Abstract
Background:
Carotid ultrasound appears to be useful in the assessment of cardiovascular risk. In this study, we have assessed the carotid intima-media thickness (CIMT) in a group of individuals without a history of cardiovascular events.
Methods:
A sample of 431subjects (189 [43.9%] males and 242 [56.1%] females) was obtained from an urban population using a stratified-cluster method in Mashhad stroke and heart atherosclerosis disorder study. None of the subjects had a history of the cardiovascular event. Carotid artery duplex ultrasound was used to determine the CIMT in all subjects, and to identify those with an abnormal value (CIMT [+]; i.e., CIMT ≥ 0.8 mm). Dietary intake of participants was assessed using a questionnaire for 24-h dietary recall. The relationship between anthropometric, biochemical and dietary data and CIMT were assessed.
Results:
The mean age of subjects was 48.7 ± 8.0 years. Of the 431 patients, 118 (27.4%) were found to be CIMT (+). Of the cardiovascular parameters assessed, only age (odds ratio [OR] [95% confidence interval (CI)], 1.11 [0.56-4.34]; P < 0.01) and male gender (OR [95% CI], 1.14 [0.63-2.23]; P < 0.05) were significant independent predictors of ultrasound defined CIMT. Crude and total energy adjusted intake were not associated with the presence of CIMT (+).
Conclusions:
It appears that within a relatively young Iranian population of individuals without a history of cardiovascular event, the presence of CIMT (+) defined by duplex ultrasound cut-off value of ≥0.8 mm, did not associate with several modifiable cardiovascular risk factors or measures of dietary intake.
Keywords: Carotid artery atherosclerosis, duplex ultrasound, intima-media thickness, nutritional intake, risk factors
INTRODUCTION
Atherosclerosis is a highly prevalent condition which may be associated with cardiovascular events such as myocardial infarction (MI) and stroke.[1] The measurement of the carotid intima-media thickness (CIMT) has been proposed as a useful measure for assessing cardiovascular risk.[2] CIMT assessment is noninvasive, inexpensive, rapid and reproducible modality that is reported to be associated with several cardiovascular risk factors and with cardiovascular end-points.[3,4,5] However, recent meta-analyses have shown inconsistent results with respect to the improvement in risk prediction of cardiovascular events in either the general population[6,7,8] or specific patient groups[9,10] by using CIMT. Furthermore, meta-analyses have not shown any consistent association between CIMT regression and a reduction of cardiovascular events.[11,12]
The association between CIMT and several cardiovascular risk and dietary factors has been investigated previously. In asymptomatic individuals with a CIMT ≥0.8 mm, body mass index (BMI), blood pressures, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides, uric acid, C-reactive protein, and fibrinogen were all significantly higher, whereas serum concentrations of Vitamin A, Vitamin E, lycopene, and beta-carotene were significantly lower compared with participants with CIMT <0.8 mm.[13] A high dietary fiber intake has been reported to be inversely associated with carotid atherosclerosis.[14] Furthermore, low plasma concentrations of Vitamin C were found to be inversely associated with CIMT in young patients with type I diabetes.[15]
In the present study, we have investigated the association between CIMT determined by duplex ultrasound and cardiovascular risk factors, including diet, in an asymptomatic Persian population.
METHODS
The study was carried out on a sample of 431 subjects (189 [43.9%] males and 242 [56.1%] females), recruited from an urban population, using a stratified-cluster method and derived from the Mashhad stroke and heart atherosclerosis disorder (MASHAD) study, Mashhad, Iran. The minimum and maximum age of the subjects was 35 and 64 years respectively. None of the subjects had a past history of a cardiovascular event (unstable angina, MI and stroke), heart failure, peripheral vascular disease including transient ischemic attack or amaurosis fugax, or a history of any previous cardiovascular interventions or surgery. Individuals with any major comorbidity such as cancer, autoimmune, infectious, and inflammatory diseases were excluded. Each subject gave informed written consent to participate in the study, which was approved by the Mashhad University of Medical Science Ethics Committee.
Anthropometric measurement
For all individuals, anthropometric parameters including weight, height, and waist circumference (WC) were measured using standard protocols. Height, body weight, and WC were measured with subjects dressed in very light clothing after an overnight fast. WC was measured at the level of the umbilicus (at the level midway between the lower rib margin and the iliac crest). Body weight was measured with standard scales to an accuracy of ± 0.1 kg and height was measured to an accuracy of ± 0.1 cm (a stadiometer was used for measuring height). Blood pressure was measured twice while patients were seated and rested for 15 min, using a standard mercury sphygmomanometer calibrated by the Iranian Institute of Standards and Industrial Research. The interval between each blood pressure measurement was at least 30 min, and in this interval patients were at rest (not doing heavy activities or running) and the average of the two measurements was taken as the blood pressure. BMI was calculated as weight (kg) divided by height squared (m2).
Routine biochemical analysis
A full fasted lipid profile, comprising TC, triglycerides, high-density lipoprotein cholesterol (HDL-C), and LDL-C, was determined for each patient. Serum lipid and fasting blood glucose (FBG) concentrations were measured by enzymatic methods.
Duplex assessment
Carotid intima-media thickness was measured by ultrasound examination of both common and internal carotid arteries using a high-resolution ultrasound scanner (Medison, SA8000 Ex, Seoul, South Korea) equipped with a linear-array transducer. The maximum intima-media thickness was measured on frozen B-mode images. CIMT was measured between the leading edge of the first echogenic line (lumen–intima interface) and the second echogenic line (upper layer of the adventitia) in the far (deeper) artery wall.[13] All measurements were made on frozen, enlarged images at the end of diastole, with the transducer in the mediolateral position.[13]
Intima-media thickness of carotid artery was examined in three areas on both the right and left carotid artery: Right common carotid artery (RCCA), right bulb (bifurcation) (RB), right internal carotid artery (RICA), left common carotid artery (LCCA), left bulb (left bifurcation) (LB) and left internal carotid artery (LICA). The presence of one or more CIMT ≥0.8 mm in one major area (RCCA, RB, RICA, LCCA, LB and LICA) was considered to be evidence of significant CIMT atherosclerosis (CIMT [+]).[13] Patients in whom CIMT was <0.8 mm were considered to have a normal duplex ultrasound-defined CIMT (CIMT [−]).[13]
Dietary assessment
Dietary information was collected using a questionnaire for 24-h recall, administered by a trained dietary interviewer in a face-to-face interview, to recall and describe every item of food and beverage consumed over the previous 24-h period.[16] Individual nutritional intakes were assessed using Dietplan6 software (Forestfield Software Ltd., UK). The dietary variables selected for the purpose of this study were crude, and total energy-adjusted intake of micronutrients.[16] An adjustment was made for total energy intake through the residual method as an alternative to using nutrient densities to control for confounding by total energy intake and to remove extraneous variation due to total energy intake. Regression analyzes were used to compute residuals of nutrient intake by removing the variation caused by total energy intake. In this procedure, the nutrient intakes of the individuals in a group are regressed on their total energy intakes. The residuals from the regression represent the differences between each individual's actual intake and the intake predicted by their total energy intake.[17,18,19] Total energy-adjusted nutrient intakes were calculated as the residuals from the regression model, with absolute nutrient intake as the dependent variable, and total energy intake as the independent variable.[17]
Statistical analysis
The data were analyzed using SPSS version 16 (SPSS, Chicago, IL), with descriptive statistics (mean, median, standard deviation, and interquartile range) being determined for all variables. Data were assessed for normality using the Kolomogorov–Smirnov test. In our comparisons, t-tests and Chi-square tests were used for quantitative and qualitative variables.
Logistic regression analysis was used to determine whether CIMT (+) was related to any of the predefined cardiovascular risk factors. To enable adjustment for potential confounding factors, we entered the following factors into the equation: Age, gender, BMI, systolic blood pressure (SBP) and diastolic blood pressure (DBP), HDL-C, TC, triglyceride, fasting blood sugar (FBG), hypertension, diabetes mellitus, and crude and total energy-adjusted intake for the dietary parameters. Correlations were assessed using Pearson correlation coefficients. Nonnormally distributed data were normalized using log-transformed before the using the Pearson correlation. A P < 0.05 was considered significant.
A person who had an FBG ≥ 126 mg/dl (≥7.0 mmol/L)[20] or had a prior diagnosis of diabetes, or used antidiabetic medication was considered to be diabetic. A person whose blood pressure was >140/90 mmHg[21] or used antihypertensive medications was considered to be hypertensive.
RESULTS
Demographic characteristics and the presence of carotid intima-media thickness (+) in males and females
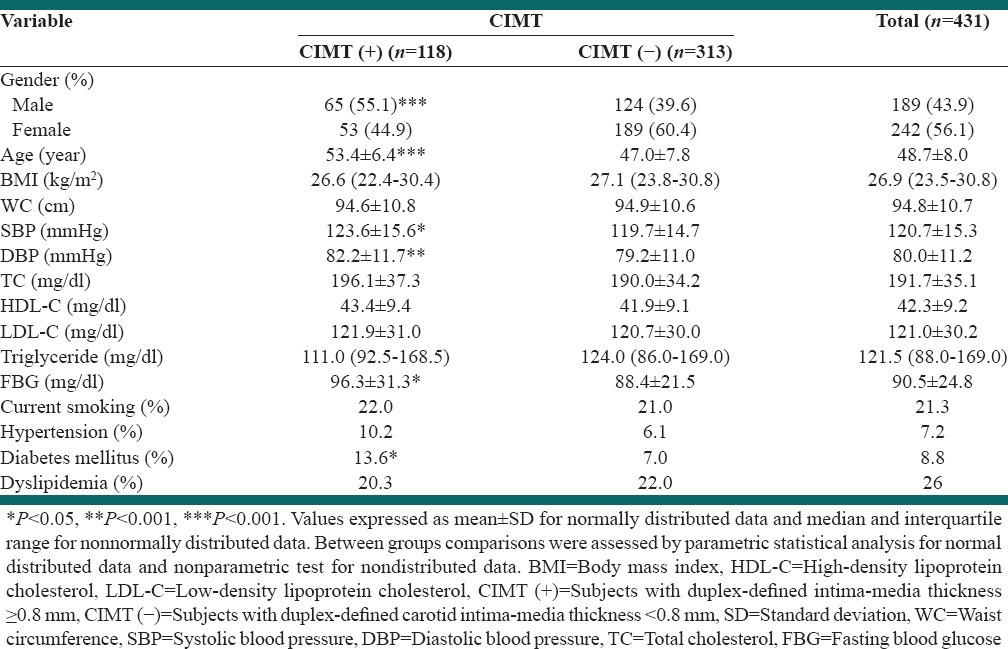
The mean age of subjects was 48.7 ± 8.0 years and the mean age was significantly higher in CIMT (+) versus CIMT (−) subject [53.4 ± 6.4 vs. 47.0 ± 7.8, P < 0.001; Table 1]. Of the subjects investigated, 313 (72.7%) were CIMT (−) and 118 (27.3%) subjects were CIMT (+). Among the 118 subjects who were CIMT (+), 65 (55.1%) were male and 53 (44.9%) were female. Of the 313 subjects who were CIMT (−), 124 (39.6%) were male and 189 (60.4%) were female [P < 0.001, Table 1].
Table 1.
Clinical and biochemical characteristics of the individuals with and without CIMT (+)
Comparisons of cardiovascular risk factors in carotid intima-media thickness (+) and carotid intima-media thickness (-) groups
Body mass index and WC of the subjects between two groups were not significantly different [P > 0.05, Table 1]. The proportion of current smokers did not differ between the CIMT (-) and CIMT (+) groups.
The average of SBP (P < 0.05) and DBP (P < 0.01) were higher in CIMT (+) subjects compared to CIMT (−) [Table 1].
Serum fasting HDL-C, LDL-C, triglyceride and TC concentrations were not significantly different for the CIMT (+) group compared to the CIMT (-), while FBG was significantly higher in CIMT (+) compared to CIMT (−) group [P < 0.05, Table 1].
The proportion of hypertensive and dyslipidemic subjects did not differ between the CIMT (+) and CIMT (−) groups; however the percentage of diabetic patients was significantly higher in CIMT (+) group compared with CIMT (−) [P < 0.05, Table 1].
Comparison of the crude and total energy-adjusted intake of nutrients between the carotid intima-media thickness (+) and carotid intima-media thickness (−) subjects
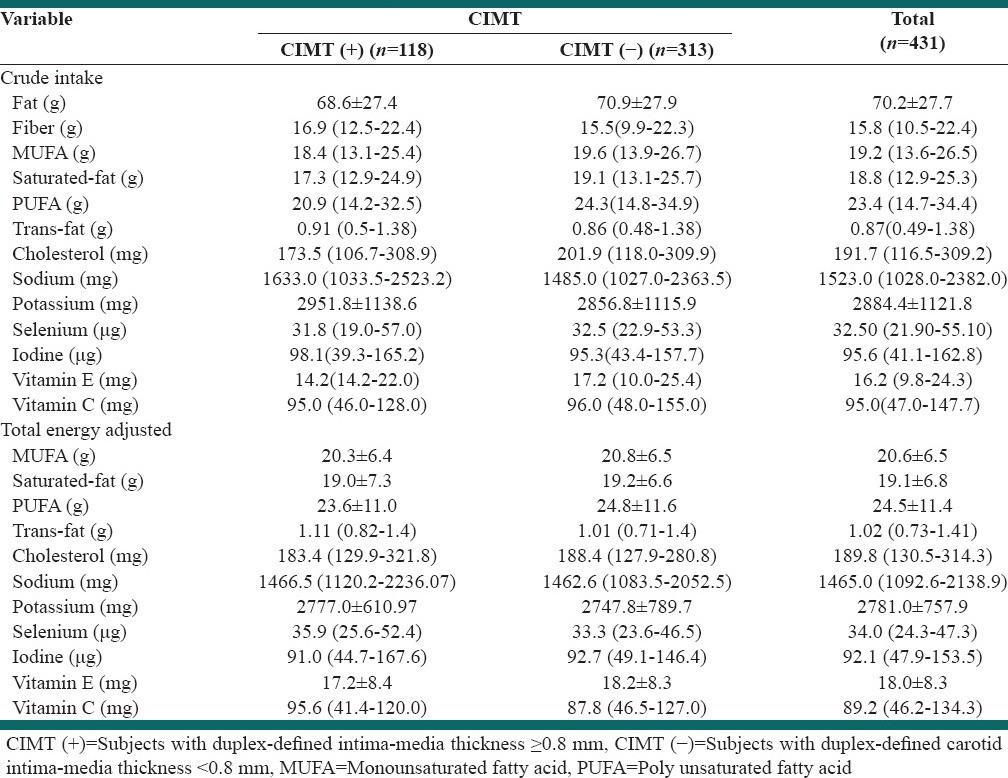
Table 2, shows the crude intake and total energy adjusted intake of the nutrients intakes. We found no difference in crude or total energy adjusted intake of nutrients between the two groups (P > 0.05).
Table 2.
Nutritional intake characteristics of the patients with and without CIMT (+)
Logistic regression analysis of different associated factors with abnormal carotid intima-media thickness
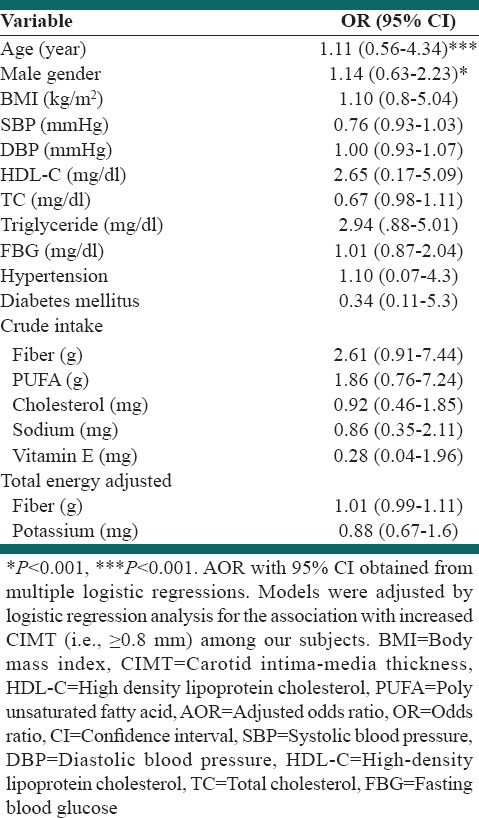
Multiple logistic regression analysis was performed to assess the relative importance of selected parameters in determining the presence of duplex defined CIMT (+) [Table 3]. Age and male gender proved to be significant predictors of CIMT (+) [Table 3; P < 0.001 and P < 0.01, respectively], with age being the strongest predictor.
Table 3.
Regression logistic between CIMT with clinical and biochemical factors
Assessment of correlation between cardiovascular risk factors and crude and total energy-adjusted intake of nutrients and maximum of carotid intima-media thickness
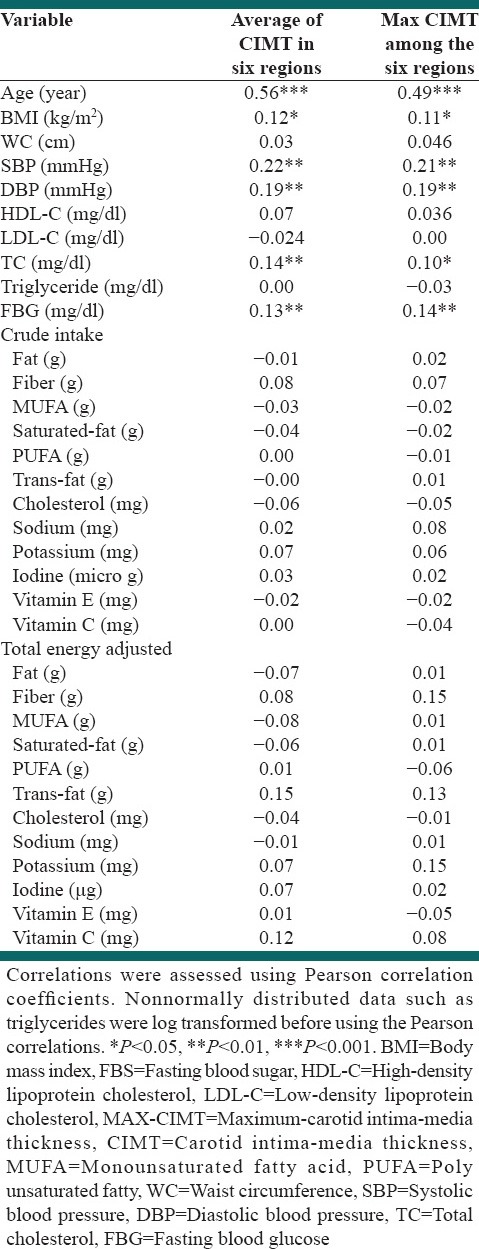
A strong positive correlation was found between age and the average CIMT for the six regions examined (i.e., [RCCA, RB, RICA, LCCA, LB and LICA] [AV-CIMT-6] [r = 0.56, P < 0.001]) and between age and the maximum CIMT for the six regions (MAX-CIMT-6) (r = 0.49, P < 0.001). Weaker positive associations were found between AV-CIMT and MAX-CIMT-6 and BMI (r = 0.12, P < 0.05; r = 0.11, P < 0.05 respectively), SBP (r = 0.22 P < 0.001; r = 0.21, P < 0.001 respectively) and DBP (r = 0.19, P < 0.001; r = 19, P < 0.001 respectively), TC (r = 0.14, P < 0.01; r = 0.1, P < 0.05 respectively) and FBG (r = 0.13, P < 0.01; r = 14, P < 0.01 respectively), whilst there was no correlation between crude and total energy adjusted intake of all considered nutrients and AV-CIMT-6 and MAX-CIMT-6 [Table 4].
Table 4.
Correlation (r) between CIMT and individual cardiovascular risk factors as well as crude and energy adjusted nutrient intake
DISCUSSION
This is the first report of a large CIMT study in subjects without manifest cardiovascular events from Iranian, although there have been several studies investigating CIMT in Iranian subjects with specific diseases, including: Non-alcoholic fatty liver disease,[22] diabetes,[23] kidney diseases[24,25] and in candidates for cardiac catheterization[26] and coronary artery bypass graft.[27] The results of this current study indicated that age, male gender, SBP and DBP, FBG and diabetes mellitus were associated with increased CIMT (i.e., CIMT [+]) in univariate analysis. Multivariate analysis showed that age and male gender were independent predictors of a CIMT ≥ 0.8 mm in asymptomatic individuals without manifest cardiovascular events. Strong correlations between age and AV-CIMT and MAX-CIMT-6 were found in this study as well as modest correlations between AV-CIMT and MAX-CIMT-6 and SBP, DBP, TC and FBS. Riccioni et al. investigated 640 subjects who were asymptomatic in terms of carotid artery disease.[13] Individuals with coronary syndrome were included in Riccioni et al. study but comprised < 1% of all participants. The population in our study was about 7 years younger than for this previous study, but the percentage of male participants was similar (43.9% vs. 46.4%). The percentage of individuals with normal CIMT in our population was substantially higher than for the study of Riccioni et al. (72.6% vs. 45.4%). The association between cardiovascular risk factors was found to be similar.[13]
Cardiovascular risk factors and carotid intima-media thickness
Calmarza et al. showed that age, male sex, BMI, SBP and oxidized LDL, but not the routine lipid profile components, were related to CIMT in a Spanish population.[28] However, 10% of this study population had established cardiovascular disease and the mean age of the participants was 15 years higher than for our population. In our study, the components of the traditional lipid profile did not show any difference between normal and abnormal CIMT; however, the level of serum TC showed a modest correlation with CIMT. Furthermore, the percentage of dyslipidemic patients did not differ between two groups of the patients.
In respect to serum triglyceride and TC, our results are in accordance with those reported by Riccioni et al.[13] Our data are consistent with that for the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study.[29]
The association between FBG and presence of CIMT (+) did not remain following multivariate analysis in our study. Furthermore, only a modest positive correlation was found between FBG and CIMT. The reported association between glycemic status and CIMT has previously been inconsistent.[30,31,32,33]
It is important to note that the level of lipid profile and FBG were potentially affected by medications in the diabetic and dyslipidemic patients. Furthermore, the level of SBP and DBP was lower than actual levels due to anti-hypertensive therapy. The median of CIMT in hypertensive (P < 0.05) and diabetic (P < 0.05) subjects were significantly higher than normal subjects; however, this matter was not consistent in dyslipidemic participants (data not shown).
According to our results, only male gender and age were independent factors for the presence of CIMT (+) in a relatively low cardiovascular risk population without manifest history of cardiovascular events. There was a low prevalence of subclinical carotid atherosclerosis in our relatively young Iranian population without manifest cardiovascular events compared to Western investigations.[13] The lack of association between modifiable cardiovascular risk factors and presence of CIMT (+) shows that application of CIMT would be best applied to an older population, and specifically the male sub-group.
The distribution of carotid involvement (i.e., CIMT [+]) in different regions of the artery is variable probably due to nonlinear mechanical behaviour of these segments.[34] According to our results, LCCA had the highest average of CIMT and also the number of CIMT (+) in LCCA was highest compared to other regions (number of CIMT [+] in LCCA: 48, in RCCA: 34, in RB: 43, in LB: 45, in RICA: 17 and in LICA: 11) and we found that age was only independent predictive factor of CIMT (+) in LCCA compared to subjects with CIMT (−) (data not shown). In general, the left carotid artery was more often affected, and it seems that the involvement of CCAs is more prominent in our study compared with the young and middle-aged ischemic stroke patients of Fromm et al.[35] study in whom the ICA was most often involved.
Nutrient intake and carotid intima-media thickness
In the present study, we have assessed the dietary intake of fat, fiber, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), saturated fat, trans-fat, cholesterol, sodium, potassium, iodine, selenium, Vitamins C and E. We found no significant difference between either the crude or total energy adjusted intake of these nutrients in subjects with and without CIMT (+).
In the primary prevention of cardiovascular disease with Mediterranean diets study, in men between 55 and 75 years of age and women between 65 and 75 years of age, who had type 2 diabetes mellitus, or three or more of the following risk factors: BMI ≥ 25 kg/m2, hypertension, abnormal LDL-C or treatment with lipid-lowering drugs, abnormal HDL-C, current smoking or family history of early-onset cardiovascular disease,[14] there was a weak inverse association between dietary fiber intake and CIMT.[14]
In the MESA study, of individuals who were aged 45-84 years and free of diabetes and cardiovascular disease, there was no association between micronutrients (heme iron, nonheme iron, Mg, Zn, Vitamin C and Vitamin E) and CIMT, with the exception of an inverse association between Mg and CIMT.[36]
Epidemiological studies and controlled trial data indicate that variations in the relative amounts of saturated, monounsaturated and polyunsaturated dietary fats influence the risk of cardiovascular diseases in part by influencing lipid metabolism.[37] Saturated fatty acids appear to increase the risk of cardiovascular risk, whereas unsaturated fatty acids have been shown to protect against cardiovascular diseases. Trans unsaturated and saturated fatty acids appear to increase the risk of cardiovascular diseases.[37,38] He et al. in a multiethnic group of 5488 adults aged 45-84 years and free of clinical cardiovascular diseases showed that the dietary intake of long-chain n-3 PUFAs was associated with a lower prevalence of subclinical atherosclerosis classified by common CIMT, although significant changes in internal CIMT were not observed.[39] Some of our results regarding fat intake are not consistent with Merchant et al.,[40] who showed that saturated and trans fat intakes were independently associated with CIMT. However, PUFA, MUFA, cholesterol, and total fat intakes were unrelated to CIMT in their study, which is in agreement with our findings.[40]
In this study, we decided to use 24-h dietary assessment which has been used in our other associated cardiovascular studies in the Iranian population,[41,42] however, some authors believe that four repeat 24-h recalls or even eight repeat 24-h recalls can be better measurements in cardiovascular studies using medium sample sizes.[43] Our dietary assessment may be one of the limitations of our study. However, further Iranian-based studies should be performed in order to provide a valid dietary assessment for Iranian societies that can represent a habitual diet of Iranian especially useful in studies with cardiovascular endpoints.
It is important to note that only 15 out of 118 CIMT (+) were between 35 and 45 (young patients) and 103 subjects were between 45 and 64 years (middle-aged patients). In young patients even univariate association between modifiable risk factors and presence of CIMT (+) was not found. We observed a similar trend regarding the association between modifiable risk factors and presence of CIMT (+) in middle-aged patients (data not shown). We showed that male gender was an independent predictor of CIMT (+) development, but our subgroup analysis in male and female participant separately confirmed the lack of association between modifiable risk factor/nutritional intake and CIMT (+) (data not shown).
The main message from these findings is that in Persian population for relatively young population, without history of cardiovascular events, the presence of traditional modifiable risk factors are not an indication for using carotid duplex screening. Moreover, general recommendations from Western societies also does not encourage duplex investigation for asymptomatic young population, and it seems that only male patients >65 years old with features of atherosclerosis may benefit from duplex screening.[44,45] Our longitudinal long-term cohort (MASHAD study) will provide sufficient important data regarding the outcomes of our sample population in the future. Development of any adverse cardiovascular event in a period of 15 years will be investigated for this group of patients. Further carotid duplex assessment of male patients (particularly those >65 years) with a history of cardiovascular events in Iranian is strongly recommended. According to the presence of race-dependent diversity of carotid artery disease[46] and also our results from Iranian population, we do believe that the Western-based vascular screening recommendations should be tested in non-Western societies.
CONCLUSIONS
The most obvious finding to emerge from this study is that duplex-ultrasound defined subclinical atherosclerosis does not associate with traditional modifiable cardiovascular risk factors in an Iranian population without the overt history of cardiovascular diseases. However, age and male gender independently predict an abnormal CIMT (i.e., CIMT [+]). Furthermore, crude and total energy adjusted energy intake of several types of nutrients did not show any relation with the presence of CIMT (+).
ACKNOWLEDGMENETS
The Mashhad University of Medical Science Research Council supported this research project.
Footnotes
Source of Support: Mashhad University of Medical Science Research Council
Conflict of Interest: None declared.
REFERENCES
- 1.Collins TC, Beyth RJ. Process of care and outcomes in peripheral arterial disease. Am J Med Sci. 2003;325:125–34. doi: 10.1097/00000441-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bots ML, Sutton-Tyrrell K. Lessons from the past and promises for the future for carotid intima-media thickness. J Am Coll Cardiol. 2012;60:1599–604. doi: 10.1016/j.jacc.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 3.van den Oord SC, Sijbrands EJ, ten Kate GL, van Klaveren D, van Domburg RT, van der Steen AF, et al. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis. 2013;228:1–11. doi: 10.1016/j.atherosclerosis.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Bauer M, Caviezel S, Teynor A, Erbel R, Mahabadi AA, Schmidt-Trucksäss A. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly. 2012;142:w13705. doi: 10.4414/smw.2012.13705. [DOI] [PubMed] [Google Scholar]
- 5.Touboul PJ, Grobbee DE, den Ruijter H. Assessment of subclinical atherosclerosis by carotid intima media thickness: Technical issues. Eur J Prev Cardiol. 2012;19:18–24. doi: 10.1177/2047487312448990. [DOI] [PubMed] [Google Scholar]
- 6.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet. 2012;379:2053–62. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 9.den Ruijter HM, Peters SA, Groenewegen KA, Anderson TJ, Britton AR, Dekker JM, et al. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: The USE-IMT initiative. Diabetologia. 2013;56:1494–502. doi: 10.1007/s00125-013-2898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: A systematic review. Diabet Med. 2006;23:609–16. doi: 10.1111/j.1464-5491.2005.01725.x. [DOI] [PubMed] [Google Scholar]
- 11.Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G, et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events. A meta-analysis of 41 randomized trials? J Am Coll Cardiol. 2010;56:2006–20. doi: 10.1016/j.jacc.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger ZD, Valle JA, Dandekar VK, Chan PS, Ko DT, Nallamothu BK. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction. A critical review and meta-regression analysis? Am Heart J. 2010;160:701–14. doi: 10.1016/j.ahj.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Riccioni G, D’Orazio N, Palumbo N, Bucciarelli V, Ilio Ed, Bazzano LA, et al. Relationship between plasma antioxidant concentrations and carotid intima-media thickness: The Asymptomatic Carotid Atherosclerotic Disease in Manfredonia Study. Eur J Cardiovasc Prev Rehabil. 2009;16:351–7. doi: 10.1097/HJR.0b013e328325d807. [DOI] [PubMed] [Google Scholar]
- 14.Buil-Cosiales P, Irimia P, Ros E, Riverol M, Gilabert R, Martinez-Vila E, et al. Dietary fibre intake is inversely associated with carotid intima-media thickness: A cross-sectional assessment in the PREDIMED study. Eur J Clin Nutr. 2009;63:1213–9. doi: 10.1038/ejcn.2009.45. [DOI] [PubMed] [Google Scholar]
- 15.Odermarsky M, Lykkesfeldt J, Liuba P. Poor vitamin C status is associated with increased carotid intima-media thickness, decreased microvascular function, and delayed myocardial repolarization in young patients with type 1 diabetes. Am J Clin Nutr. 2009;90:447–52. doi: 10.3945/ajcn.2009.27602. [DOI] [PubMed] [Google Scholar]
- 16.Mahan LK, Escott-Stump S, Reymond JL. 13th ed. St Louis: Elsevier; 2012. Krause's Food and the Nutrition Care Process. [Google Scholar]
- 17.Freire RD, Cardoso MA, Gimeno SG, Ferreira SR Japanese-Brazilian Diabetes Study Group. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care. 2005;28:1779–85. doi: 10.2337/diacare.28.7.1779. [DOI] [PubMed] [Google Scholar]
- 18.Willett W, Stampfer MJ. Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 20.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American diabetes association and 1999 world health organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–12. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 21.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi A, Sedani HH, Ghasemi-Rad M. Evaluation of carotid intima-media thickness and flow-mediated dilatation in middle-aged patients with nonalcoholic fatty liver disease. Vasc Health Risk Manag. 2011;7:661–5. doi: 10.2147/VHRM.S26011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alizadeh A, Roudbari A, Heidarzadeh A, Babaei Jandaghi A, Bani Jamali M. Ultrasonic measurement of common carotid intima-media thickness in type 2 diabetic and non-diabetic patients. Iran J Radiol. 2012;9:79–82. doi: 10.5812/iranjradiol.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salama DS, Narooinejad M, Saffari S, Khak M. Comparison of intima-media thickness of the common carotid artery in dialysis and kidney transplant recipient patients. Exp Clin Transplant. 2011;9:26–31. [PubMed] [Google Scholar]
- 25.Ossareh S, Alaei A, Saedi D. Carotid intima-media thickness in maintenance hemodialysis patients: Role of cardiovascular risk factor. Iran J Kidney Dis. 2011;5:169–74. [PubMed] [Google Scholar]
- 26.Alaee A, Khademloo M. Evaluation of correlation between carotid artery intima media wall thickness and coronary artery stenosis in Sari, north of Iran. Pak J Biol Sci. 2008;11:2360–3. doi: 10.3923/pjbs.2008.2360.2363. [DOI] [PubMed] [Google Scholar]
- 27.Shakouri P, Nezami N, Tarzamni MK, Rashid RJ. The elusive link between high sensitivity C-reactive protein and carotid subclinical atherosclerosis in coronary artery bypass grafting candidates: A cross-sectional study. Cardiovasc Ultrasound. 2008;6:23. doi: 10.1186/1476-7120-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calmarza P, Trejo JM, Lapresta C, López P. LDL oxidation and its association with carotid artery intima-media thickness and other cardiovascular risk factors in a sample of Spanish general population. Angiology. 2014;65:357–62. doi: 10.1177/0003319713488639. [DOI] [PubMed] [Google Scholar]
- 29.Bauer M, Delaney JA, Möhlenkamp S, Jöckel KH, Kronmal RA, Lehmann N, et al. Comparison of factors associated with carotid intima-media thickness in the Multi-ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR) J Am Soc Echocardiogr. 2013;26:667–73. doi: 10.1016/j.echo.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonora E, Kiechl S, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M, et al. Impaired glucose tolerance, Type II diabetes mellitus and carotid atherosclerosis: Prospective results from the Bruneck Study. Diabetologia. 2000;43:156–64. doi: 10.1007/s001250050024. [DOI] [PubMed] [Google Scholar]
- 31.Faeh D, William J, Yerly P, Paccaud F, Bovet P. Diabetes and pre-diabetes are associated with cardiovascular risk factors and carotid/femoral intima-media thickness independently of markers of insulin resistance and adiposity. Cardiovasc Diabetol. 2007;6:32. doi: 10.1186/1475-2840-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
- 33.Kowall B, Ebert N, Then C, Thiery J, Koenig W, Meisinger C, et al. Associations between blood glucose and carotid intima-media thickness disappear after adjustment for shared risk factors: The KORA F4 study. PLoS One. 2012;7:e52590. doi: 10.1371/journal.pone.0052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamenskiy AV, Dzenis YA, MacTaggart JN, Lynch TG, Jaffar Kazmi SA, Pipinos II. Nonlinear mechanical behavior of the human common, external, and internal carotid arteries in vivo. J Surg Res. 2012;176:329–36. doi: 10.1016/j.jss.2011.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fromm A, Haaland ØA, Naess H, Thomassen L, Waje-Andreassen U. Risk factors and their impact on carotid intima-media thickness in young and middle-aged ischemic stroke patients and controls: The Norwegian Stroke in the Young Study. BMC Res Notes. 2014;7:176. doi: 10.1186/1756-0500-7-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Jenny NS, Jiang R, et al. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141:1508–15. doi: 10.3945/jn.111.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katan MB, Grundy SM, Willett WC. Should a low-fat, high-carbohydrate diet be recommended for everyone. Beyond low-fat diets? N Engl J Med. 1997;337:563–6. [PubMed] [Google Scholar]
- 38.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 39.He K, Liu K, Daviglus ML, Mayer-Davis E, Jenny NS, Jiang R, et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr. 2008;88:1111–8. doi: 10.1093/ajcn/88.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merchant AT, Kelemen LE, de Koning L, Lonn E, Vuksan V, Jacobs R, et al. Interrelation of saturated fat, trans fat, alcohol intake, and subclinical atherosclerosis. Am J Clin Nutr. 2008;87:168–74. doi: 10.1093/ajcn/87.1.168. [DOI] [PubMed] [Google Scholar]
- 41.Motamed S, Ebrahimi M, Safarian M, Ghayour-Mobarhan M, Mouhebati M, Azarpazhouh M, et al. Micronutrient intake and the presence of the metabolic syndrome. N Am J Med Sci. 2013;5:377–85. doi: 10.4103/1947-2714.114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazeminezhad R, Tajfard M, Latiff LA, Mouhebati M, Esmaeily H, Ferns GA, et al. Dietary intake of patients with angiographically defined coronary artery disease and that of healthy controls in Iran. Eur J Clin Nutr. 2014;68:109–13. doi: 10.1038/ejcn.2013.205. [DOI] [PubMed] [Google Scholar]
- 43.Raina SK. Limitations of 24-hour Recall method: Micronutrient intake and the presence of the metabolic syndrome. N Am J Med Sci. 2013;5:498. doi: 10.4103/1947-2714.117329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazemi-Bajestani SM, van der Vlugt M, de Leeuw FE, Blankensteijn JD, Bredie SJ. A high prevalence of carotid artery stenosis in male patients older than 65 years, irrespective of presenting clinical manifestation of atherosclerotic diseases. Angiology. 2013;64:281–6. doi: 10.1177/0003319712445374. [DOI] [PubMed] [Google Scholar]
- 45.Lim LS, Haq N, Mahmood S, Hoeksema L ACPM Prevention Practice Committee. American College of Preventive Medicine. Atherosclerotic cardiovascular disease screening in adults: American College Of Preventive Medicine position statement on preventive practice. Am J Prev Med. 2011;40:381 e1–10. doi: 10.1016/j.amepre.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Rockman CB, Hoang H, Guo Y, Maldonado TS, Jacobowitz GR, Talishinskiy T, et al. The prevalence of carotid artery stenosis varies significantly by race. J Vasc Surg. 2013;57:327–37. doi: 10.1016/j.jvs.2012.08.118. [DOI] [PubMed] [Google Scholar]


