2006.pto_497.x. [DOI] [PubMed] [Google Scholar]
- 15.Elmastas M, Ozturk L, Gokce I, Erenler R, Aboul-Enein HY. Determination of antioxidant activity of marshmallow flower (Althaea officinalis L.) Anal Lett. 2004;37:1859–69. [Google Scholar]
- 16.Shah S, Akhtar N, Akram M, Shah PA, Saeed T, Ahmed K, et al. Pharmacological activity of Althaea officinalis L. J Med Plants Res. 2011;5:5662–6. [Google Scholar]
- 17.Hage-Sleiman R, Mroueh M, Daher CF. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm Biol. 2011;49:327–33. doi: 10.3109/13880209.2010.516754. [DOI] [PubMed] [Google Scholar]
- 18.Valiei M, Shafaghat A, Salimi F. Chemical composition and antimicrobial activity of the flower and root hexane extracts of Althaea officinalis in Northwest Iran. J Med Plants Res. 2011;5:6972–6. [Google Scholar]
- 19.Romero F, Pérez M, Chávez M, Parra G, Durante P. Effect of uric acid on gentamicin-induced nephrotoxicity in rats-role of matrix metalloproteinases 2 and 9. Basic Clin Pharmacol Toxicol. 2009;105:416–24. doi: 10.1111/j.1742-7843.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–70. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padmini MP, Kumar JV. A histopathological study on gentamycin induced nephrotoxicity in experimental albino rats. IOSR J Dent Med Sci. 2012;1:14–7. [Google Scholar]
- 22.Erdem A, Gündogan NU, Usubütün A, Kilinç K, Erdem SR, Kara A, et al. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant. 2000;15:1175–82. doi: 10.1093/ndt/15.8.1175. [DOI] [PubMed] [Google Scholar]
- 23.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 24.Bairati I, Meyer F, Jobin E, Gélinas M, Fortin A, Nabid A, et al. Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int J Cancer. 2006;119:2221–4. doi: 10.1002/ijc.22042. [DOI] [PubMed] [Google Scholar]


