Abstract
We have previously shown that Mycobacterium tuberculosis attenuates cell surface expression of major histocompatibility complex class II molecules in response to gamma interferon (IFN-γ) by a mechanism dependent on intracellular sequestration of α,β dimers. In this study we examined whether intracellular alkalinization due to mycobacterial urease could account for the defect in intracellular trafficking of class II molecules. Phagocytosis of wild-type Mycobacterium bovis BCG was associated with secretion of ammonia intracellularly, which increased substantially upon addition of exogenous urea to the culture medium. Increased intracellular ammonia, due to urea degradation by the bacterium, correlated with inhibition of class II surface expression. Conversely, no ammonia was detected in cells infected with a urease-negative mutant strain of M. bovis BCG, which also displayed a reduced effect on surface expression of class II molecules. A direct cause-effect relationship between urease and class II molecule trafficking was established with experiments where cells ingesting beads coated with purified urease showed an increased ammonia level and decreased surface expression of class II in response to IFN-γ. In contrast to BCG, infection of macrophages with Mycobacterium smegmatis, which expresses relatively greater urease activity in cell-free culture, had a marginal effect on both the intracellular level of ammonia and class II expression. The limited effect of M. smegmatis was consistent with a failure to resist intracellular killing, suggesting that urease alone is not sufficient to resist macrophage microbicidal mechanisms and that this is required for a more distal effect on cell regulation. Our results demonstrate that alkalinization of critical intracellular organelles by pathogenic mycobacteria expressing urease contributes significantly to the intracellular retention of class II dimers.
The integration of various multidisciplinary studies over the past decade has contributed to a better understanding of the pathogenesis of tuberculosis. In particular, there has been a substantial increase in our understanding of the host immune response to tuberculosis and the requirements for protective immunity (14, 30).
The usual response of macrophages to infection is to implement a range of complex mechanisms that act rapidly to neutralize and kill ingested microorganisms (39). This allows macrophages to process and present microbial antigens to antigen-specific T cells in the context of major histocompatibility complex (MHC) cell surface molecules (5, 38). Activated T cells with enhanced capacity to secrete gamma interferon (IFN-γ) in turn activate macrophage bactericidal mechanisms along with enhanced surface expression of MHC class II molecules and other costimulatory molecules (3, 13, 47). However, cells infected with Mycobacterium tuberculosis show a distinctly different phenotype. In particular, mononuclear phagocytes infected with pathogenic mycobacteria, such as M. tuberculosis or its variant, Mycobacterium bovis BCG, have a diminished capacity to present antigen to T helper cells (16, 37) and are poor responders to IFN-γ in terms of the expression of MHC class II molecules (20, 22, 28, 29). As the ability of M. tuberculosis to evade the host immune response contributes largely to its success as a pathogen, the mechanisms underlying attenuation of macrophage class II expression and antigen presentation function are of significant interest.
IFN-γ regulates the expression of class II genes primarily at the level of transcription, and this requires induction of the class II transactivator (CIITA) (40); expression of the CIITA gene itself is dependent on STAT1 signaling (6, 7, 43). Our investigators have previously provided direct evidence that IFN-γ-induced STAT1 activation and expression of the CIITA gene occur normally in macrophages infected with M. tuberculosis. Additional analysis showed normal steady-state levels of class II molecules in infected cells, indicating distal effects on class II expression likely involving maturation and transport to the cell surface (20).
Newly synthesized class II α and β chains associate with invariant (Ii) chain and then exit the endoplasmic reticulum, subsequently localizing to an acidic endosomal-lysosomal compartment referred to as the MHC class II compartment (MIIC) (5). In the MIIC, the Ii chain is removed and class II molecules mature into peptide-loaded stable dimers. Removal of the Ii chain and peptide loading are believed to be critical for appropriate class II molecule export to the cell surface (24). These events are likely candidates for disruption in cells harboring live mycobacteria (20), and alkalinization of the endosomal-lysosomal compartment by mycobacteria is a potential mechanism to explain retarded maturation of class II dimers in infected cells. Mycobacterial urease, an enzyme that hydrolyzes urea into carbon dioxide and ammonia (27), has the potential to be active within the host cell, thereby leading to inadequate acidification of the MIIC and processing of class II complexes. Consequently, immature class II molecules may be retained in the endocytic compartment. This hypothesis is supported by the finding that treatment of cells with the weak base ammonium chloride also inhibits maturation of class II molecules (20). Inhibition of at least some proteolytic events by alkalinization is also consistent with the finding that mycobacteria resist intracellular degradation (2, 8).
In the present study, we examined further the basis for diminished trafficking of class II molecules to the cell surface in cells infected with pathogenic mycobacteria. The results obtained provide evidence for direct involvement of mycobacterial urease in preventing normal expression of MHC class II molecules at the cell surface.
MATERIALS AND METHODS
Reagents and chemicals.
RPMI 1640, Hanks balanced salt solution (HBSS), phorbol myristate acetate (PMA), protease inhibitor mixture, phenylmethylsulfonyl fluoride, and trypsin-EDTA were obtained from Sigma-Aldrich (Oakville, Ontario, Canada). Latex beads (4-μm diameter) were from Interfacial Dynamics Inc. (Portland, Oreg.). Anti-human HLA-DR monoclonal antibody (MAb; clone HL38; immunoglobulin G1 [IgG1]) and irrelevant IgG1 MAb were from Caltag Laboratories (San Francisco, Calif.). Anti-phospho-STAT1 (Tyr-701) MAb was from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 goat anti-mouse IgG and horseradish peroxidase-conjugated goat anti-mouse IgG were from Sigma. Human recombinant IFN-γ was a generous gift of Genentech (South San Francisco, Calif.).
Mycobacteria strains.
A urease-negative isogenic mutant strain of BCG was constructed by allelic exchange involving replacement of the ureC gene with a kanamycin-disrupted copy (ureC::aph) (42). The urease-negative mutant (BCGUre−) was used along with the parental strain M. bovis BCG wild type (BCGUre+). M. smegmatis (mc2155) was provided by R. Stokes. The green fluorescent protein (GFP) expression plasmid pSC301 was provided by Y. Av-Gay and used to transform BCGUre+ and M. smegmatis organisms as previously described (10). Positive clones were checked for green fluorescence at λ excitation and emission wavelengths of 360 and 520 nm in a VersaFluor fluorometer (Bio-Rad Laboratories, Hercules, Calif.). Mycobacterial strains were grown in Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose solution (OADC; Difco) and 0.05% (vol/vol) Tween 80 (Sigma) at 37°C to an A600 of 0.5 on a rotating platform (50 rpm). Bacteria were harvested by centrifugation, and pellets were suspended in complete medium plus 10% glycerol. Mycobacteria cultures were stored in aliquots (∼5 × 108 cells/vial) at −70°C. Prior to infection, bacteria were grown for 48 h in 7H9-OADC and opsonized as follows: 109 mycobacteria were suspended in 1 ml of RPMI containing 50% human serum (AB+) and PPD− and rocked for 30 min at 37°C. Bacteria were then pelleted and suspended in 1 ml of RPMI, and clumps were disrupted by multiple passages through a 25-gauge needle. To evaluate the phagocytosis of mycobacteria by THP-1 cells, bacteria (109/ml) were labeled by incubation with rhodamine isothiocyanate (RITC; Sigma) at 1 μg/ml in 7H9 for 2 h at 37°C. Thereafter, RITC-labeled bacteria were washed twice with 7H9 and then opsonized and used for infection as described above. CFU counts and analysis of proteins secreted in culture medium supplemented with [35S]methionine showed that both control and labeled bacteria expressed similar viabilities and protein profiles, indicating that RITC labeling did not alter bacterial metabolism (data not shown).
Differentiation and infection of THP-1 cells.
The monocytic cell line THP-1 (American Type Culture Collection, Rockville, Md.) was cultured in RPMI supplemented with 5% fetal calf serum (Life Technologies, Burlington, Ontario, Canada), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were seeded at a density of 105/cm2 and allowed to adhere and differentiate in the presence of PMA (20 ng/ml) at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Depending on the quantity of cell material needed, 3-, 6-, or 10-cm-diameter cell culture dishes (Becton Dickinson, Franklin Lakes, N.J.) were used. Cells were then washed three times with HBSS, and adherent monolayers were replenished with culture medium without antibiotics and infected with opsonized mycobacteria (bacterium/cell ratio of 50:1). After a period of 3 h, partially attached, noningested bacteria were removed by 5-min treatment with trypsin-EDTA and extensive washing with HBSS. This resulted in an infection rate of 80 to 90% with an approximate range of 5 to 15 bacteria per cell. For quantitative evaluation of phagocytosis, THP-1 monolayers were incubated with opsonized RITC-labeled bacteria for 3 h at 37°C. After treatment with trypsin-EDTA and washing with HBSS, the number of cells loaded with bacteria was measured by flow cytometry.
Coating of latex microspheres with urease.
Latex beads were coated with urease from Bacillus, which is closely related to mycobacterial urease (9). Beads were washed twice with 25 mM morpholineethanesulfonic acid buffer (pH 5.5), and pellets were resuspended in the same buffer containing 500 μg of either Bacillus pasteuri urease (∼200 U/mg of protein; Sigma) or bovine serum albumin (BSA; control)/ml. After overnight incubation at room temperature on a shaker, latex beads were washed twice with HBSS and resuspended in RPMI. Urease activity of coated beads was routinely verified prior to each experiment by 1 h of incubation at 37°C in broth medium containing urea and phenol red (42).
Cell staining and flow cytometry.
To measure cell surface expression of MHC class II, cells were incubated with anti-HLA-DR MAb or irrelevant isotype-matched IgG1 for 20 min and then washed twice and labeled with FITC-conjugated F(ab′)2 goat anti-mouse IgG for 20 min. All staining and washing procedures were performed at room temperature in HBSS containing 0.1% NaN3 and 1% fetal calf serum. To control for cell viability, cells were incubated with propidium iodide (0.5 μg/ml in staining buffer) for 10 min. The cells were then washed twice and fixed in 2% paraformaldehyde in staining buffer. The combination of 2% paraformaldehyde plus 0.1% NaN3 killed both free and cell-associated bacteria. Cell fluorescence was analyzed using a FACSCalibur flow cytometer (Becton Dickinson). Viable cells were identified by exclusion of propidium iodide. Relative fluorescence intensities of 10,000 cells were recorded as single-parameter histograms (log scale; 1,024 channels; four decades), and the mean fluorescence intensity (MFI) was calculated for each histogram. Results are expressed as the MFI index, which corresponds to the following ratio: (MFI of cells plus specific Ab)/(MFI of cells plus irrelevant isotype-matched IgG).
Fluorescence microscopy.
PMA-treated THP-1 cells were adhered to tissue culture-treated coverslips (Fisher Scientific, Nepean, Ontario, Canada) in 24-well plates and infected with opsonized RITC-labeled bacteria. After IFN-γ treatment, intracellular staining was performed as follows: cells were washed with phosphate-buffered saline (PBS), fixed for 15 min at 37°C with 2.5% paraformaldehyde-PBS, and then washed three times and permeabilized in PBS containing 0.2% saponin and 10% normal goat serum for 5 min. Cells were then incubated with anti-HLA-DR MAb in PBS-saponin-normal serum for 30 min at room temperature, washed, and stained with FITC-conjugated goat anti-mouse IgG. At the end of the staining procedure, samples were washed three times with PBS and once with distilled water. Coverslips were then mounted in FluorSave (Calbiochem-Novabiochem Corp., La Jolla, Calif.) to minimize photobleaching, and slides were examined using an epifluorescence microscope (Zeiss Axioplan II; Carl Zeiss Inc., Thornwood, N.Y.). A 60× oil objective with numerical aperture of 1.4 was used, and images were captured such that the xyz dimensions were 0.2 μm3. Images were recorded using a charge-coupled device camera and Northern Eclipse software with digital deconvolution capabilities (Empix Imaging Inc., Mississauga, Ontario, Canada). The green and red images were assigned to individual RGB channels, and the images collected in the red and green channels were merged to visualize the relative positional distribution of the two fluorochromes. Red and green overlapping fluorescence was reflected by a yellow signal. To control for nonspecific binding, secondary Ab was used alone, which gave negligible signals in the absence of primary Ab.
Western blotting for STAT1.
Control and infected cells were treated with IFN-γ for 15 min and then washed with HBSS, and whole-cell lysates were prepared as described previously (20). Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (50 μg/lane) and immunoblotting with anti-phospho-STAT1. Blots were developed by enhanced chemiluminescence.
RNA isolation and RT-PCR.
RNA preparation, cDNA synthesis, and PCR conditions were described previously (20, 32). Sequences of oligonucleotide primers used in PCR amplifications were as follows: DRA sense, CGA GTT CTA TCT GAA TCC TGA CCA; DRA antisense, GTT CTG CTG CAT TGC TTT TGC GCA; β-actin sense, CAC CCC GTG CTG CTG ACC GAG GCC; β-actin antisense, CCA CAC GGA GTA CTT GCG CTC AGG. Controls included in the reverse transcriptase PCRs (RT-PCRs) were no RNA and RNA without RT.
Measurement of intracellular ammonia.
Control and THP-1 cells ingesting bacteria or coated beads were washed twice with HBSS and scraped in 100 μl of HBSS. Cell suspensions were then sonicated for 5 s in a Sonic Dismembrator 60 (Fisher Scientific) in ice, and cell debris and membranes were removed by centrifugation at 12,000 × g for 30 min at 4°C. Soluble fractions were then assayed for ammonia by the fluorometric method described by Sugawara and Oyama (44). In brief, 50-μl-size samples adjusted to 50 μg of protein were added to 3 ml of 50 mM phosphate buffer (pH 8) and received 100 μl of a mixture of 5 μM o-phthaldehyde and 5 μM dithiothreitol in ethanol. After 3 h of incubation at room temperature in the dark, fluorescence was measured at λ excitation and emission wavelengths of 360 and 520 nm. Ammonia concentration was calculated by reference to a standard curve using a range (1 to 100 nM) of NH4SO4 concentrations.
Statistical analysis.
All data are expressed as the mean ± the standard deviation (SD). Statistical analysis was performed using Student's t test. P values of <0.05 were considered to be significant.
RESULTS
MHC class II expression and IFN-γ signaling in cells infected with M. bovis BCG.
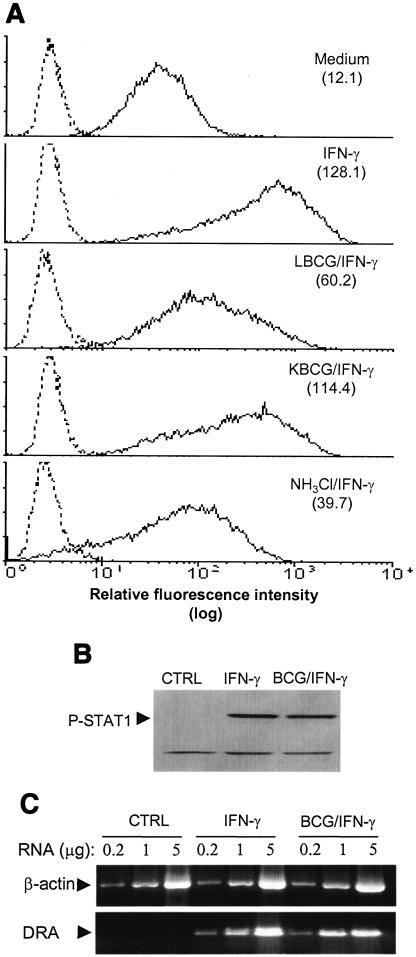
Initial experiments examined surface expression of MHC class II molecules by PMA-differentiated THP-1 cells in response to infection with M. bovis BCG. Cell surface labeling and flow cytometry analysis indicated that expression of HLA-DR in response to IFN-γ was significantly reduced in infected cells (53% decrease) (Fig. 1A). In contrast, constitutive and IFN-γ-induced expression of class I molecules and the expression of additional unrelated surface markers, such as CD18 and transferrin receptor, were unchanged in infected cells (data not shown), indicating that the inhibitory effect of BCG on MHC class II expression is selective. Compared to live BCG, phagocytosis of heat-killed (2 h, 60°C) BCG led to only a partial attenuation (12% decrease) of IFN-γ-induced HLA-DR expression, indicating that attenuation of class II molecule expression is mostly dependent on metabolically active bacteria. Similar data were obtained with bacteria killed by gentamicin (50 μg/ml; 30 min) (data not shown), suggesting that the lack of class II inhibition in the presence of killed bacteria was not due to protein inactivation at the surface of the bacterium.
FIG. 1.
M. bovis BCG inhibits surface expression of HLA class II molecules despite normal STAT1 signaling and MHC gene expression. (A) PMA-treated THP-1 cells were incubated with opsonized live (LBCG) or heat-killed M. bovis BCG (KBCG) at a bacterium/cell ratio of 50:1, or with NH4Cl (20 mM) for 24 h, and then IFN-γ (200 U/ml) was added for 24 h. Cells were incubated for 20 min at 4°C with anti-HLA-DR or irrelevant MAb. After two washes, cells were labeled with FITC-conjugated F(ab′)2 goat anti-mouse IgG for 20 min, washed and fixed, and analyzed by flow cytometry. Results are expressed as histograms of fluorescence intensity; solid lines represent cells stained with anti-HLA-DR MAb, and dashed lines represent cells stained withirrelevant isotype-matched IgG. Values in the top right of each panel indicate MFI indices, which correspond to the ratio the MFI of cells incubated with specific Ab to the MFI of cells stained with irrelevant isotype-matched IgG. The data shown are representative of results obtained in three separate experiments. (B) Control and infected cells were washed with HBSS and incubated with IFN-γ (200 U/ml) for 15 min at 37°C. Cell lysates were generated with modified radioimmunoprecipitation assay buffer, and soluble material was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were probed with anti-phospho-STAT1 MAb and developed by using enhanced chemiluminescence. The Ab recognized a second protein band below STAT1, which indicated equal protein loading from all treatment samples. The data shown are from one of two independent experiments that yielded similar results. (C) Differentiated cells were incubated in medium alone (CTRL) or with opsonized BCG organisms (bacterium/cell ratio of 50:1) for 24 h, and then IFN-γ (200 U/ml) was added for an additional 24 h. RNA was isolated, and RT-PCR was carried out using various RNA concentrations as described elsewhere (20, 32). The data shown are from one of two independent experiments that yielded similar results.
Similar to viable BCG, incubation of cells with ammonium chloride, which is known to inhibit maturational processing of class II molecules (20), significantly decreased (70%) surface expression of class II (Fig. 1A).
The transcription of MHC class II genes in response to IFN-γ is known to be dependent upon signaling through the IFN-γ-activated DNA-binding protein STAT1 (11, 18). To examine whether attenuation of IFN-γ-induced expression of class II molecules by BCG was related to impaired signaling through the STAT1 pathway, infected and noninfected THP-1 cells were incubated with IFN-γ for 15 min and cell lysates were analyzed for tyrosine phosphorylation of STAT1. IFN-γ-induced tyrosine phosphorylation of STAT1 was not influenced by infection with BCG (Fig. 1B). In other experiments, total RNA was isolated from IFN-γ-stimulated control and BCG-infected cells, and RT-PCR was performed with primers for DRA and β-actin and different dilutions of RNA. The results shown in Fig. 1C demonstrate that mRNA levels for DRA induced by IFN-γ were apparently normal in cells infected with BCG, and further analysis of DNA bands by densitometry showed that infection induced less than 10% diminution of DRA mRNA (data not shown). These findings indicate that BCG infection of human monocytic cells affects surface expression of class II molecules at a posttranscriptional level, contrasting with a previous report showing decreased CIITA and MHC II mRNA expression in murine macrophages infected with M. tuberculosis (34).
Intracellular sequestration of MHC class II molecules in cells infected with M. bovis BCG.
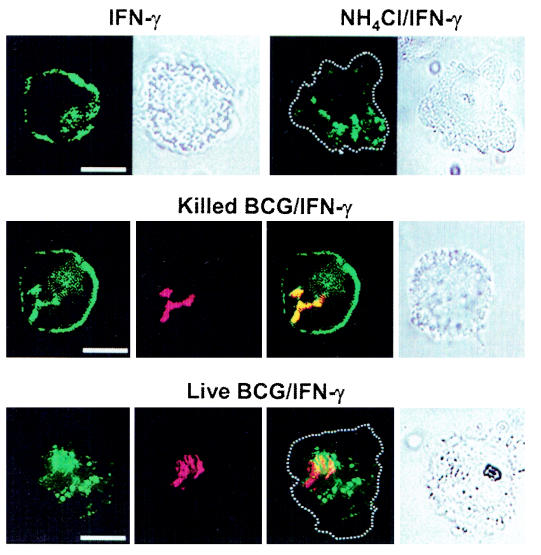
Differentiated THP-1 cells were infected with RITC-labeled BCG (red fluorescence) and stained with Ab to HLA-DR and FITC-labeled secondary Ab (green fluorescence). Samples were then examined with digital confocal microscopy. In IFN-γ-treated control cells and in cells infected with killed BCG, the staining pattern of HLA-DR showed a random distribution throughout the cell with a notably bright peripheral staining (Fig. 2). In contrast, cells either infected with live BCG or treated with ammonium chloride showed vesicles of various sizes retained within the cytosolic compartment in a perinuclear distribution. Peripheral and cell membrane staining was negligible. In addition, colocalization of class II with live BCG was markedly less than that observed with killed bacteria. As previously observed in cells infected with M. tuberculosis (20), defective cell surface targeting of class II molecules in BCG-infected cells appears to be related to intracellular sequestration.
FIG. 2.
Intracellular accumulation of class II molecules in ammonia-treated and BCG-infected cells. THP-1 cells adhering to tissue culture coverslips were treated with NH4Cl (20 mM) or infected with live or killed RITC-labeled BCG for 24 h. Cells were then stimulated with IFN-γ for 24 h, fixed, permeabilized, and stained with anti-DR MAb and FITC-labeled secondary Ab as described in Materials and Methods. Labeled cells were analyzed with digital confocal microscopy, and optical sections (0.2 μm) were scanned for green and red fluorescence. The images are displayed in panels with green (HLA-DR), red (bacteria), and yellow signals, the latter depicting colocalization of green with red. Dotted lines represent the cell membrane delimitation deducted from bright-field images. Bar, 10 μm.
Urease-defective mutant M. bovis BCG is less potent in reducing class II expression: contribution of exogenous urea.
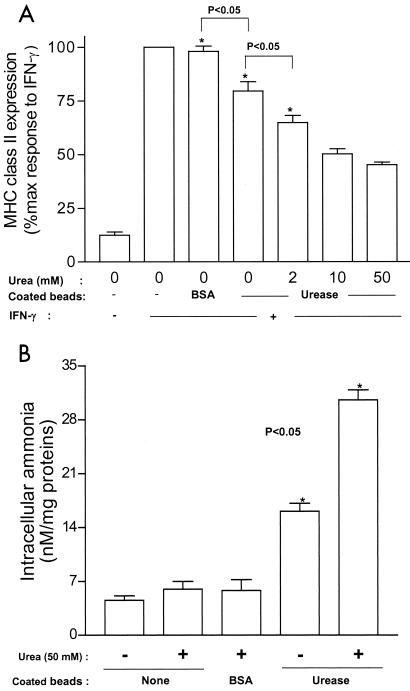
The similarities between live BCG and ammonium chloride in blocking surface expression of class II suggested the hypothesis that mycobacteria may express urease in infected cells, leading to alkalinization, via ammonia, of essential compartments where class II molecules are processed before export to the cell surface. To investigate the effects of mycobacterial urease expression in infected cells, differentiated THP-1 cells were infected with either BCGUre+ or mutant BCGUre−for 24 h followed by an additional 24-h stimulation with IFN-γ. Cells were then stained for class II molecules and analyzed by flow cytometry. Results obtained from five independent experiments (Fig. 3A) demonstrated that mutant BCGUre−was significantly attenuated in its capacity to block class II expression (35.2% ± 2.0% inhibition) in comparison to results with wild-type BCGUre+ (55.5% ± 1.6%). Given that urease-positive mycobacteria are able to produce substantial quantities (up to 20 mM) of ammonia in cell-free culture (17), we anticipated that this would also occur in cells infected with BCGUre+ and that this would be accompanied by more substantial inhibition of class II expression by BCGUre+ in comparison to that with BCGUre−. One factor that might have explained the limited difference between wild-type and mutant BCG could have been limited availability of the urease substrate within the host cell, thereby constraining ammonia production. Indeed, addition of 50 mM urea to the culture medium, during infection and treatment with IFN-γ, led to dramatic attenuation (79.1% ± 4.5% decrease) of class II expression in cells infected with BCGUre+ compared to results in the absence of urea (55.5% ± 3.8% decrease) (Fig. 3A). Conversely, addition of exogenous urea did not potentiate inhibition of class II surface expression in cells infected with BCGUre−. Similarly, the addition of urea did not alter class II expression in control noninfected cells treated with IFN-γ.
FIG.3.
BCGUre+ produces ammonia within the host cell and expresses a higher anti-class II activity. (A) Adherent THP-1 cells were infected with opsonized BCGUre+ or BCGUre− at a bacterium/cell ratio of 50:1, for 24 h in the absence or presence of urea. Cells were then incubated with IFN-γ (200 U/ml) for an additional 24 h. Cells were stained for surface class II and analyzed by flow cytometry as described in the legend to Fig. 1. MFI indices were deducted from fluorescence histograms, and results are expressed as the percentage of maximal class II surface expression (i.e., class II expression by cells treated with IFN-γ). (B) Adherent THP-1 cells were incubated with opsonized RITC-labeled BCGUre+ or BCGUre− at a bacterium/cell ratio of 50:1, for 3 h at 37°C. Partially attached, noningested bacteria were removed by 5-min treatment with trypsin-EDTA and extensive washing with HBSS. Samples were analyzed by flow cytometry. Results are expressed as histograms of fluorescence intensity. Dashed lines represent autofluorescence background (cells alone), and solid lines represent cells exposed to fluorescent bacteria. The percentages of positive cells are indicated in each panel. (C) Control and THP-1 cells ingesting bacteria were washed twice with HBSS and sonicated for 5 s. Cell debris and membranes were removed by centrifugation, and supernatants were assayed for ammonia by the fluorometric method described in Materials and Methods. Values in panel A are the mean ± SD of five independent experiments; in panel B values represent one of five independent experiments that yielded similar results; values in panel C are the mean ± SD of three independent experiments.
To exclude the possibility that differences between bacterial strains on MHC class II expression could have been accounted for by variable rates of infection, in each experiment parallel cell cultures were infected with the same batch of either BCGUre+ or BCGUre−cells freshly labeled with RITC. Bacterial uptake was then measured by flow cytometry. Only experiments in which both BCGUre+ and BCGUre−displayed similar infection rates, as shown in Fig. 3B, were analyzed.
To establish a correlation between attenuation of class II expression and capacity to generate intracellular ammonia, lysates were prepared from control cells and cells infected with either wild-type or mutant BCG for 24 h, and these were assayed for ammonia content. Figure 3C shows that after subtraction of background ammonia from control cells there was nearly no production of ammonia in cells infected with BCGUre− while substantial levels of ammonia (11.7 ± 1.2 nM/mg of protein) were detected in cells infected with BCGUre+. Moreover, this increased markedly (19.8 ± 0.8 nM/mg of protein) when cells were incubated in the presence of exogenous urea. These data suggest that infection with BCG expressing urease is accompanied by ammonia production within the host cell, with the capacity to affect class II trafficking to the cell surface.
Urease-coated latex beads inhibit surface expression of class II molecules.
Latex beads coated with urease from B. subtilis, which is closely related to mycobacterial ureases (9), were used to examine more directly the role of urease in class II inhibition. THP-1 cells were incubated with coated beads and stimulated with IFN-γ. BSA-coated beads were used as a control. Cells were then stained with anti-class II Ab and examined by flow cytometry. The results shown in Fig. 4A indicate that cells that had ingested urease-coated beads had diminished surface expression of class II (27% inhibition) and that the addition of various concentrations of urea (2 to 50 mM) to the system gradually potentiated the inhibitory effect of urease-coated beads (35 to 55% inhibition). In addition, inhibition of IFN-γ-induced class II expression by exogenous urease correlated with the level of intracellular ammonia produced (Fig. 4B). Taken together, these data confirm those data observed with BCG strains and suggest that (i) expression of urease within the host cell is specifically contributing to inhibition of class II, via ammonia production, and (ii) that this effect is dependent on the availability of the substrate, urea.
FIG. 4.
Ingestion of latex beads coated with urease increased intracellular levels of ammonia and attenuated cell surface expression of class II molecules. (A) Adherent THP-1 cells were exposed to latex beads coated with urease (or BSA [control]) at a bead/cell ratio of 5:1. Untreated cells were used as an additional control. After a period of 3 h of phagocytosis and a 1-h chase, cells were stimulated with IFN-γ (200 U/ml) for 24 h in the absence or presence (hatched bar) of 50 mM urea. Cells were then stained with anti-class II and analyzed by flow cytometry as described in the legend to Fig. 1. Results are expressed as the mean ± SD (n = 3) of maximal class II surface expression. (B) Control and cells ingesting coated beads were incubated in the absence or presence of various concentrations of urea for 5 h (3-h phagocytosis and 2-h chase). Cells were washed twice with HBSS and sonicated for 5 s. Cell debris and membranes were removed by centrifugation, and supernatants were assayed for ammonia by the fluorometric method described in Materials and Methods. Results are expressed as the mean ± SD (n = 3) nanomolar concentration of ammonia per milligram of protein.
Urease action is distal to bacterial survival within macrophages.
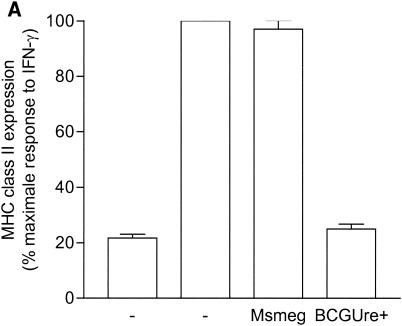
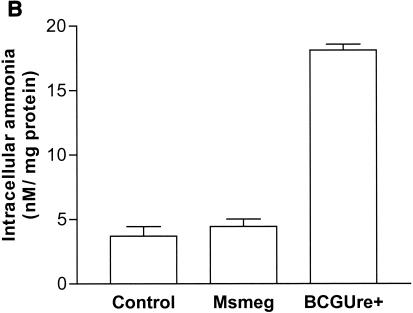
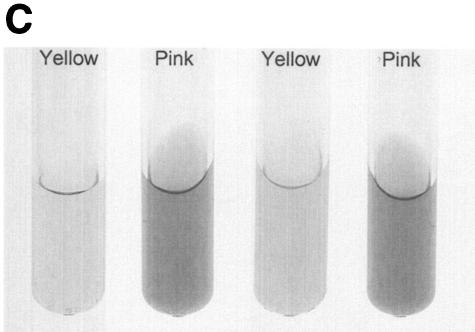
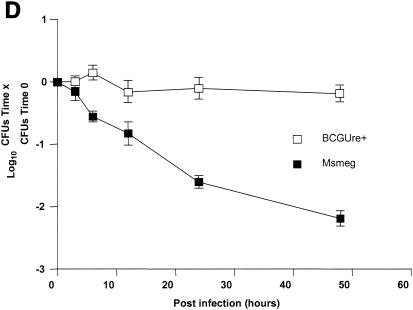
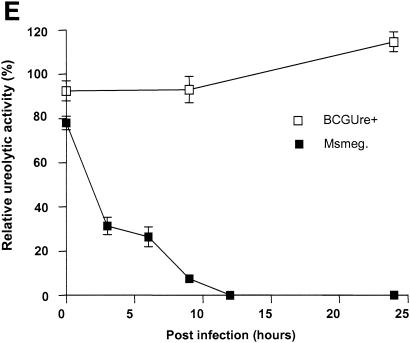
Urease is also expressed by some nonpathogenic mycobacteria, such as M. smegmatis, M. phlei and M. vaccae (23). This suggested the possibility that nonpathogenic, urease-positive mycobacterial strains could also down regulate expression of MHC class II. To address this hypothesis, THP-1 cells were infected with either BCGUre+ or M. smegmatis in the presence of exogenous urea and stimulated with IFN-γ. Cells were then stained for class II and analyzed by flow cytometry. Figure 5A shows that M. smegmatis had a limited effect on class II expression (∼10% inhibition) compared to BCGUre+ (75% inhibition). Parallel experiments examining phagocytosis of fluorescent BCG and M. smegmatis by flow cytometry showed similar internalization levels for both bacteria (data not shown), indicating that the lack of inhibition of class II molecules by M. smegmatis could not be assigned to a reduced bacterial internalization. Additional data obtained from the ammonia assay showed that in contrast to BCGUre+, which produced up to 20 mM ammonia/mg of protein, the production of ammonia that could be specifically assigned to infection with M. smegmatis was negligible (Fig. 5B). This was the case despite the fact that M. smegmatis did express urease activity in vitro (Fig. 5C). This discrepancy between urease production in vitro and in vivo was likely explained by the inability of M. smegmatis to survive in vivo. Thus, measurement of CFU corresponding to bacteria isolated from infected THP-1 cells at various times showed rapid intracellular clearance of M. smegmatis (up to 90% within 48 h), whereas BCGUre+ organisms were resistant to microbicidal activity of macrophages (less than 10% clearance). Additional experiments examined whether bacteria must be alive to express urease activity within the host cell. Macrophages were infected with either BCGUre+ or M. smegmatis expressing GFP, and bacteria were isolated at different time points over 24 h. Based on the GFP signal, the ureolytic activity of bacteria grown within the host cell was compared to that of an equivalent number of GFP-expressing bacteria grown in culture medium. Figure 5E shows that M. smegmatis ureolytic activity nearly completely disappeared at the 9-h time point. In contrast, BCG organisms isolated from macrophages maintained their capacity to hydrolyze urea at all time points.
FIG. 5.
Urease-positive, nonpathogenic M. smegmatis has a minor effect on class II expression and intracellular ammonia level. (A) Differentiated THP-1 cells were incubated with opsonized BCGUre+ or M. smegmatis (Msmeg), at a bacterium/cell ratio of 50:1, for 24 h in presence of 50 mM urea, and then IFN-γ (200 U/ml) was added for 24 h. Controls were cells alone and cells treated with IFN-γ. Cells were stained for surface class II and analyzed by flow cytometry as described in the legend to Fig. 1. MFI indices were deducted from fluorescence histograms, and results are expressed as the mean ± SD (n = 3) of maximal class II surface expression. (B) THP-1 cells were infected as described for panel A. After 24 h of incubation, cells were washed twice with HBSS and sonicated for 5 s. Cell debris and membranes were removed by centrifugation, and supernatants were assayed for ammonia by the fluorometric method described in Materials and Methods. Results are expressed as the nanomolar concentration of ammonia per milligram of protein and are the mean ± SD of three independent experiments. (C) Urease test tubes (broth medium plus urea and phenol red) were inoculated with 109 M. smegmatis, BCGUre− or BCGUre+ cells and incubated at 37°C for 12 h. Urease-mediated ammonia production changes the color of the medium from yellow to dark pink. (D) THP-1 cells were infected with M. smegmatis or BCGUre+ for 3 h at 37°C in culture medium. Partially attached, noningested bacteria were removed by 5 min of treatment with trypsin-EDTA and extensive washing with HBSS. Culture plates were reincubated in medium supplemented with streptomycin and penicillin. At the time points indicated, bacteria were released by treatment with 0.1% Triton X-100 in PBS, and serial dilutions were plated on 7H11 plates in triplicate. The results are expressed as described in reference 41. For each time point, data are the mean ± SD of CFU counts obtained from two independentexperiments. (E) BCGUre+ and GFP-expressing M. smegmatis cells were isolated from infected THP-1 at different time points as described for panel D and tested for ureolytic activity in broth medium plus urea and phenol red. The absorbance of the pink color resulting from medium alkalinization was measured in an enzyme-linked immunosorbent assay reader at 540 nm. For each time point, relative urease activity was calculated as follows: 100 × (absorbance due to GFP-expressing bacteria isolated from macrophages)/(absorbance resulting from an equal number of GFP-expressing bacteria grown in cell-free culture medium). Results are expressed as the mean ± SD of values obtained from two independent experiments.
Taken together, these observations suggest that ureolytic activity is not essential for multiplication and persistence of mycobacterium and that the enzyme participates actively in class II inhibition only when urease-positive bacteria are able to circumvent intracellular killing.
DISCUSSION
This study examined the hypothesis that mycobacterial urease may be a virulence determinant that disrupts trafficking of MHC class II molecules through the endocytic pathway. Building on the observation that M. tuberculosis cells as well as alkalinization of the endosomal-lysosomal compartment prevent surface expression of MHC class II α and β chains, leading to their intracellular sequestration (20), a BCG urease-negative mutant generated by allelic exchange (42) was used in this study along with the human monocytic cell line THP-1 to investigate the effect of ureolytic activity on MHC class II expression. THP-1 cells display many characteristics of the mature monocyte (45) and, when differentiated with PMA, represent a versatile human macrophage model for studying several cell activities, including class II expression and antigen presentation (4, 19, 20, 31).
Initial experiments introduced the THP-1/BCG model and showed that IFN-γ-induced cell surface expression of HLA-DR molecules is markedly attenuated in cells infected with viable wild-type M. bovis BCG and cells treated with ammonium chloride (Fig. 1). Confocal scanning of 0.2-μm sections showed intense intracellular expression of class II molecules in cells alkalinized or infected with BCG, which is consistent with normal IFN-γ signaling and a minimal effect on class II gene expression (Fig. 2). These findings established that the variant M. bovis BCG inhibits class II expression at the posttranscriptional level, presumably by interfering with intracellular trafficking of class II proteins.
Class II and Ii chains are coordinately transported and processed until proteolytic cleavage of Ii in an acidic compartment immediately before class II surface expression (12, 46). Thus, there is an essential requirement for degradative dissociation of Ii from newly synthesized class II α,β dimers prior to transport to the cell surface (24). Ii degradation is inhibited either by cysteine protease inhibitors, such as leupeptin (33), or lysosomotropic agents, such as chloroquine and ammonium chloride (21, 24). By virtue of their ability to produce ammonia in vitro (17), we hypothesized that pathogenic mycobacteria could affect the activities of endosomal enzymes with acidic pH optima. By using a sensitive fluorometric method capable of ammonia detection down to nanomolar concentrations (44), we found that wild-type BCG was capable of ammonia production within the host cell while the mutant BCGUre−was not. Moreover, ammonia production correlated with a marked decreased of class II surface expression in cells infected with BCGUre+ compared to those infected with the mutant BCGUre−(Fig. 3). Within the urease gene cluster in BCG, there are two more genes, UreF and UreG, located downstream of the kanamycin-disrupted UreC in BCGUre− and these genes are also involved in the expression of urease activity (9, 42). Therefore, no polar effect on other functions that may have contributed to the phenotype of limited inhibition of class II expression could be assigned to BCGUre−.
To directly investigate the role of urease on class II expression, we performed experiments using latex beads coated with purified urease. This approach demonstrated that urease-coated beads were also able to express ureolytic activity within macrophages, leading ultimately to reduced class II expression in response to IFN-γ (Fig. 4), consistent with the findings made with wild-type BCG.
The second question addressed in this study concerned the availability of urea as substrate for mycobacterial urease. Supplementing culture medium with 50 mM urea during infection significantly enhanced the inhibitory effect of BCGUre+ on class II expression. Similarly, intracellular levels of ammonia increased and surface class II decreased in cells ingesting urease-coated beads in the presence of exogenous urea (Fig. 3 and 4), suggesting that urease-dependent ammonia production would be dependent on arginine degradation into urea within the host cell. In macrophages, the metabolism of arginine is determined by the expression of the arginine-metabolizing enzymes inducible nitric oxide synthase and two isoforms of arginase (arginase I and II) (1). Nitric oxide synthase is induced by Th1 cytokines (IL-1, tumor necrosis factor alpha, and IFN-γ), while arginases are induced by Th2 cytokines, such as IL-4, IL-10, IL-13, and transforming growth factor β (1, 25). Thus, through dominant Th2 cytokine stimulations, the arginase-dependent catabolism of endogenous arginine into urea would provide specific substrate for mycobacterial urease-dependent intracellular alkalinization. Consistent with this hypothesis, it has recently been found that macrophages from mouse lines more prone to a Th2 response (BALB/c and DBA/2) not only do not produce NO, but also, instead, increase arginine metabolism to urea in response to lipopolysaccharide. Macrophages from mouse lines prone to a Th1 response (C57BL/6 and B10D2) do not (25, 26). These differences in macrophage responses have been termed M1 (Th1 mouse) and M2 (Th2 mouse) (25, 26). Therefore, further investigation based on the M1-M2 concept would provide further insight on the relevance of urease in the virulence of mycobacteria.
The finding that the BCGUre−mutant retained the capacity to inhibit MHC class II, although significantly less than BCGUre+, suggested that mycobacteria might use mechanisms other than urease to inhibit class II expression. In this regard, other laboratories have shown that long-term exposure (48 to 72 h) to the purified mycobacterial 19-kDa antigen (p19), a pathogen-associated molecular pattern, has the capacity to partially inhibit IFN-γ-induced surface expression of MHC class II (15, 35). Moreover, recent findings suggest that this may be related to attenuation of MHC gene transcription by p19 (36). However, studies using mycobacterial p19 knockout strains are necessary to validate the contribution of this putative virulence factor to defective MHC expression during infection.
The urease gene is absent from some nonpathogenic mycobacteria, such as M. xenopi, M. gordonae, and M. triviale, but it is present in others, such as M. smegmatis, M. phlei, and M. vaccae (23). Moreover, detailed characterization of mycobacterial urease showed that in the case of M. smegmatis the level of urease activity is 10-fold greater than it is in M. tuberculosis and M. bovis BCG (9). Therefore, we examined the level of ammonia produced by pathogenic and nonpathogenic mycobacteria within the host cells and showed that M. smegmatis, although capable of urea degradation in vitro, has only a marginal capacity to produce ammonia compared to BCG. This appeared to be relevant to lack of survival of M. smegmatis within macrophages, whereas wild-type BCG resists intracellular killing (Fig. 5). This finding suggested that expression of urease alone appeared insufficient to explain the greater virulence of pathogenic mycobacteria, consistent with previous studies showing marginal differences in intramacrophagic survival between the wild-type strain and its urease-negative mutant (41). Therefore, in light of the contrast between M. smegmatis and M. bovis BCG in use of urease, it is likely that once ingested by the macrophage, BCG uses initially urease-independent strategies to evade intracellular killing by the innate immune response. Subsequently, it may use urease activity to deactivate specialized functions, such as class II expression and antigen presentation. Since BCGUre−has a decreased effect on class II surface expression, it could be a good candidate strain to express heterologous antigen to construct recombinant vaccines designed to function in the context of MHC class II presentation.
In summary, the data presented above indicate that (i) M. bovis BCG markedly attenuates the cell surface expression of class II molecules in response to IFN-γ despite normal STAT1 signaling and expression of DRA; (ii) class II molecules are synthesized in infected cells but fail to transit to the cell membrane; (iii) mycobacterial urease, via ammonia secretion, contributes to defective intracellular transport and maturation of MHC class II gene products; and (iv) the intracellular availability of urea is a limiting factor in urease-dependent inhibition of class II expression.
Acknowledgments
We thank N. E. Reiner and M. Grigg for critically reviewing the manuscript. We also thank Yossef Av-Gay for providing pSC301 vector, R. Stokes for providing M. smegmatis cultures, and Genentech Inc. for the gift of human recombinant IFN-γ.
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR), grant MOP-43891, and an establishment grant from the Michael Smith Foundation for Health Research (MSFHR), grant CI-SCH-26. Z.H. was supported by scholar awards from the CIHR and MSFHR. K.S., A.D., and A.T. were supported by the BC Lung Association and TBVets Charitable Foundation.
Editor: J. B. Bliska
REFERENCES
- 1.Bansal, V., and J. B. Ochoa. 2003. Arginine availability, arginase, and the immune response. Curr. Opin. Clin. Nutr. Metab. Care 6:223-228. [DOI] [PubMed] [Google Scholar]
- 2.Barker, L. P., K. M. George, S. Falkow, and P. L. Small. 1997. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect. Immun. 65:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, S. 1984. Interferons as modulators of human monocyte-macrophage differentiation. I. Interferon-gamma increases HLA-DR expression and inhibits phagocytosis of zymosan. J. Immunol. 132:1249-1254. [PubMed] [Google Scholar]
- 4.Brett, S. J., J. Rhodes, F. Y. Liew, and J. P. Tite. 1993. Comparison of antigen presentation of influenza A nucleoprotein expressed in attenuated AroA− Salmonella typhimurium with that of live virus. J. Immunol. 150:2869-2884. [PubMed] [Google Scholar]
- 5.Busch, R., and E. D. Mellins. 1996. Developing and shedding inhibitions: how MHC class II molecules reach maturity. Curr. Opin. Immunol. 8:51-58. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C. H., J. D. Fontes, M. Peterlin, and R. A. Flavell. 1994. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J. Exp. Med. 180:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, C. H., and R. A. Flavell. 1995. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J. Exp. Med. 181:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 1995. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 177:5644-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley, S. C., and Y. Av-Gay. 2001. Monitoring promoter activity and protein localization in Mycobacterium spp. using green fluorescent protein. Gene 264:225-231. [DOI] [PubMed] [Google Scholar]
- 11.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 12.Deussing, J., W. Roth, P. Saftig, C. Peters, H. L. Ploegh, and J. A. Villadangos. 1998. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. USA 95:4516-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueiredo, F., T. J. Koerner, and D. O. Adams. 1989. Molecular mechanisms regulating the expression of class II histocompatibility molecules on macrophages. Effects of inductive and suppressive signals on gene transcription. J. Immunol. 143:3781-3786. [PubMed] [Google Scholar]
- 14.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 15.Gehring, A. J., R. E. Rojas, D. H. Canaday, D. L. Lakey, C. V. Harding, and W. H. Boom. 2003. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 71:4487-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gercken, J., J. Pryjma, M. Ernst, and H. D. Flad. 1994. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect. Immun. 62:3472-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, A. H., P. D. Hart, and M. R. Young. 1980. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 286:79-80. [DOI] [PubMed] [Google Scholar]
- 18.Greenlund, A. C., M. A. Farrar, B. L. Viviano, and R. D. Schreiber. 1994. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 13:1591-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslberger, A. G., G. Kohl, D. Felnerova, U. B. Mayr, S. Furst-Ladani, and W. Lubitz. 2000. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J. Biotechnol. 83:57-66. [DOI] [PubMed] [Google Scholar]
- 20.Hmama, Z., R. Gabathuler, W. A. Jefferies, G. de Jon, and N. E. Reiner. 1998. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J. Immunol. 161:4882-4893. [PubMed] [Google Scholar]
- 21.Humbert, M., P. Bertolino, F. Forquet, C. Rabourdin-Combe, D. Gerlier, J. Davoust, and J. Salamero. 1993. Major histocompatibility complex class II-restricted presentation of secreted and endoplasmic reticulum resident antigens requires the invariant chains and is sensitive to lysosomotropic agents. Eur. J. Immunol. 23:3167-3172. [DOI] [PubMed] [Google Scholar]
- 22.Kaye, P. M., M. Sims, and M. Feldmann. 1986. Regulation of macrophage accessory cell activity by mycobacteria. II. In vitro inhibition of Ia expression by Mycobacterium microti. Clin. Exp. Immunol. 64:28-34. [PMC free article] [PubMed] [Google Scholar]
- 23.Levy-Frebault, V. V., and F. Portaels. 1992. Proposed minimal standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int. J. Syst. Bacteriol. 42:315-323. [DOI] [PubMed] [Google Scholar]
- 24.Loss, G. E., Jr., and A. J. Sant. 1993. Invariant chain retains MHC class II molecules in the endocytic pathway. J. Immunol. 150:3187-3197. [PubMed] [Google Scholar]
- 25.Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166-6173.10843666 [Google Scholar]
- 26.Mills, C. D. 2001. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit. Rev. Immunol. 21:399-425. [PubMed] [Google Scholar]
- 27.Mobley, H. L., M. D. Island, and R. P. Hausinger. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59:451-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohagheghpour, N., D. Gammon, A. van Vollenhoven, Y. Hornig, L. E. Bermudez, and L. S. Young. 1997. Mycobacterium avium reduces expression of costimulatory/adhesion molecules by human monocytes. Cell. Immunol. 176:82-91. [DOI] [PubMed] [Google Scholar]
- 29.Mshana, R. N., R. C. Hastings, and J. L. Krahenbuhl. 1988. Infection with live mycobacteria inhibits in vitro detection of Ia antigen on macrophages. Immunobiology 177:40-54. [DOI] [PubMed] [Google Scholar]
- 30.Murray, P. J. 1999. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 7:366-372. [DOI] [PubMed] [Google Scholar]
- 31.Nadler, S. G., B. M. Rankin, P. Moran-Davis, J. S. Cleaveland, and P. A. Kiener. 1994. Effect of interferon-gamma on antigen processing in human monocytes. Eur. J. Immunol. 24:3124-3130. [DOI] [PubMed] [Google Scholar]
- 32.Nandan, D., and N. E. Reiner. 1997. TGF-β attenuates the class II transactivator and reveals an accessory pathway of IFN-γ action. J. Immunol. 158:1095-1101. [PubMed] [Google Scholar]
- 33.Neefjes, J. J., and H. L. Ploegh. 1992. Inhibition of endosomal proteolytic activity by leupeptin blocks surface expression of MHC class II molecules and their conversion to SDS resistance alpha beta heterodimers in endosomes. EMBO J. 11:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noss, E. H., C. V. Harding, and W. H. Boom. 2000. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell. Immunol. 201:63-74. [DOI] [PubMed] [Google Scholar]
- 35.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 36.Pai, R. K., M. Convery, T. A. Hamilton, W. H. Boom, and C. V. Harding. 2003. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171:175-184. [DOI] [PubMed] [Google Scholar]
- 37.Pancholi, P., A. Mirza, N. Bhardwaj, and R. M. Steinman. 1993. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260:984-986. [DOI] [PubMed] [Google Scholar]
- 38.Pieters, J. 1997. MHC class II restricted antigen presentation. Curr. Opin. Immunol. 9:89-96. [DOI] [PubMed] [Google Scholar]
- 39.Reiner, N. E. 1994. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol. Today 15:374-381. [DOI] [PubMed] [Google Scholar]
- 40.Reith, W., V. Steimle, and B. Mach. 1995. Molecular defects in the bare lymphocyte syndrome and regulation of MHC class II genes. Immunol. Today 16:539-546. [DOI] [PubMed] [Google Scholar]
- 41.Reyrat, J. M., G. Lopez-Ramirez, C. Ofredo, B. Gicquel, and N. Winter. 1996. Urease activity does not contribute dramatically to persistence of Mycobacterium bovis bacillus Calmette-Guerin. Infect. Immun. 64:3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyrat, J. M., F. X. Berthet, and B. Gicquel. 1995. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guerin. Proc. Natl. Acad. Sci. USA 92:8768-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steimle, V., C. A. Siegrist, A. Mottet, B. Lisowska-Grospierre, and B. Mach. 1994. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265:106-109. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara, K., and F. Oyama. 1981. Fluorogenic reaction and specific microdetermination of ammonia. J. Biochem. (Tokyo) 89:771-774. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 46.Villadangos, J. A., R. A. Bryant, J. Deussing, C. Driessen, A. M. Lennon-Dumenil, R. J. Riese, W. Roth, P. Saftig, G. P. Shi, H. A. Chapman, C. Peters, and H. L. Ploegh. 1999. Proteases involved in MHC class II antigen presentation. Immunol. Rev. 172:109-120. [DOI] [PubMed] [Google Scholar]
- 47.Young, H. A., and K. J. Hardy. 1995. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 58:373-381. [PubMed] [Google Scholar]