Abstract
Background:
Appetite lowering characteristics of dairy have attracted scientists to look for its effect on energy intake particularly among children. In the present study, we tried to assess the effect of low-fat milk on total and short-term energy intake among obese boys in a randomized three-way cross-over clinical trial.
Methods:
A total of 34 obese 10-12-year-old boys were randomized to consume three beverages (low-fat milk, apple juice, or water) with a fixed energy breakfast for two consecutive days, 1 week apart. Ad libitum lunch was provided for subjects 5 h later. The energy intake from breakfast till lunch and total energy intake on intervention days, and 2 days after intervention were compared. Generalized linear model repeated measures procedure in which test beverages were considered as repeated factors.
Results:
Energy intake from breakfast till lunch was lower when low-fat milk consumption was included in the breakfast compared with water and apple juice (adjusted mean ± standard error: Low-fat milk = 1484.33 ± 15.30 Kcal, apple juice = 1543.39 ± 20.70 Kcal, water = 1606.6 ± 19.94 Kcal; P < 0.05). The energy intake on a day before interventions, total energy intake on intervention days, and 2 days after intervention was not statistically different between intervention periods (P > 0.05).
Conclusions:
One serving of low-fat milk might affect the energy intake in a short-term period. The possible effect of frequent consumption of dairy products on long-term energy intake among children is needed to be examined.
Keywords: Apple juice, children, energy intake, low-fat milk, obesity
INTRODUCTION
Today, obesity is becoming an epidemic among children.[1] According to the latest report by WHO, the prevalence of childhood obesity is estimated to be about 43 million, worldwide.[2] Kelishadi et al. showed that obesity is affecting 8.8% of Iranian children based on the criteria explained by Center for Disease Control (CDC).[3] Management of childhood obesity has become very important for health care providers because new researches have revealed its connections with several chronic conditions including metabolic syndrome, cardiovascular disease, type 2 diabetes, and nonalcoholic fatty liver disease.[4]
Extra energy intake and sedentary lifestyle are two well-known determinants of childhood obesity.[5] Increasing the sense of satiety among children has been targeted by many researchers to reduce the total energy intake and in turn to control their weight gain.[6] Dietary factors, including nutrients (calcium and vitamin D), food groups (such as whole grains and dairy products) and glycemic index and load are widely studied for their effects on the total energy intake.[7] Dairy is one of the food groups with a high level of protein and calcium content that is widely studied for its possible role on controlling obesity, body fat and waist circumstance.[8] The effect of dairy consumption on the energy intake in the subsequent meal is mostly examined among the adult population, but the reports of these studies were contradictory;[9,10,11,12] however, studies have led to more inconsistent results when taking the total energy intake into consideration. For instance, Almiron-Roig and Drewnowski comparing the effects of orange juice, low-fat milk (1%), regular cola, and carbonated water consumption on the short-term energy intake reported that energy containing preload decreased hunger and increased fullness compared with water but energy intake at next meal was not significantly different between all four beverages. They also showed that the total energy intake in participants who consumed orange juice, low-fat milk (1%), and regular cola was higher than subjects who consumed water.[13] Soenen and Westerterp-Plantenga when assessed the effect of sucrose, high-fructose corn syrup (HFCS), milk and diet drink (orange flavored custom-made beverages) consumption on satiety and energy intake of ad libitum meal, found that although energy intake of test meal was significantly lower after intake of energy containing beverages in comparison with diet drink but the total energy intake (energy intake from preload plus energy intake of test meal) was higher after intake of sucrose, HFCS and milk compared with the diet preload.[14] Tsuchiya et al. showed that when subjects consumed fruit drink, dairy fruit drink, liquid yogurt and semi-solid yogurt as the snack, the total energy intake (breakfast, snack, test meal) was not significantly different.[11] We are aware of the only study trying to assess the effect of dairy intake compared to isoenergetic drinks including glucose beverage and a half milk/glucose beverage on appetite and short-term energy intake.[15] Brindal et al. showed that the three iso-energetic beverages had same effects on appetite and the energy intake 3 h after the ingestion of preloads among 10-12 years children. To the best of our knowledge, no study has examined the effect of dairy intake on the total daily energy intake among children; therefore, the present study was designed to assess the effect of consuming 240 ml low-fat milk in breakfast compared with 240 ml of apple juice (with the same volume and energy) and 240 ml of water on the total daily energy intake in the context of a randomized three-way cross-over clinical trial.
METHODS
Study design and participants
Participants were recruited from an elementary school located in the 4th educational district of Isfahan, Iran. A total of 43 obese 10-12-year-old boys were enrolled to study and 34 children were eligible to enter the study. Eligibility criteria for entering to study were included: Having a body mass index (BMI) above the 95th percentile based on WHO BMI-for-age charts, not having dietary history of being on diet during or not intending to go on diet during study period, not having any intolerance to cow's milk; not having any history of congenital or metabolic diseases using information from parents and regularly consume breakfast. Subjects were excluded if they refused to participate in a study at least in two of the intervention periods. Parents were asked to fill an informed consent before the start of the study. Height and weight were measured before intervention days. Characteristics of participants in this study were shown in Table 1. The study protocol was registered in the Iranian Registry of Clinical Trials (registration no.: IRCT2013022312571N1).
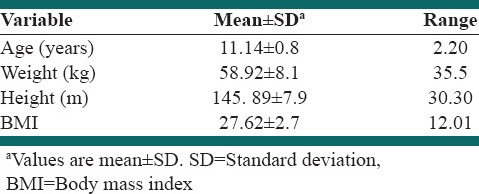
Table 1.
Characteristics of study subjects
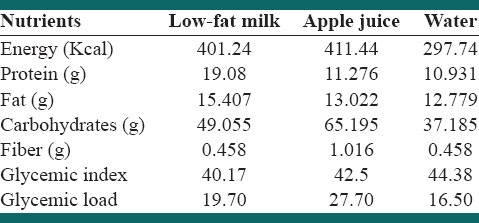
This was a three-way repeated measurement randomized, controlled cross-over trial in which each individual served as his own control. Participants were randomly assigned into 6 rolling methods to receive one of the three iso-volumic drinks (low-fat milk [1.5% fat], apple juice, water) along with a fixed energy breakfast. Intervention periods lasted for 2 consecutive days, and there were 7 days wash-out period between interventions. On the intervention days, boys attended to school, while they were in fasted state at 7 a.m. Subjects were given breakfast meal that was included 65 g of an Iranian whole-wheat bread (called sangak), 15 g of low-fat cheese and 12 g of walnut with one of the three test beverages: Low fat milk, apple juice or water. Subjects completely ingested their breakfast till 7:30 a.m. The energy and nutrient profile of breakfast meals be represented in Table 2. After breakfast, the boys were prohibited to eat and drink anything until lunchtime except water and a small portion of the fruit (apple) that contained approximately 10 gr of carbohydrates. Lunch meal was provided 5 h after breakfast at 12:30 p.m. that was the same list between three groups. An ad libitum lunch was provided for subjects that consisted of rice, chicken, boiled potato, yogurt, cola beverage, pasta, tomato sauce and botteled water for the first days of the intervention periods and rice, chicken, potato, roast ground, yogurt, botteled water and colla beverage for all second intervention days. Subjects were allowed to freely select their food. Lunch was served in the school hall. Subjects were asked to continue to eat until they felt absolutely full. The boys were given 30 min to eat their lunch. The foods that were given to boys were weighed before serving and after they finishing their lunch.
Table 2.
Nutrient profile of the intervention preloads along with the fixed content breakfast
Procedures and variables assessment
Anthropometric measurements
A wall-mounted stadiometer was used to measure the height, and weight was assessed using a digital scale, whereas subjects are wearing minimal clothing. BMI was computed as weight (kg) divided by the square of height (m2). Anthropometric measurements were performed three times for each person and value that was shown at least twice for each subject was recorded.
Assessment of energy intake and physical activity
Subjects were asked to maintain their physical activity and usual diet during the study period. To check this, parents were asked to record their children's physical activity 1 day before the intervention, during the intervention days and 1 day after the intervention. Physical activity information was converted to metabolic equivalent-hour/day. The dietary intakes consumed by participants on intervention days from breakfast till lunch was measured by weighted dietary record done by a trained nutritionist. Parents were also asked to complete a food record 2 days before intervention, during the intervention, and 2 days after intervention days. Dietary intakes were converted to grams and then converted to energy using nutritionist IV software (version 3.5.2, Axxya Systems, Redmond, Washington, USA).
Statistical analysis
Randomized allocation of participants into rolling methods and all statistical analysis were performed using IBM SPSS software, version 20 (IBM SPSS, Tokyo, Japan). Descriptive statistics is represented as means ± standard deviations (SDs), otherwise stated. The normal distribution of all continuous variables was checked using Kolmogorov–Smirnov test. Mean energy intakes (1) from breakfast till lunch (breakfast, morning snack, and lunch); (2) on whole intervention days (3) until two days after intervention and (4) two days before intervention days. Were compared among test beverages using generalized linear model repeated measures procedure in which test beverages and testing occasions were considered as repeated factors. Participants’ age, BMI, and rolling program as between subjects’ variables and physical activity and total energy intake, as within subject variables were adjusted in all models. P < 0.05 were considered as statistically significant.
RESULTS
All of the 34 participants who had inclusion criteria and enrolled to study, completed the three periods of interventions. Mean ± SD of participant's age was 11.14 ± 0.8 years. Their mean weight, height, and BMI were 58.9 ± 8.1 kg, 145.9 ± 7.9 cm, and 27.62 ± 2.7 kg/m2, respectively.
Energy intakes were normally distributed. Figure 1 depicts the participants’ age, BMI, rolling program, physical activity and total energy intake adjusted mean ± standard error energy intake from breakfast till lunch based on test beverages. Energy intake was 122.25 ± 15.91 Kcal lower when subjects consumed low-fat milk in comparison with the days that they consumed water at breakfast (P < 0.001). Low-fat milk also led to 59.06 ± 18.28 Kcal lower energy intake compared with apple juice (P = 0.01).
Figure 1.
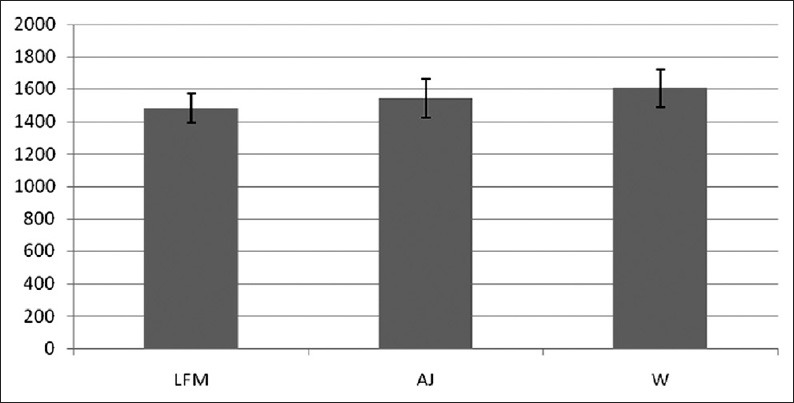
Age, BMI, rolling program, physical activity and total energy intake adjusted Mean ±SD of energy intake from breakfast till lunch based on test meals
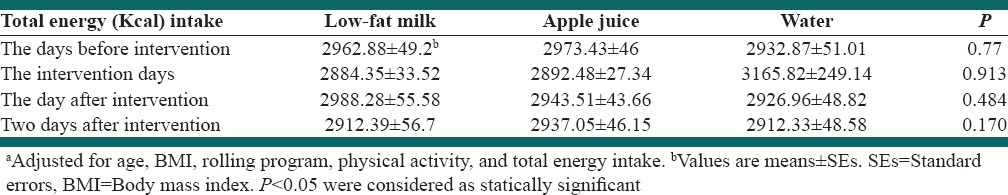
Total adjusted energy intake on the day before intervention, intervention days, the day after intervention and 2 days after intervention were not significantly different between test beverages (P > 0.05). Details are represented in Table 3.
Table 3.
Adjusted energy intake (Kcal) based on intervention periodsa
DISCUSSION
The present three-way cross-over trial among 10-12 years old boys showed that intake of low-fat milk in breakfast lead to decrease energy intake from breakfast till lunch in comparison with apple juice and water. However, total whole day energy intake was not significantly different between the three interventions. In contrast with this study, Soenen and Westerterp-Plantenga showed that the intake of energy containing preload including milk, sucrose, HFCS lead to increased total energy intake from time that subjects consumed the beverages till they consumed ad libitum test meal in comparison with diet preload.[14] Also, Dougkas et al. demonstrated that although the dairy snacks lead to decrease energy intake at next meal in comparison with water, however, when the energy of dairy snacks and water were added to the energy of next meal that subjects consumed, the total energy intake (snack plus next meal) in dairy groups was higher than in water group.[16] DellaValle et al. found that when test meal was served as a caloric beverages including regular cola, juice and milk or noncaloric beverages including water and diet cola or without any beverages, energy intake from test meal was not significantly different but when the energy from beverages was added to the energy that consumed from next meal the energy intake was higher for caloric beverages than noncaloric beverages.[17] Tsuchiya et al. showed that when subjects consumed fruit drink, dairy fruit drink, liquid yogurt and semi-solid yogurt as the snack, the energy intake at next meal was not significantly different. The situation did not change when the energy intake from breakfast, snack and test meal was added.[11]
Studies showed that dairy can affect the energy intake through its protein content. Diets with higher content of protein can increase diet-induced thermogenesis (DIT) than the carbohydrate or fat;[18,19] because the high amount of ATP is needed to synthesize protein.[20] Studies have shown that diets which produced higher DIT lead to reduced satiety compared with diets with lower DIT.[21] Also, studies found that whey protein lead to increased DIT compared with casein,[22] on the other hand Hursel et al. concluded that casein has higher satiating properties than whey protein.[23] Furthermore, it is proposed that the intake of casein lead to increased lipid oxidation compared to whey protein when supplied in small amounts.[24]
Protein might also stimulate the secretion of gastrointestinal (GI) hormones with have satiating properties and reduce the rate of gastric emptying such as cholecystokinin (CCK), glucagon-like peptide-1. The level of this hormone in circulatory system affects the intake of the next meal.[25,26] Dairy is one of the high protein dietary group that consisted of 80% casein and 20% whey protein that is high in branched chain amino acids and it is associated with stimulation of CCK secretion and induced satiety. It also affects the motility of GI tract and reduced the rate of gastric emptying.[27,28,29,30]
Whey and isoleucine that are highly found in milk lead to down regulate expression of genes that play a role in lipogenesis like acetyl-coenzyme A (acetyl-COA) carboxylase, cholesterol absorption and synthesis like 3-hydroxy-3-methyl-glutaryl-COA redoctase; therefore, dairy product could be involved in controlling energy intake thorough these pathways.[31] The other component of dairy that might help in controlling the energy intake is calcium. The mechanisms to justify the effects of calcium intake on satiety and energy intake are not fully cleared, but studies reported that presence of calcium represents availability of energy for the body.[32] A recent study showed that high calcium diet lead to increasing peptide YY (PYY) concentration that it may contribute to increasing sense of satiety.[33] Investigation was showed that PYY regulate a region in the brain that is contribute to behavior of appetite in human, so it can reduce food intake.[34] Some studies showed that calcium intake can lead to weight loss through a decreased lipogenesis, increased lipolysis and fat oxidation[35] but few study assessed the effect of calcium intake on regulating of appetite. It is also proposed that medium chain triglycerides in dairy products affect the DIT and lead to decrease energy intake and satiety.[36]
The present study has some limitations. One of them is that all of the participants were taking their breakfast together and were not blinded about the beverages. Participants may have changed their habitual diet because they were aware that the food intake of them during the whole day was recorded by their parents. Only obese boys were included in the present study. Therefore the generalization of our results to children must be done with caution.
CONCLUSIONS
Including one serving of low-fat milk in the breakfast of obese boys might affect their short-term energy intake in their subsequent meal; however, this effect might not remain for the whole day. Studies with longer intervention periods which include more dairy in the child's diet through the day are highly recommended.
ACKNOWLEDGMENTS
The authors would like to thank all the children, their parents, the staff of Hassane Jafarian Elementary School and Isfahan Department of Education for their close cooperation and hospitality.
Footnotes
Source of Support: Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Lob-Corzilius T. Overweight and obesity in childhood – a special challenge for public health. Int J Hyg Environ Health. 2007;210:585–9. doi: 10.1016/j.ijheh.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 2.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–64. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Kelishadi R, Ardalan G, Gheiratmand R, Majdzadeh R, Hosseini M, Gouya MM, et al. Thinness, overweight and obesity in a national sample of Iranian children and adolescents: CASPIAN Study. Child Care Health Dev. 2008;34:44–54. doi: 10.1111/j.1365-2214.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60:222–8. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 5.Karnik S, Kanekar A. Childhood obesity: A global public health crisis. Int J Prev Med. 2012;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Chapelot D, Payen F. Comparison of the effects of a liquid yogurt and chocolate bars on satiety: A multidimensional approach. Br J Nutr. 2010;103:760–7. doi: 10.1017/S000711450999225X. [DOI] [PubMed] [Google Scholar]
- 7.Rouhani MH, Salehi-Abargouei A, Azadbakht L. Effect of glycemic index and glycemic load on energy intake in children. Nutrition. 2013;29:1100–5. doi: 10.1016/j.nut.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Abargouei AS, Janghorbani M, Salehi-Marzijarani M, Esmaillzadeh A. Effect of dairy consumption on weight and body composition in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Int J Obes (Lond) 2012;36:1485–93. doi: 10.1038/ijo.2011.269. [DOI] [PubMed] [Google Scholar]
- 9.Dove ER, Hodgson JM, Puddey IB, Beilin LJ, Lee YP, Mori TA. Skim milk compared with a fruit drink acutely reduces appetite and energy intake in overweight men and women. Am J Clin Nutr. 2009;90:70–5. doi: 10.3945/ajcn.2008.27411. [DOI] [PubMed] [Google Scholar]
- 10.Harper A, James A, Flint A, Astrup A. Increased satiety after intake of a chocolate milk drink compared with a carbonated beverage, but no difference in subsequent ad libitum lunch intake. Br J Nutr. 2007;97:579–83. doi: 10.1017/S0007114507339846. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya A, Almiron-Roig E, Lluch A, Guyonnet D, Drewnowski A. Higher satiety ratings following yogurt consumption relative to fruit drink or dairy fruit drink. J Am Diet Assoc. 2006;106:550–7. doi: 10.1016/j.jada.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Almiron-Roig E, Grathwohl D, Green H, Erkner A. Impact of some isoenergetic snacks on satiety and next meal intake in healthy adults. J Hum Nutr Diet. 2009;22:469–74. doi: 10.1111/j.1365-277X.2009.00978.x. [DOI] [PubMed] [Google Scholar]
- 13.Almiron-Roig E, Drewnowski A. Hunger, thirst, and energy intakes following consumption of caloric beverages. Physiol Behav. 2003;79:767–73. doi: 10.1016/s0031-9384(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 14.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–94. doi: 10.1093/ajcn/86.5.1586. [DOI] [PubMed] [Google Scholar]
- 15.Brindal E, Baird D, Slater A, Danthiir V, Wilson C, Bowen J, et al. The effect of beverages varying in glycaemic load on postprandial glucose responses, appetite and cognition in 10-12-year-old school children. Br J Nutr. 2013;110:529–37. doi: 10.1017/S0007114512005296. [DOI] [PubMed] [Google Scholar]
- 16.Dougkas A, Minihane AM, Givens DI, Reynolds CK, Yaqoob P. Differential effects of dairy snacks on appetite, but not overall energy intake. Br J Nutr. 2012;108:2274–85. doi: 10.1017/S0007114512000323. [DOI] [PubMed] [Google Scholar]
- 17.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44:187–93. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Mikkelsen PB, Toubro S, Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: Comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr. 2000;72:1135–41. doi: 10.1093/ajcn/72.5.1135. [DOI] [PubMed] [Google Scholar]
- 19.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J Am Coll Nutr. 2004;23:373–85. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr. 1990;52:72–80. doi: 10.1093/ajcn/52.1.72. [DOI] [PubMed] [Google Scholar]
- 21.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502. doi: 10.1038/sj.ejcn.1600782. [DOI] [PubMed] [Google Scholar]
- 22.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hursel R, van der Zee L, Westerterp-Plantenga MS. Effects of a breakfast yoghurt, with additional total whey protein or caseinomacropeptide-depleted alpha-lactalbumin-enriched whey protein, on diet-induced thermogenesis and appetite suppression. Br J Nutr. 2010;103:775–80. doi: 10.1017/S0007114509992352. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzen J, Frederiksen R, Hoppe C, Hvid R, Astrup A. The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur J Clin Nutr. 2012;66:622–7. doi: 10.1038/ejcn.2011.221. [DOI] [PubMed] [Google Scholar]
- 25.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond) 2007;31:1696–703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]
- 26.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–83. doi: 10.1210/jc.2005-1856. [DOI] [PubMed] [Google Scholar]
- 27.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr. 2011;141:1626–34. doi: 10.3945/jn.111.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–57. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 29.Burton-Freeman BM. Glycomacropeptide (GMP) is not critical to whey-induced satiety, but may have a unique role in energy intake regulation through cholecystokinin (CCK) Physiol Behav. 2008;93:379–87. doi: 10.1016/j.physbeh.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Froetschel MA. Bioactive peptides in digesta that regulate gastrointestinal function and intake. J Anim Sci. 1996;74:2500–8. doi: 10.2527/1996.74102500x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Reimer RA. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition. 2009;25:340–9. doi: 10.1016/j.nut.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert JA, Joanisse DR, Chaput JP, Miegueu P, Cianflone K, Alméras N, et al. Milk supplementation facilitates appetite control in obese women during weight loss: A randomised, single-blind, placebo-controlled trial. Br J Nutr. 2011;105:133–43. doi: 10.1017/S0007114510003119. [DOI] [PubMed] [Google Scholar]
- 33.Jones KW, Eller LK, Parnell JA, Doyle-Baker PK, Edwards AL, Reimer RA. Effect of a dairy- and calcium-rich diet on weight loss and appetite during energy restriction in overweight and obese adults: A randomized trial. Eur J Clin Nutr. 2013;67:371–6. doi: 10.1038/ejcn.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009;587:19–25. doi: 10.1113/jphysiol.2008.164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougkas A, Reynolds CK, Givens ID, Elwood PC, Minihane AM. Associations between dairy consumption and body weight: A review of the evidence and underlying mechanisms. Nutr Res Rev. 2011;24:72–95. doi: 10.1017/S095442241000034X. [DOI] [PubMed] [Google Scholar]
- 36.Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68:226–34. doi: 10.1093/ajcn/68.2.226. [DOI] [PubMed] [Google Scholar]


