Abstract
Background:
Sumac (Rhus coriaria L.) has been used in traditional treatment of some diseases. The aim of this study was to determine the effect of sumac (R. coriaria L.) powder on insulin resistance (IR), malondialdehyde (MDA), high sensitive C-reactive protein (hs-CRP), and paraoxonase 1 (PON1) activity in type 2 diabetic patients.
Materials and Methods:
A double-blind randomized placebo controlled trial on 41 type 2 diabetic volunteers was conducted. Participants randomly assigned into 3 g per day sumac powder (n = 22) or placebo (n = 19) groups for 3 months. IR was assessed using the homeostatic model assessment of IR (HOMA-IR), which including measurement of insulin by immunoassay method and measurement of glucose by enzymatic method. MDA and PON1 activity were measured colorimetrically, hs-CRP turbidimetrically.
Results:
There were a significant increase in PON1 activity (from 84.72 ± 30.59 to 92.91 ± 32.63) and significant decrease in insulin (from 7.09 ± 4.28 to 5.32 ± 3.22), HOMA-IR (from 2.56 ± 1.58 to 1.67 ± 0.94), MDA (from 2.71 ± 0.73 to 1.97 ± 0.49), and also hs-CRP (from 18.49 ± 16.96 to 15.89 ± 16.70) in the sumac group at the end of study compared with initial values (P < 0.05). Furthermore, there were significant differences in MDA and PON1 between the two groups at the end of the study (P < 0.05). Furthermore, the mean of differences of insulin, HOMA-IR, MDA, hs-CRP and PON1 activity between groups were significant (P < 0.05).
Conclusion:
We concluded that daily intake of 3 g sumac for 3 months may be beneficial for diabetic patients to make them less susceptible to cardiovascular disease.
Keywords: High sensitive C-reactive protein, insulin resistance, malondialdehyde, paraoxonase 1, sumac (Rhus coriaria L.), type 2 diabetes
INTRODUCTION
Type 2 diabetes is a well-known endocrine and metabolic disorder, which has reached epidemic proportions worldwide and represents a serious public health concern.[1] The prevalence of type 2 diabetes is reported to be more than 14% in Tehran, Iran, with an estimated incidence of new cases in about 1% of population per year.[2]
Since ancient times, spices and herbs have also been used in traditional treatment of some diseases. Now-a-days, several experimental studies and to a lesser extent, clinical trials have also emphasized the role of herbs in the treatment of a variety of disorders.[3] Rhus coriaria L. is a shrub known as “sumac,” belonging to the family of Anacardiaceae. It is widespread in Spain, Southern Italy and Turkey and from the Middle Eastern countries to Afghanistan.[4] In Iran, R. coriaria is traditionally used as a table spice especially along with rich dishes.[5] The research efforts on sumac extracts to date indicate a promising potential for this plant family to provide renewable bioproducts with the following reported desirable bioactivities: Antifibrogenic, antifungal, antiinflammatory, antimalarial, antimicrobial, antimutagenic, antioxidant, antithrombin, antitumorigenic, antiviral, cytotoxic, hypoglycemic, and leukopenic.[6]
It is well-documented that reactive oxygen species (ROS) play a key role in the etiology of a number of human diseases including cancer, age-related disorders, neurodegenerative diseases and diabetes. Therefore, strong efforts have been made over the last decades to identify dietary components, which protect against the consequences of oxidative damage and numerous ROS-protective phytochemicals have been detected.[7] Previous studies have suggested that extract of R. coriaria L. fruits may be a source of natural antioxidants.[8] The antioxidant activity of fruit extracts of R. coriaria has been demonstrated in cell-free models of oxidative stress.[4] A number of in vitro and animal investigations were published concerning antioxidant effects of sumac[7,9,10] that it is problematic to extrapolate such findings to human. Oral administration of ethanolic extract of R. coriaria fruits increased superoxide dismutase (SOD) and catalase (CAT) levels without changes in the transcript levels of insulin in rats.[5] According to our knowledge, the only human study was carried out in healthy humans, and the results indicate that the protection against oxidative DNA-damage and suggest that gallic acid (GA) is may be an account for sumac effects.[7] GA is one of the main constituents of sumac,[6] which possesses potent antioxidant properties.[9] Sumac is also a rich source of hydrolysable tannins. Tannin and its derivatives are strong antioxidants.[8]
Paraoxonase 1 (PON1) is an esterase associated with high-density lipoprotein (HDL) particle containing clusterin and apolipoprotein A-I in human serum, which protects lipoprotein particles from free radical oxidation and oxidative stress.[11] Serum PON1 activity are low in diabetic patients.[12] We didn’t find any study about the effect of sumac on PON1 and high sensitive C-reactive protein (hs-CRP) in diabetes. In this present study, we have investigated the effect of sumac (R. coriaria L.) powder on insulin resistance (IR), malondialdehyde (MDA), hs-CRP and PON1 activity in type 2 diabetic patients.
MATERIALS AND METHODS
Study design and participants
We recruited study participants between June 2011 and November 2011 from Iranian Diabetes Association, Tehran, Iran. Sample size was determined based on the difference in blood glucose level (d: 50 mg/dl) from the study of Jung et al.[13] by considering the type I error level α = 0.05 with test power of 80% and. The sample size was computed as 19 per group. Regarding a possible loss to follow-up, a safety margin of 20% was determined, and therefore, 23 patients were allocated in each group. Participants were selected based on inclusion criteria (age: 20-60 years, hemoglobin A1c 6-8%, at least 2 years duration of diabetes, diabetes was diagnosed with endocrinologist) who were all nonsmokers, no alcohol and nonusers of antioxidant supplements at least 3 months before the intervention. Of a total of 87 participants initially selected, 46 participants who met the above inclusion criteria [Figure 1] were recruited and were randomly allocated either to the placebo or sumac groups by using computer's random numbers. Investigators and participants were blinded to group assignment and to capsule content. Patients were excluded if they had a reluctance to continue working with, a change in the type or dose of medication, changes in diet or physical activity daily, taking any antioxidant supplement, consuming <80% of supplements delivered to the patient at the baseline, recent receiving insulin, pregnancy and lactation, kidney, liver, thyroid diseases, and anemia.
Figure 1.
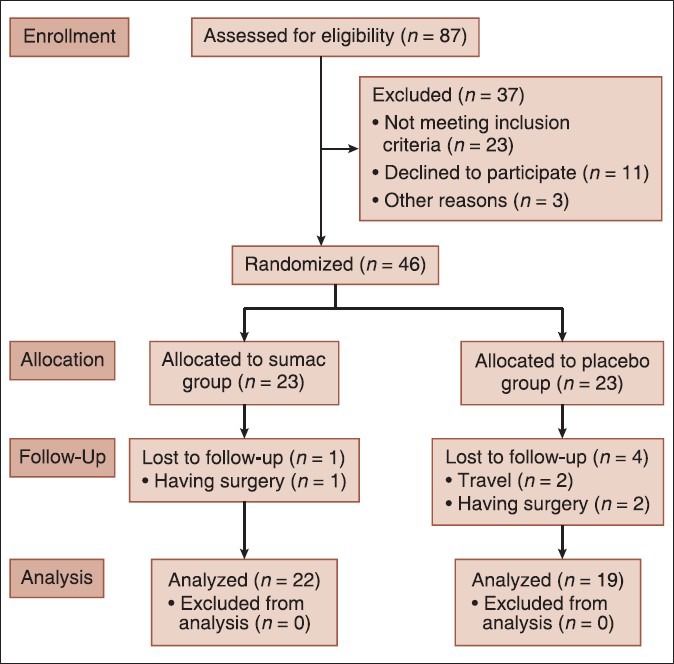
Follow of participants throughout the intervention
The Ethics Committee of the Iran University of Medical Sciences approved the study, and all participants signed consent form.
Procedures
Purified sumac was bought from East Azarbaijan Province located in Iran. During the intervention, each participant consumed 3 g pure sumac powder or the same amount of lactose over 3 months. All of the capsules were made by faculty of pharmacy, Tehran University of Medical Sciences. During the intervention, each participant consumed six capsules three times a day tow capsules with each meal (breakfast, lunch and dinner) over 3 months. Dietary intake was asked from participants to complete a 24-h diet recall questionnaire, in the three 1st days of the study (an off day and two working days) and the three last days of the study. Physical activity was measured by an International Physical Activity Questionnaire[14] at low, medium and high levels, at the baseline and the end of the 3 months intervention. Moreover, anthropometric measurements assessed before and after study. The patients were followed-up by telephone each week. After 12-14 h under fasting condition, the blood sample was collected from each participant in the beginning and the end of the intervention.
After serum preparation, IR was assessed using the homeostatic model assessment of IR (HOMA-IR) which including measurement of insulin by immunoassay method using commercially available kit (Roche, Germany) and measurement of glucose by enzymatic method using commercially available kit (Elitech, France) which performed on Hitachi 717 auto analyzer. MDA was determined using a spectrophotometric method as described by Satoh.[15] Hs-CRP was measured by turbidimetric method using commercially available kit (Roche, Germany) on the Cobas analyzer. PON1 activity was also measured by colorimetric method.[16]
Statistical analysis
The data are presented as means ± standard deviation. Statistical significance was set at P < 0.05. All analyses were performed using SPSS statistical software (version 16, SPSS). All data was assessed for normality of distribution before statistical analysis via Kolmogorov–Smirnov test. The parametric test was used for those variables that were normally distributed. Significant differences between groups were determined by using an independent t test. Paired t-test was used for comparing means of the variables within a group before and after intervention. All variables had a normal distribution except hs-CRP and Mann–Whitney test was used for comparing. We also calculated the mean of differences before and after intervention between groups by using an independent t-test.
RESULTS
From 46 patients, three participants were lost for having surgery, and two participants were excluded due to travel. Of the 41 diabetic patients, 22 (7 males and 15 females) were in the sumac group, and 19 (9 males and 10 females) were in the placebo group.
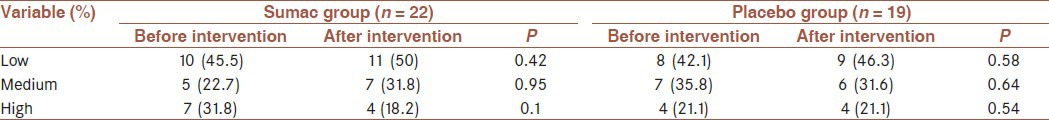
The percent of participants in sumac group who used metformin, glibenclamide and both agents was 51%, 6% and 43%, respectively and in the placebo group was 38%, 4% and 58%, respectively. Baseline characteristics did not significantly differ between the two groups [Table 1]. There were no significant differences in energy and micronutrients intake between groups at baseline [Table 2] and did not change during the study that were tested by independent samples t-test. Physical activity levels were tested by Chi-square test that did not significantly differ between groups at baseline and did not change during the study [Table 3].
Table 1.
Baseline characteristics in sumac and placebo groups
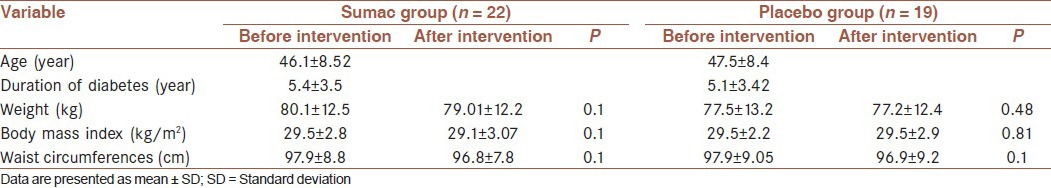
Table 2.
Total energy and micronutrients intake in sumac and placebo groups
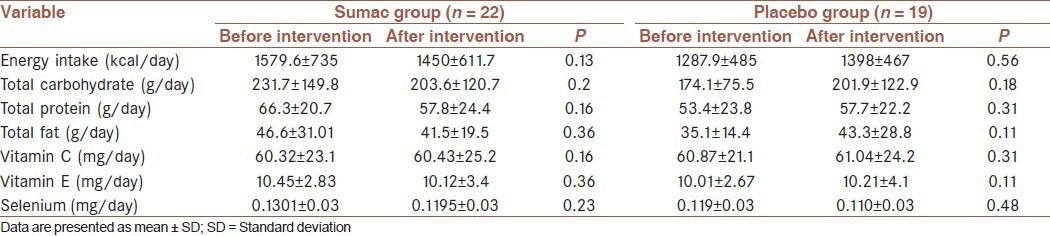
Table 3.
Physical activity levels in sumac and placebo groups
Any complications or side-effects of sumac in the treatment group did not report.
Effect of sumac powder on insulin and homeostatic model assessment of insulin resistance
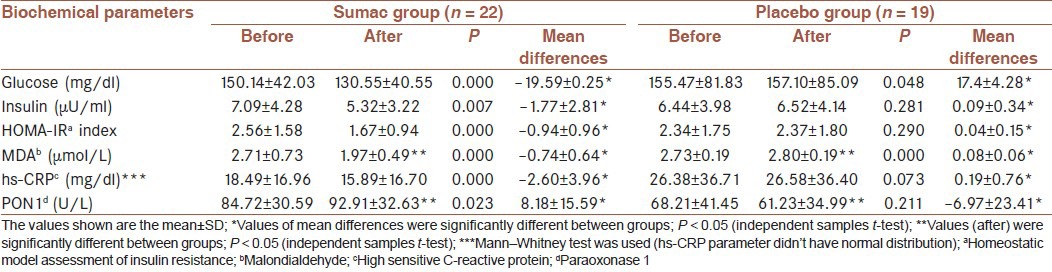
Results of insulin and HOMA-IR are presented in Table 4. In the sumac group, there was a significant decrease in insulin (P < 0.05) and HOMA-IR (P < 0.05) after intervention compared with initial values. The mean of differences were significant between groups (P < 0.05).
Table 4.
Serum glucose, insulin, HOMA-IR, MDA, hs-CRP and PON1 activity in sumac and placebo groups before and after the intervention
Effect of sumac powder on malondialdehyde
As shown in Table 4, MDA was decreased (P < 0.05) in sumac group and increased (P < 0.05) in the placebo group after intervention compared with initial values. There was a significant difference in MDA between the two groups at the end of the study (P < 0.05) [Table 4]. The mean of differences of MDA between groups were significant (P < 0.05).
Effect of sumac powder on high sensitive C-reactive protein and paraoxonase 1
In the sumac group, there was a significant decrease in hs-CRP (P < 0.05), a significant increase in PON1 (P < 0.05) after intervention compared with initial values [Table 4].
There was a significant difference in PON1 between the two groups at the end of the study (P < 0.05) [Table 4]. The mean of differences of hs-CRP and PON1 were significant between groups (P < 0.05).
DISCUSSION
The results of this study demonstrate that intake of 3.0 g/daily sumac powder for 3 months leads to a significant increase in PON1 activity and a significant decrease in serum insulin, MDA and hs-CRP in type 2 diabetic patients. Furthermore, the mean of differences of insulin, HOMA-IR, MDA, hs-CRP and PON1 activity between groups were significant (P < 0.05). There were no significant differences in any of the baseline parameters between the two groups, which made them comparable in all variables at baseline.
Insulin resistance is a fundamental metabolic disorder that independently increases the risk for coronary heart disease.[17] Accurate prediction of the presence of IR is crucial in clinical practice so as to identify and treat patients at risk for cardiovascular disorders.[18] The significant decrease in insulin and HOMA-IR in our study was a favorite result for decreasing the cardiovascular disease (CVD) risk. However, the effects of sumac on insulin in animal has been examined but in human have not been reported. Mohammadi et al. reported a single dose of administration of the extract of R. coriaria L. fruits made no changes in the transcript levels of insulin in rats and antihyperglycemic effects of R. coriaria fruits may be related to modulation of insulin secretion or action.[5] Evidence from various studies indicates that flavonoids may preserve β-cell function by reducing oxidative stress-induced tissue damage and therefore protect against the progression of IR to type 2 diabetes.[19] It seems that a diet full of antioxidant and phytochemical have beneficial effects on increasing insulin sensitivity,[20] thereby preventing the progression to overt type 2 diabetes.
Data obtained from the literature indicated that only certain food components are protective against ROS in humans and animals.[7] Sumac has been found to show antioxidant capacity.[9] Both in vitro and vivo studies observed that sumac (R. coriaria L.) could inhibit oxidative stress.[7,9,10] The antioxidant activity of fruit extracts of R. coriaria has been demonstrated in cell-free models of oxidative stress (inhibition of superoxide anion formation from the xanthine/xanthine oxidase system, inhibition of lipid peroxidation, etc.,)[4] Results from Candan and Sökmen study suggest that the methanolic extracts of R. coriaria L. fruits have considerable antioxidant activity against free radicals and lipid peroxidation in vitro.[10] The only human study was published by Chakraborty et al. that showed the antioxidant effects of the extract that protects healthy humans against oxidative DNA-damage and suggest that GA is may be an account for sumac effects.[7] Decrease of MDA in our study is similar to the findings of in vitro and animal study.[10,21] Similarly, treatment with the fraction of sumac containing sulfuretin significantly decreased MDA formation in rats.[22]
Sumac extract was reported to be a source of natural antioxidants. The methanolic extracts of R. retinorrhaea Steud. showed a remarkable radical scavenging effect even at low concentrations. A crude extract of R. coriaria exhibits interesting antioxidant properties, expressed by the capacity to either scavenge superoxide radical or uncompetitively inhibit xanthine oxidase.[10] One of the main constituents of sumac is GA,[6] which possesses potent antioxidant properties.[9] Sumac contains various substances, and exactly tannin and GAs may be responsible for the antioxidant ability of this plant.[9] Rhus verniciflua stokes contains six compounds that this extract could contribute to the antioxidant activities and inhibition of intracellular ROS level.[21] R. coriaria L., which contains many polyphenols, may prevent diabetes by inhibiting ROS, but further studies are required to show the most active constituent of sumac fruit.
The significant decrease in hs-CRP in our study can prevent individuals at high risk of developing ischemic heart disease (IHD).[23] Choi et al. study was consistent with our results that oral administration of the extract of R. verniciflua slightly decreased CRP factor in rats and also oral administration (30 mg/kg) of sulfuretin and fustin, which were isolated from the extract significantly decreased CRP factor.[22] Elevated plasma hs-CRP has also been associated with obesity, IR, and hyperglycemia, suggesting that IR, type 2 diabetes, and IHD may be consequences of the ongoing acute phase response, reflecting a chronic adaptation of the immune system.[23] In our study, it seems that the hs-CRP-reducing effect of sumac was due to the decrease of insulin level. A limitation of our study is other unmeasured inflammatory factors. This present study is probably the first study documenting a lowering effect of sumac on hs-CRP level in human. Therefore, more experiments should be performed on human trials to prove this idea.
Significant increase in serum PON1 activity in sumac group compared with placebo group provides beneficial effects against atherosclerosis by protection of HDL from peroxidation and plasma membranes from free radical injury.[24] This effect may be one of the antiatherosclerotic activities of sumac, and protection of low-density lipoprotein (LDL) against oxidation by sumac may also be related to its inducing effect on PON1. The studies have demonstrated that dietary interventions with flavonoids modulate PON1 activity.[24,25] Polyphenols from pomegranate juice increase the stability of binding of PON1 to HDL, thus preserving its activity[25] and the grape seed extract increased paraoxonase activities in streptozotocin-induced diabetic rats.[24] Moreover, recent investigations of human cell lines have revealed upregulation of PON1 expression and increased arylesterase activity in hepatic cells exposed to resveratrol, an ingredient of common red wine.[26,27] Similarly, rats fed quercetin showed upregulation of PON1 hepatic expression and increased paraoxonase activity, which was associated with improved protection against LDL oxidation.[28] Overall, both paraoxonase and arylesterase were lower in diabetic patients and a flavonoid-rich diet is positively associated with PON1 arylesterase activity in them.[12] Flavonoids can achieve this beneficial effect via several mechanisms. In vitro copper-induced loss of PON1 arylesterase activity in micellar solution is prevented by coincubation with the flavonoids quercetin and glabridin, but not with α-tocopherol. These flavonoids act by protecting the free sulfhydryl group of the enzyme from oxidation.[12]
CONCLUSION
This study is the first clinical trial of the effects of sumac on IR, MDA, hs-CRP and PON1 activity in type 2 diabetic patients. We concluded that daily intake of 3 g sumac for 3 months may be beneficial for diabetic patients to make them less susceptible to CVD. However, further investigations on the effect of sumac consumption in healthy and diabetic humans are needed to support this suggestion.
LIMITATIONS OF THE STUDY
Our trial had some limitations. First, in spite of using both genders in our study along with the greater number of participants, significant differences might have been recognized better between groups. Second, we have not measured the antioxidants enzymes such as CAT and SOD (because of budget and financial limitations).
AUTHOR'S CONTRIBUTION
STR contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FS contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. NK contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. AR contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. SPH contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. SMM contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENT
We would like to thank all volunteers who participated in this study. This study was financed by the Research Institute for Islamic and Complementary Medicine of Iran the University of Medical Sciences (Project Number: 90-01-116-13338). IRCT number was 201201172709N24.
Footnotes
Source of Support: Research Institute for Islamic and Complementary Medicine of Iran University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Shidfar F, Ebrahimi SS, Hosseini S, Heydari I, Shidfar S, Hajhassani G. The Effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, Homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran J Pharm Res. 2012;11:643–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Shidfar F, Heydari I, Hajimiresmaiel SJ, Hosseini S, Shidfar S, Amiri F. The effects of cranberry juice on serum glucose, apoB, apoA-I, Lp(a), and Paraoxonase-1 activity in type 2 diabetic male patients. J Res Med Sci. 2012;17:355–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Al Mofleh IA. Spices, herbal xenobiotics and the stomach: Friends or foes? World J Gastroenterol. 2010;16:2710–9. doi: 10.3748/wjg.v16.i22.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beretta G, Rossoni G, Santagati NA, Facino RM. Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta. Planta Med. 2009;75:1482–8. doi: 10.1055/s-0029-1185797. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi S, Montasser Kouhsari S, Monavar Feshani A. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru. 2010;18:270–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Rayne S, Mazza G. Biological activities of extracts from sumac (Rhus spp.): A review. Plant Foods Hum Nutr. 2007;62:165–75. doi: 10.1007/s11130-007-0058-4. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty A, Ferk F, Simic T, Brantner A, Dusinská M, Kundi M, et al. DNA-protective effects of sumach (Rhus coriaria L.), A common spice: Results of human and animal studies. Mutat Res. 2009;661:10–7. doi: 10.1016/j.mrfmmm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. Against oxidative stress cytotoxicity. Food Chem Toxicol. 2010;48:854–8. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Capcarova M, Slamecka J, Abbas K, Kolesarova A, Kalafova A, Valent M, et al. Effects of dietary inclusion of Rhus coriaria on internal milieu of rabbits. J Anim Physiol Anim Nutr (Berl) 2012;96:459–65. doi: 10.1111/j.1439-0396.2011.01164.x. [DOI] [PubMed] [Google Scholar]
- 10.Candan F, Sökmen A. Effects of Rhus coriaria L (Anacardiaceae) on lipid peroxidation and free radical scavenging activity. Phytother Res. 2004;18:84–6. doi: 10.1002/ptr.1228. [DOI] [PubMed] [Google Scholar]
- 11.Shidfar F, Ehramphosh E, Heydari I, Haghighi L, Hosseini S, Shidfar S. Effects of soy bean on serum paraoxonase 1 activity and lipoproteins in hyperlipidemic postmenopausal women. Int J Food Sci Nutr. 2009;60:195–205. doi: 10.1080/09637480701669463. [DOI] [PubMed] [Google Scholar]
- 12.Lixandru D, Mohora M, Coman A, Stoian I, van Gils C, Aerts P, et al. Diet and paraoxonase 1 enzymatic activity in diabetic foot patients from Romania and Belgium: Favorable association of high flavonoid dietary intake with arylesterase activity. Ann Nutr Metab. 2010;56:294–301. doi: 10.1159/000298879. [DOI] [PubMed] [Google Scholar]
- 13.Jung CH, Zhou S, Ding GX, Kim JH, Hong MH, Shin YC, et al. Antihyperglycemic activity of herb extracts on streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2006;70:2556–9. doi: 10.1271/bbb.60238. [DOI] [PubMed] [Google Scholar]
- 14.Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Hoshiarrad A, et al. Vitamin D3 and the risk of CVD in overweight and obese women: A randomised controlled trial. Br J Nutr. 2012;108:1866–73. doi: 10.1017/S0007114512000098. [DOI] [PubMed] [Google Scholar]
- 15.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 16.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–6. [PubMed] [Google Scholar]
- 17.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, et al. ApoB/apoA-I ratio: An independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J. 2007;28:2637–43. doi: 10.1093/eurheartj/ehm360. [DOI] [PubMed] [Google Scholar]
- 18.Ying X, Qian Y, Jiang Y, Jiang Z, Song Z, Zhao C. Association of the apolipoprotein B/apolipoprotein A-I ratio and low-density lipoprotein cholesterol with insulin resistance in a Chinese population with abdominal obesity. Acta Diabetol. 2012;49:465–72. doi: 10.1007/s00592-012-0419-9. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–84. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 20.Hinderliter AL, Babyak MA, Sherwood A, Blumenthal JA. The DASH diet and insulin sensitivity. Curr Hypertens Rep. 2011;13:67–73. doi: 10.1007/s11906-010-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung CH, Jun CY, Lee S, Park CH, Cho K, Ko SG. Rhus verniciflua stokes extract: Radical scavenging activities and protective effects on H2O2-induced cytotoxicity in macrophage RAW 264.7 cell lines. Biol Pharm Bull. 2006;29:1603–7. doi: 10.1248/bpb.29.1603. [DOI] [PubMed] [Google Scholar]
- 22.Choi J, Yoon BJ, Han YN, Lee KT, Ha J, Jung HJ, et al. Antirheumatoid arthritis effect of Rhus verniciflua and of the active component, sulfuretin. Planta Med. 2003;69:899–904. doi: 10.1055/s-2003-45097. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–8. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 24.Kiyici A, Okudan N, Gökbel H, Belviranli M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J Med Food. 2010;13:725–8. doi: 10.1089/jmf.2009.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhrman B, Volkova N, Aviram M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition. 2010;26:359–66. doi: 10.1016/j.nut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Gouédard C, Barouki R, Morel Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler Thromb Vasc Biol. 2004;24:2378–83. doi: 10.1161/01.ATV.0000146530.24736.ce. [DOI] [PubMed] [Google Scholar]
- 27.Curtin BF, Seetharam KI, Dhoieam P, Gordon RK, Doctor BP, Nambiar MP. Resveratrol induces catalytic bioscavenger paraoxonase 1 expression and protects against chemical warfare nerve agent toxicity in human cell lines. J Cell Biochem. 2008;103:1524–35. doi: 10.1002/jcb.21543. [DOI] [PubMed] [Google Scholar]
- 28.Gong M, Garige M, Varatharajalu R, Marmillot P, Gottipatti C, Leckey LC, et al. Quercetin up-regulates paraoxonase 1 gene expression with concomitant protection against LDL oxidation. Biochem Biophys Res Commun. 2009;379:1001–4. doi: 10.1016/j.bbrc.2009.01.015. [DOI] [PubMed] [Google Scholar]


