Abstract
The lack of an experimental model has significantly limited the understanding of the pathogenesis of Chlamydia trachomatis infections in males. In an attempt to establish a model using the natural route of infection, we inoculated male mice in the meatus urethra. To establish the 50% infectious dose (ID50), C3H/HeN (H-2k) male mice were inoculated in the meatus urethra with doses ranging from 101 to 107 inclusion-forming units (IFU) of C. trachomatis mouse pneumonitis biovar (MoPn) and were euthanized at 10 days postinfection (p.i.). Approximately 50% of the animals inoculated with 5 × 104 IFU had positive cultures of the urethra, urinary bladder, epididymides, and/or testes. Subsequently, to characterize the course of the infection, a group of animals was inoculated with 106 IFU/mouse (20 times the ID50). Positive cultures from the urethra, urinary bladder, epididymides, and testes were obtained from the animals. The infection peaked in the first 2 weeks p.i. and subsequently declined over the 7 weeks of observation. C. trachomatis-specific antibodies were first detected in serum by 2 weeks p.i. and rose over the period of observation. The titers of immunoglobulin G2a (IgG2a) were 16-fold higher than those of IgG1. A lymphoproliferative assay using splenocytes and local lymph nodes showed a strong cell-mediated immune response. Levels of gamma interferon were significantly higher than those of interleukin-4 in the supernatants from stimulated lymphocytes. An acute inflammatory infiltrate consisting of polymorphonuclear leukocytes was detected in the urethra at 1 week p.i. At 3 weeks p.i., a mixed acute and chronic inflammatory infiltrate was observed in the urethra that by 5 to 6 weeks was mainly composed of mononuclear cells. Similar findings were also observed in the urinary bladder, although the inflammatory infiltrate was delayed by approximately a week relative to that in the urethra. Sections of the epididymides showed a focal acute inflammatory infiltrate at 2 weeks p.i. Immunohistochemical staining demonstrated multiple chlamydial inclusions in the epithelium of the urethra and urinary bladder. No chlamydial inclusions were observed in the epididymides or testes. In conclusion, inoculation of male mice in the meatus urethra with C. trachomatis MoPn results in an infection of the genitourinary tract that closely parallels that described in humans. This model should help to characterize the pathogenesis of chlamydial infections in males and to test therapeutic and preventive measures.
It is estimated that, annually, two million cases of symptomatic acute urethritis occur in males in the United States and, recently, an increase in the incidence of male urethritis in several countries has been noted (1, 9, 19, 20, 36, 37, 39). Of these cases, approximately 50% are due to Chlamydia trachomatis. In addition, 50 to 70% of the infections due to C. trachomatis in males do not produce symptoms (18, 36, 39). In certain individuals, following an episode of urethritis other complications may develop. For example, it is estimated that approximately 50,000 cases of epididymitis occur yearly in the United States due to C. trachomatis (3, 4, 41). Although very rare, abscess formation and infarction of the testicle may occur (3, 4). The role that C. trachomatis may play in male infertility is not well understood. Acute inflammation of the epididymides could lead to decreased fertility even in the absence of occlusion (6, 11, 13, 31). Furthermore, the role that C. trachomatis may have in Reiter's syndrome, sexually acquired reactive arthritis, proctitis, prostatitis, and granulomatous bowel disease requires further investigation (6, 24, 33, 36, 39).
Data on the immunopathogenesis of genitourinary tract infections in males, and in particular those with C. trachomatis, are limited. For example, Pudney and Anderson (32) characterized the immunobiology of the human penile urethra and concluded that it contains all the necessary components for antigen presentation and humoral and cellular immune responses. In contrast, Pate et al. (29) collected penile urethral swabs from males with PCR-confirmed C. trachomatis infection and looked for the presence of cytokines, including interleukin-1β (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, and IL-18. In addition, they measured transforming growth factor β, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and antibodies to Chlamydia. In comparison with the control group, they only detected increased levels of IL-8 and immunoglobulin A (IgA) and IgG Chlamydia-specific antibodies. Based on these results, the authors concluded that the male genitourinary tract should be considered a weak inductive site.
The characterization of chlamydial infections in males has been limited in great part due to the lack of an experimental model that mimics the natural infection. Up to now, the mouse pneumonitis biovar (MoPn) has been used in a female model for the characterization of the immunopathogenesis of C. trachomatis infections (22, 35). However, there is no parallel model to study infections in males. To implement a new model of chlamydial infection of the male genitourinary tract, we chose to work with C3H/HeN (H-2k) mice. The female animals of this strain have been found to be highly susceptible to infection with C. trachomatis MoPn (7). Here, we report that inoculation of male C3H/HeN mice, in the meatus urethra with C. trachomatis MoPn, produces an infection that closely parallels that described in humans.
MATERIALS AND METHODS
Organisms.
The C. trachomatis MoPn strain Nigg II was purchased from the American Type Culture Collection (Manassas, Va.) and was grown in HeLa-229 cells (25, 26). Stocks of elementary bodies (EB) were prepared as described by Caldwell et al. (5) and stored at −70°C in SPG (0.2 M sucrose, 20 mM sodium phosphate [pH 7.2], and 5 mM glutamic acid).
Inoculation of mice.
Seven- to eight-week-old male C3H/HeN (H-2k) mice were purchased from Charles River Laboratories (Wilmington, Mass.). The animals were housed in isolation cubicles at a constant temperature of 24°C with a cycle of 12 h of light and 12 h of darkness and were fed mouse chowder ad libitum. For inoculation, the animals were anesthetized and, while on their backs, the prepuce was pulled back and by using a 4× magnifying glass the inoculum was placed on the meatus urethra. Mice were kept on their backs until the inoculum was observed to reflux into the urethra. Groups of 5 to 10 mice were inoculated with doses ranging from 101 to 107 IFU of C. trachomatis MoPn in 2 μl of SPG. A control group was sham infected with 2 μl of SPG. The experiments were repeated twice. The animal protocols were approved by the University of California Irvine Animal Care and Use Committee.
Organ culture.
To determine the infectious dose that would infect 50% of the animals (ID50), mice were euthanized at 10 days postinfection (p.i.). To follow the course of the infection the animals were euthanized at different days up to 7 weeks p.i. The penile and membranous urethra, urinary bladder, epididymides, and testes were harvested and placed in 2 ml of SPG. The tissues were homogenized using a Stomacher Lab-Blender 80 (Tekmar Co., Cincinnati, Ohio), and duplicates of 10-fold dilutions were inoculated by centrifugation (1,000 × g; 1 h at 24°C) onto McCoy cells grown in 48-well tissue culture plates. Each well was inoculated with 100 μl of the homogenate. The cells were incubated for 30 h at 37°C, and the chlamydial inclusions were stained with a pool of monoclonal antibodies prepared in our laboratory (26, 27). This pool included monoclonal antibodies to the major outer membrane protein (MOMP), the 60-kDa cysteine-rich protein, a 150-kDa putative outer membrane protein, and the lipopolysaccharide (LPS) of C. trachomatis MoPn. The limit of detection was 50 IFU per organ.
Immunoassays.
Blood was collected at weekly intervals by periorbital puncture and terminally from the heart, and the sera were pooled for each group of mice. The C. trachomatis MoPn-specific antibody titer in serum was determined by an enzyme immune assay (EIA) (26). Briefly, a 96-well plate was coated with 100 μl of C. trachomatis MoPn EB in phosphate-buffered saline at a concentration of 10 μg of protein/ml. Serum (100 μl) was added to each well in twofold serial dilutions. After incubation at 37°C for 1 h, the serum was discarded and the wells were washed three times with phosphate-buffered saline. The plates were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgM, IgA, IgG, IgG1, IgG2a, IgG2b, and IgG3 (Southern Biotechnology Associates, Inc., Birmingham, Ala.). The binding was measured in an EIA reader (Labsystem Multiscan, Helsinki, Finland) using 2′-2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) as the substrate. Preinoculation sera were used as negative controls.
Immunoblot assays were performed by using 250 μg of protein from purified C. trachomatis MoPn EB or recombinant C. trachomatis serovar A heat shock protein 60 (hsp60), a generous gift of R. Morrison (Montana State University, Bozeman, Mont.) in Tricine-sodium dodecyl sulfate (SDS)-polyacrylamide gels (10% acrylamide, 0.3% bis-acrylamide [wt/vol]) (38). Following transfer to nitrocellulose membranes, the nonspecific sites were blocked with BLOTTO (bovine lacto transfer technique optimizer; 5% [wt/vol] nonfat dried milk, 2 mM CaCl2, and 50 mM Tris-HCl; pH 8.0). Serum samples were diluted 1:100 and incubated overnight at 4°C. Antibody binding was detected with horseradish peroxidase-conjugated goat anti-mouse antibody developed with 0.01% hydrogen peroxide and 4-chloro-1-naphthol (38).
A T-cell lymphoproliferative assay was performed using splenocytes and iliac and caudal lymph nodes (26). Briefly, the spleens of two to three mice were collected, teased, and enriched for T cells by passage over a nylon wool column. Lymph nodes from the same animals were collected, teased, and used without further enrichment. T-cell-enriched cells were counted, and 105 cells were aliquoted into a 96-well plate. Antigen-presenting cells were prepared by irradiating splenocytes with 3,300 rads. UV-inactivated C. trachomatis MoPn EB were added at a concentration of 10 EB to 1 antigen-presenting cell. Control wells received medium alone as a negative control or concanavalin A, as a positive control, at a concentration of 5 μg/ml. Cell proliferation was measured by addition of 1 μCi of [methyl-3H]thymidine per well. The mean count was determined for triplicate cultures.
Cytokine measurements.
Levels of IFN-γ, IL-4, and TNF-α were determined by EIA with commercial kits (BD-Pharmingen, San Diego, Calif.). T cells from the spleen and lymph nodes were stimulated as described above, and the cytokines were measured in tissue culture supernatants collected after 48 h of incubation.
Histopathology.
Mice were euthanized, their genitourinary tracts were fixed in buffered formaldehyde and processed, and the tissue sections were stained with hematoxylin and eosin.
For immunohistological analysis, tissue sections were stained with a rabbit anti-C. trachomatis MoPn serum, prepared in our laboratory, followed by a biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, Calif.) (27). The sections were counterstained with hematoxylin.
Statistics.
The two-tailed unpaired Student t test and Mann-Whitney U test were used to determine the significance of differences between the groups by using the StatView software program on a Macintosh computer (Apple, Cupertino, Calif.).
RESULTS
Determination of ID50.
To determine the ID50, groups of 5 to 10 C3H/HeN male mice were inoculated in the meatus urethra with doses of C. trachomatis MoPn ranging from 101 to 107 IFU per mouse. A control group of animals was sham infected with SPG. Animals were euthanized at 10 days p.i., and the penis, urinary bladder, epididymides, and testes were cultured for C. trachomatis. The compilation of the replicate results of the dose response is shown in Table 1. All cultures from animals inoculated with 101 IFU were negative. Forty percent of the mice inoculated with 104 IFU had at least a positive culture from one organ, while 70% of the mice inoculated with 105 IFU had at least one positive culture from the organs. Thus, for C3H/HeN male mice, the ID50 determined by the Reed-Muench formula was 5 × 104 IFU of C. trachomatis MoPn per mouse. All the animals inoculated with 106 or 107 IFU had at least one or more organs positive by culture. All sham-infected mice had negative cultures.
TABLE 1.
Culture results from male C3H/HeN mice infected in the meatus urethra with various doses of C. trachomatis MoPn
| Dose/mouse | No. of mice | % Mice with at least one organ posc | Urethraa
|
Bladdera
|
Epididymidesa
|
Testesa
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | |||
| 0 | 10 | 0 | 0 | <1.7b | 0 | <1.7 | 0 | <1.7 | 0 | <1.7 |
| 101 | 5 | 0 | 0 | <1.7 | 0 | <1.7 | 0 | <1.7 | 0 | <1.7 |
| 103 | 10 | 20 | 20 | 6.12 ± 6.67 | 0 | <1.7 | 10 | 0.3 ± 0.7 | 0 | <1.7 |
| 104 | 5 | 40 | 40 | 6.66 ± 6.95 | 40 | 2.70 ± 2.8 | 40 | 2.10 ± 2.37 | 40 | 2.18 ± 2.41 |
| 105 | 10 | 70 | 70 | 6.20 ± 6.46 | 60 | 3.76 ± 4.23 | 70 | 2.21 ± 2.37 | 60 | 1.48 ± 1.50 |
| 106 | 5 | 100 | 100 | 6.11 ± 6.25 | 80 | 5.25 ± 5.57 | 60 | 2.03 ± 2.29 | 20 | 1.60 ± 1.90 |
| 107 | 10 | 100 | 100 | 5.37 ± 5.65 | 70 | 6.20 ± 6.69 | 70 | 2.50 ± 2.75 | 50 | 1.34 ± 1.40 |
Mean log10 IFU recovered per organ ± 1 standard deviation.
Log10 50 = 1.7 (limit of detection).
Pos, positive.
Characterization of the course of infection.
To determine the course of the infection, mice were infected with 106 C. trachomatis IFU, a dose corresponding to approximately 20 times the ID50. As shown in Table 2, at 1 week p.i. all mice had positive cultures from the urethra and remained positive, for the most part, for the first 4 weeks of observation. During the first 4 weeks p.i., most of the mice also had positive cultures from the bladder. The epididymides and testes yielded positive cultures mainly for the first 2 weeks p.i. A significant number of mice (67%) still had positive cultures from the urethra at 7 weeks p.i. On the other hand, cultures from the bladder, epididymides, and testes at that point were for the most part negative. The highest yield of C. trachomatis IFU, for the four organs cultured, was obtained during the first 2 weeks p.i. and subsequently declined (Table 2).
TABLE 2.
Culture results from male C3H/HeN mice infected in the meatus urethra with 106 IFU of C. trachomatis MoPn
| Wk postinfection | No. of mice euthanized | % Mice with at least one organ posc | Urethraa
|
Bladdera
|
Epididymidesa
|
Testesa
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | % Pos | IFU ± 1 SD | |||
| 1 | 8 | 100 | 100 | 7.53 ± 7.42 | 63 | 4.82 ± 5.03 | 50 | 4.59 ± 5.03 | 25 | 3.26 ± 3.71 |
| 2 | 8 | 100 | 100 | 5.77 ± 5.93 | 100 | 6.93 ± 7.00 | 75 | 3.32 ± 3.59 | 38 | 2.48 ± 2.70 |
| 3 | 8 | 88 | 88 | 3.56 ± 3.77 | 88 | 5.94 ± 6.25 | 13 | 2.24 ± 2.69 | 13 | 1.00 ± 1.45 |
| 4 | 8 | 100 | 88 | 2.52 ± 2.51 | 63 | 1.68 ± 1.85 | 0 | <1.7b | 0 | <1.7 |
| 5 | 9 | 56 | 33 | 1.71 ± 2.12 | 33 | 2.65 ± 3.12 | 11 | 0.35 ± 0.82 | 0 | <1.7 |
| 7 | 9 | 67 | 67 | 2.92 ± 3.22 | 11 | 1.65 ± 2.12 | 0 | <1.7 | 0 | <1.7 |
Mean log10 IFU recovered per organ ± 1 standard deviation.
Log10 50 = 1.7 (limit of detection).
Pos, positive.
Assessment of the immune response.
Results of the EIA performed to detect serum antibodies to C. trachomatis MoPn EB are shown in Table 3. Chlamydia-specific IgM antibodies were first detected 1 week p.i. and peaked by the third week. IgG antibodies were first detected during the second week p.i. and increased progressively for the first 4 weeks. A peak IgG antibody titer was obtained at 4 weeks p.i. and remained at the same level for the 7 weeks of observation. Levels of IgG1 and IgG2a paralleled those of total IgG. Titers of IgG2a, however, were 16-fold higher than those of IgG1 (25,600 versus 1,600). During the second week p.i., IgA antibodies were detected in serum, and they reached a titer of 1,600 by the fifth week.
TABLE 3.
Mean ELISA antibody titers in serum of male C3H/HeN mice following inoculation in the meatus urethra with 106 IFU of C. trachomatis MoPn
| Wks postinfection | ELISA titer to C. trachomatis MoPn EB
|
||||||
|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | IgM | |
| 0 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| 1 | <100 | <100 | <100 | <100 | <100 | <100 | 100 |
| 2 | <100 | <100 | <100 | 400 | 100 | 100 | 100 |
| 3 | 3,200 | <100 | 1,600 | 6,400 | 1,600 | 800 | 400 |
| 4 | 51,200 | 1,600 | 25,600 | 12,800 | 3,200 | 800 | 200 |
| 5 | 51,200 | 1,600 | 25,600 | 12,800 | 6,400 | 1,600 | 100 |
| 7 | 51,200 | 1,600 | 25,600 | 12,800 | 6,400 | 1,600 | 100 |
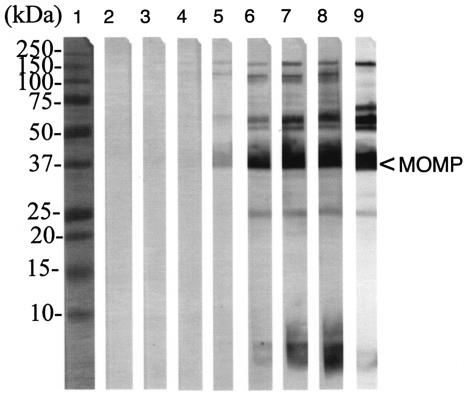
Figure 1 shows the results of the Western blotting performed with the serum samples collected p.i. using EB as the antigen. A faint band, corresponding to the MOMP, was first detected at 3 weeks p.i. At 4 weeks p.i., several bands with a molecular mass in the range of 75 to 200 kDa, the doublet corresponding to the 60-kDa cysteine-rich protein (crp), the 60-kDa hsp, MOMP, the 28-kDa protein, and LPS were detected. A similar pattern was observed with sera collected at 7 weeks p.i. Reactivity to the 60-kDa hsp was confirmed using a recombinant hsp60 as the antigen (data not shown).
FIG. 1.
Immunoblot of EB probed with serum samples collected from male mice infected in the meatus urethra with C. trachomatis. Lane 1, molecular weight standards; lanes 2 to 8, serum samples collected at weeks 1 to 7 p.i.; lane 9, EB probed with monoclonal antibodies to the 150,000, 60,000 (crp), 40,000, and 28,000 MW proteins and to LPS.
To characterize the local and systemic cellular immune responses to the C. trachomatis infection, T-cell-enriched splenocytes and lymphocytes from the iliac and caudal lymph nodes were tested for their proliferative response to EB. As a control, splenocytes and lymph nodes from the sham-infected males were used. As shown in Table 4, a strong proliferative response to EB was observed with both the T-cell-enriched splenocytes and the lymph nodes from the C. trachomatis-infected mice in comparison to the control animals.
TABLE 4.
T-cell responses of C3H/HeN male mice at 7 weeks postinoculation after infection with C. trachomatis MoPn in the meatus urethraa
| Group | T-cell-enriched splenocytes
|
Cells from iliac and caudal lymph nodes
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proliferative response to (103 cpm ± 1 SD):
|
In vitro cytokine productionb to EB (pg/ml)
|
Proliferative response to (103 cpm ± 1 SD):
|
In vitro cytokine productionc to EB (pg/ml)
|
|||||||
| EB | Medium | IFN-γ | IL-4 | TNF-α | EB | Medium | IFN-γ | IL-4 | TNF-α | |
| Infected | 11.4 ± 2.0e | 0.3 ± 0.06 | 3,897 ± 514f | 0.9 | 352 ± 3f | 2.9 ± 0.3e | 0.4 ± 0.1 | 2,167 ± 14f | <0.1 | 35 ± 8f |
| Controld | 0.6 ± 0.2 | 0.3 ± 0.1 | 397 ± 15 | 0.6 | 52 ± 2 | 0.5 ± 0.1 | 0.3 ± 0.05 | 4 ± 9 | <0.1 | 8 ± 5 |
Results are means of triplicate cultures. Data correspond to one of the experiments representative of duplicate experiments. UV-inactivated C. trachomatis MoPn EB were added at a 10:1 ratio to the T cells.
A total of 2.5 × 106 T-cell-enriched splenocytes were stimulated
A total of 1.5 × 106 cells from lymph nodes were stimulated.
Sham infected with SPG.
P < 0.05, by the Mann-Whitney U test, compared to the sham-infected group.
P < 0.05, by Student's t test, compared to the sham-infected group.
Levels of IFN-γ, IL-4, and TNF-α were measured in the supernatants from stimulated lymphocytes from the spleen and lymph nodes. As shown in Table 4, high levels of IFN-γ were detected in the supernatants of the lymphocytes from the infected mice in comparison to those in the sham-infected mice (P < 0.05). In contrast, levels of IL-4 were not significantly different between the C. trachomatis-infected and the mock-infected animals. Levels of TNF-α were also significantly higher in the C. trachomatis-infected mice when compared to the controls.
Histopathological analyses.
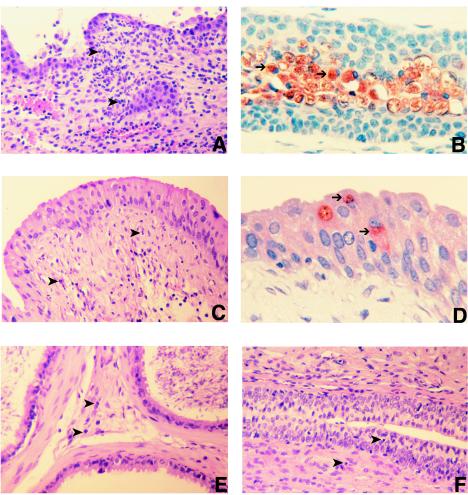
Tissue sections from the urethra, urinary bladder, epididymides, and testes from the mice inoculated with 106 C. trachomatis IFU were prepared for histopathological analysis (Fig. 2). At 1 week p.i., the urethra showed focal areas with an acute inflammatory infiltrate consisting of polymorphonuclear neutrophils (PMN) mainly located in the lamina propria but also infiltrating the epithelium (data not shown). This same type of inflammatory infiltrate was more pronounced in the urethra by the second week p.i. At that point, multiple chlamydial inclusions could be observed in the epithelium of the urethra. Tissue sections collected at 4 weeks p.i. showed a decrease in the number of PMN and a moderate infiltrate of chronic inflammatory cells consisting of lymphocytes and monocytes (data not shown). A few plasma cells were also detected in the urethra. At 5 weeks p.i., the infiltrate was mainly composed of chronic inflammatory cells, although foci of PMN could still be detected. By 6 to 7 weeks p.i., most of the sections of the urethra appeared normal, although focal areas with inflammatory infiltrates were still detected (data not shown). Similar changes were observed in the urinary bladder; however, the inflammatory infiltrate was delayed by approximately a week relative to the changes observed in the urethra. Thus, in the urinary bladder, the peak of the acute inflammatory response was observed at 2 to 3 weeks p.i. Chlamydial inclusions were observed in the epithelium of the urinary bladder. The acute infiltrate was subsequently replaced by chronic inflammation that slowly disappeared over the 7 weeks of observation. In the epididymides, we detected only a few foci of PMN at 2 weeks p.i. Most of the sections of the epididymides, however, did not show pathological changes. No significant morphological changes were seen in the testes. No chlamydial inclusions were detected in the epididymides or in the testes.
FIG. 2.
Histological sections from tissues of male mice infected with C. trachomatis MoPn. (A, C, and E) Sections of the urethra (A), bladder (C), and epididymides (E), stained with hematoxylin and eosin, from mice inoculated with 106 IFU of C. trachomatis MoPn and that were euthanized at 2 weeks p.i. (B and D) Immunochemical staining with C. trachomatis MoPn polyclonal antibody of the urethra (B) and bladder (D) from the same group of mice as shown in panels A, C, and E. (F) Urethra, stained with hematoxylin and eosin, from a mouse inoculated with 106 IFU and euthanized at 5 weeks p.i. Inflammatory cells are marked with arrowheads, and chlamydial inclusions are indicated with arrows.
DISCUSSION
The lack of an experimental model and the limited access to human specimens from the genitourinary tract have hindered our understanding of the pathogenesis of C. trachomatis infections in males. We have shown here that inoculation of male C3H/HeN mice in the meatus urethra with C. trachomatis MoPn results in an infection of the genitourinary tract that closely parallels, in length of time of positive cultures and tissue involvement, that described in humans (2, 36). This is, to our knowledge, the first time that a model of the natural route of infection of the genital tract of the male mouse was achieved with a sexually transmitted pathogen. This model should provide an opportunity to characterize the pathogenesis of chlamydial infections in males and to determine the components of the innate and adaptive immune systems that are involved in controlling this organism.
Two species of Chlamydia, C. trachomatis and C. psittaci, have been utilized to analyze genitourinary tract infections in male animal models. For example, Taylor-Robinson et al. (42) inoculated three chimpanzees in the urethra with human isolates of C. trachomatis and were able to culture the organism from the urethras of two of the three animals. To determine the role of C. trachomatis in epididymitis, Moller and Mardh (21) infected two grivet monkeys in the spermatic cord with the C. trachomatis K serovar. By 7 days p.i., both animals had significant swelling of the epididymides and spermatic cord that were shown to be infiltrated with PMN and lymphocytes. To assess the feasibility of using rodents as an experimental model, Kuzan et al. (17) inoculated the epididymides of B6D2F1 mice with 2.8 × 108 IFU of C. trachomatis serovar E. A severe inflammatory infiltrate, consisting of PMN and lymphocytes, was observed at 3 days p.i. and subsided by 3 weeks p.i. Also, Jantos et al. (15) inoculated the vas deferens of Wistar rats with 4 × 107 IFU of C. trachomatis MoPn and detected severe inflammation of the infected epididymides followed by atrophy of the ipsilateral testis. Mount et al. (23) infected male guinea pigs in the urethra with the C. psittaci guinea pig inclusion conjunctivitis (GPIC) agent and detected inclusions in the epithelium of the urethra and Chlamydia-specific antibodies in serum. Also, Rank et al. (34), by placing a drop of the inoculum in the meatus urethra or by intraurethral injection, induced urethritis and cystitis in guinea pigs with GPIC. Jantos et al. (14), after inoculating the vas deferens of rats with the GPIC agent, recovered Chlamydia from the epididymides and the prostate for a period of several weeks.
Here, we first determined the ID50 by inoculating 7- to 8-week-old male C3H/HeN mice in the meatus urethra with doses ranging from 101 to 107 IFU of C. trachomatis MoPn. Approximately 5 × 104 IFU were necessary to infect 50% of the animals, an ID50 that closely parallels the one determined in female C3H/HeN mice using the vaginal route (28). Thus, it appears that males and females of this strain have similar susceptibilities to a chlamydial genital infection. In this male model, inoculation with C. trachomatis in the meatus urethra resulted not only in urethritis but also in infection of the epididymides and testes. Animals inoculated with 106 IFU of C. trachomatis showed extensive involvement of the urethra with heavy inflammatory infiltrate and multiple chlamydial inclusions in the epithelial layer. The level of infection of the epididymides and testes was low, as shown by the number of IFU recovered and the focal inflammatory infiltrate detected.
The pathogenesis of epididymitis and possible sequelae, e.g., infertility, has not been defined. Epididymitis and orchitis could be the result of an ascending intraluminar infection or could occur by dissemination through the hematogenous or lymphatic route (2). It is interesting that in our model, as in the guinea pig model, we were able to observe chlamydial inclusions and inflammation in the urinary bladder (34). In humans, chlamydial cystitis has not been described. This may be due to the fact that a biopsy is rarely obtained during an acute urinary tract infection. However, urine collected directly from the bladder has yielded positive results, suggesting that chlamydial cystitis most likely occurs in humans (40). On the other hand, the possibility that epididymitis is not always the result of intracanalicular dissemination is supported by the fact that it has been observed in patients that have undergone contraceptive vasectomy (2). In addition to an obstruction of the epididymides, C. trachomatis may affect fertility by other mechanisms. For example, decrease in sperm motility and alteration of sperm capacitation by C. trachomatis have been reported in in vitro assays (11). These mechanisms may be important because, in general, chlamydial epididymitis elicits a minimal inflammatory response and, thus, obstruction is unlikely to occur (10). Therefore, using this model, the pathogenic mechanisms and the role that C. trachomatis may have in orchitis and male infertility could be evaluated.
This model should also help define the role of the innate and adaptive components of the immune system that contribute to controlling the infection. Several experimental models have shown that males can develop a strong protective immune response against a chlamydial infection. For example, Digiacomo et al. (8) inoculated the urethra of two sexually mature male baboons (Papio cynocephalus) with C. trachomatis serovar D and/or I. The animals were subsequently challenged with the same serovar or the alternate serovar and were found to be protected. Similarly, Howard et al. (12) and Patterson and Rank (30) initiated the characterization of the immune response to reinfection in the guinea pig model. Male animals inoculated in the urethra with the GPIC agent developed urethritis. When the male guinea pigs were subsequently challenged, they were found to be more resistant to reinfection, and for a longer period of time, than female guinea pigs. Therefore, vaccinating males may block transmission and, thus, potentially eradicate the infection.
In these experiments we have shown that following inoculation in the meatus urethra with C. trachomatis MoPn, male mice developed a strong humoral and cell-mediated immune response. IgM and IgG Chlamydia-specific antibodies were detected in serum, and EB were shown to elicit a significant proliferative response in lymphocytes from the local lymph nodes and the spleens of infected mice. A high IgG2a/IgG1 ratio and a predominance of IFN-γ production over IL-4 indicated that the mice developed a strong local and systemic Th1 response. In the murine female model, following a chlamydial genital infection, a predominant Th1 response has been described (22, 35). The findings reported here show that a similar situation occurs in this male murine model. Furthermore, we also determined that the group of chlamydial antigens recognized by serum antibodies of the infected male mice parallels those described in the female model (26). This information may help guide the search for common chlamydial antigens that can be used in vaccine formulations to protect both females and males.
This murine model has some characteristics that may restrict its utilization for certain types of experiments. For example, to follow the course of the infection we euthanized mice to culture the various organs. This approach requires a large number of animals and numerous cultures. A possible alternative would be to culture urine or to do nucleic acid amplification tests. This would significantly decrease the number of animals needed to perform the experiment and may be adequate in instances when only semiquantitative data are needed. Another shortcoming of this model is the small amount of tissue that can be harvested from the genitourinary tract of male mice to perform structural or functional assays. In situ immunohistochemical analysis and nucleic acid techniques may help address this limitation.
In conclusion, chlamydial infections in males are widespread throughout the world. Unfortunately, the amount of information currently available on the pathogenesis of, and immunity to, a C. trachomatis infection in males is scarce. This is due to several factors, including the difficulty in obtaining adequate samples from humans with these infections and the limitations of our current experimental models. Nonhuman primates are the obvious choice for characterizing these infections. However, the restricted availability and high costs of these animals makes working with them quite difficult (16). The guinea pig model offers a valuable alternative, but the current lack of reagents and of well-defined strains of animals may be a limitation. Infection of the genital tract of female mice with C. trachomatis MoPn has provided much-needed insights into the immunopathogenesis of these diseases. Implementation of a similar system of inoculating male mice by the natural route of infection could add invaluable information to our current limited knowledge of C. trachomatis infections in human males.
Acknowledgments
This work was supported by Public Health Service grant AI-32248 from the National Institute of Allergy and Infectious Diseases.
Editor: A. D. O'Brien
REFERENCES
- 1.Adler, M. W. 2003. Sexual health: health of the nation. Sex. Transm. Infect. 79:84-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, R. E. 1999. Acute epididymitis, p. 847-848. In K. K. Holmes et al. (ed.), Sexually transmitted diseases. McGraw Hill, New York, N.Y.
- 3.Berger, R. E., E. R. Alexander, J. P. Harnisch, C. A. Paulsen, G. D. Monda, J. Ansell, and K. K. Holmes. 1979. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J. Urol. 121:750-754. [DOI] [PubMed] [Google Scholar]
- 4.Berger, R. E., E. R. Alexander, G. D. Monda, J. Ansell, G. McCormick, and K. K. Holmes. 1978. Chlamydia trachomatis as a cause of acute “idiopathic” epididymitis. N. Engl. J. Med. 298:301-304. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cengiz, T., L. Aydoganli, M. Baykam, N. A. Mungan, E. Tuncbilek, M. Dincer, K. Yakupoglu, and Z. Akalin. 1997. Chlamydial infections and male infertility. Int. Urol. Nephrol. 29:687-693. [DOI] [PubMed] [Google Scholar]
- 7.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Digiacomo, R. F., J. L. Gale, S.-P. Wang, and M. D. Kiviat. 1975. Chlamydial infection of the male baboon urethra. Br. J. Vener. Dis. 51:310-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayston, J. T., and S.-P. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87-105. [DOI] [PubMed] [Google Scholar]
- 10.Hori, S., and Y. Tsutsumi. 1995. Histological differentiation between chlamydial and bacterial epididymitis: nondestructive and proliferative versus destructive and abscess forming-immunohistochemical and clinicopathological findings. Hum. Pathol. 26:402-407. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinzadeh, S., I. A. Brewis, A. A. Pacey, H. D. M. Moore, and A. Eley. 2000. Coincubation of human spermatozoa with Chlamydia trachomatis in vitro causes increased tyrosine phophorylation of sperm proteins. Infect. Immun. 68:4872-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard, L. V., M. P. O'Leary, and R. L. Nichols. 1976. Animal model studies of genital chlamydial infections. Immunity to re-infection with guinea pig inclusion conjunctivitis agent in the urethra and eye of male guinea pigs. Br. J. Vener. Dis. 52:261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingerslev, H. J., S. Walter, J. T. Andersen, P. Brandenhoff, J. Eldrup, J. P. Geerdsen, J. Scheibel, N. Tromholt, H. M. Jensen, and T. Hjort. 1986. A prospective study of antisperm antibody development in acute epididymitis. J. Urol. 136:162-164. [DOI] [PubMed] [Google Scholar]
- 14.Jantos, C. A., J. Augustin, B. Durchfeld-Meyer, W. Baumgartner, and H. G. Schiefer. 1998. Experimental genital tract infection with Chlamydia psittaci (GPIC agent) in male rats. Infection 26:126-130. [DOI] [PubMed] [Google Scholar]
- 15.Jantos, C. A., W. Baumgartner, B. Durchfeld, and H. G. Schiefer. 1992. Experimental epididymitis due to Chlamydia trachomatis in rats. Infect. Immun. 60:2324-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, A. P., and D. Taylor-Robinson. 1982. Chlamydial genital tract infection. Experimental infection of the primate genital tract with Chlamydia trachomatis. Am. J. Pathol. 106:132-135. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuzan, F. B., D. L. Patton, S. M. Allen, and C.-C. Kuo. A proposed mouse model for acute epididymitis provoked by genital serovar E, Chlamydia trachomatis. Biol. Reprod. 40:165-172. [DOI] [PubMed]
- 18.LaMontagne, D. S., D. N. Fine, and J. M. Marrazzo. 2003. Chlamydia trachomatis infection in asymptomatic men. Am. J. Prev. Med. 24:36-42. [DOI] [PubMed] [Google Scholar]
- 19.Martin, D. H., and W. R. Bowie. 1999. Urethritis in males, p. 761-781. In K. K. Holmes et al. (ed.), Sexually transmitted diseases. McGraw Hill, New York, N.Y.
- 20.Massari, V., O. Retel, and A. Flahault. 2002. A recent increase in the incidence of male urethritis in France. Sex. Transm. Dis. 29:319-323. [DOI] [PubMed] [Google Scholar]
- 21.Moller, B. R., and P.-A. Mardh. 1980. Experimental epididymitis and urethritis in grivet monkeys provoked by Chlamydia trachomatis. Fert. Steril. 34:275-279. [DOI] [PubMed] [Google Scholar]
- 22.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mount, D. T., P. E. Bigazzi, and A. L. Barron. 1973. Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect. Immun. 8:925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munday, P. E., J. M. Carder, and D. Taylor-Robinson. 1985. Chlamydial proctitis? Genitourin. Med. 61:376-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nigg, C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 99:49-50. [DOI] [PubMed] [Google Scholar]
- 26.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal, S., E. M. Peterson, and L. M. de la Maza. 1999. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect. Immun. 67:2607-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal, S., E. M. Peterson, and L. M. de la Maza. 2001. Susceptibility of mice to vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect. Immun. 69:5203-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pate, M. S., S. R. Hedges, D. A. Sibley, M. W. Russell, E. W. Hook, and J. Mestescky. 2001. Urethral cytokine and immune responses in Chlamydia trachomatis-infected males. Infect. Immun. 69:7178-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson, T. L., and R. G. Rank. 1996. Immunity to reinfection and immunization of male guinea pigs against urethral infection with the agent of guinea pig inclusion conjunctivitis. Sex. Transm. Dis. 23:145-150. [DOI] [PubMed] [Google Scholar]
- 31.Pearson, R. C., C. D. Baumber, D. McGhie, and I. V. Thambar. 1988. The relevance of Chlamydia trachomatis in acute epididymitis in young men. Br. J. Urol. 62:72-75. [DOI] [PubMed] [Google Scholar]
- 32.Pudney, J., and D. J. Anderson. 1995. Immunobiology of the human penile urethra. Am. J. Pathol. 147:155-165. [PMC free article] [PubMed] [Google Scholar]
- 33.Quinn, T. C., S. E. Goodell, E. Mkrtichian, M. D. Schuffler, S.-P. Wang, W. E. Stamm, and K. K. Holmes. 1981. Chlamydia trachomatis proctitis. N. Engl. J. Med. 305:195-200. [DOI] [PubMed] [Google Scholar]
- 34.Rank, R. G., H. J. White, B. L. Soloff, and A. L. Barron. 1981. Cystitis associated with chlamydial infection of the genital tract in male guinea pigs. Sex. Transm. Dis. 8:203-210. [DOI] [PubMed] [Google Scholar]
- 35.Rank, R. G. 1999. Models of immunity, p. 239-296. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 36.Schachter, J. 1999. Infection and disease epidemiology, p. 139-170. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 37.Schachter, J., and C. Dawson. 1978. Human chlamydial infections. PSG Publishing Company, Inc., Littleton, Mass.
- 38.Schagger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 39.Stamm, W. E. 1999. Chlamydia trachomatis infections of the adult, p. 407-422. In K. K. Holmes (ed.), Sexually transmitted diseases. McGraw Hill, New York, N.Y.
- 40.Stamm, W. E., K. F. Wagner, R. Amsel, E. R. Alexander, M. Turck, G. W. Counts, and K. K. Holmes. 1980. Causes of acute urethral syndrome in women. N. Engl. J. Med. 303:409-415. [DOI] [PubMed] [Google Scholar]
- 41.Stratton, K. R., J. S. Durch, and R. S. Lawrence. 2000. Vaccines for the 21st century: a tool for decisionmaking, p. 149-158. Institute of Medicine, National Academy Press, Washington, D.C. [PubMed]
- 42.Taylor-Robinson, D., R. H. Purcell, W. T. London, D. L. Sly, B. J. Thomas, and R. T. Evans. 1981. Microbiological, serological, and histopathological features of experimental Chlamydia trachomatis urethritis in chimpanzees. Br. J. Vener. Dis. 57:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]