Abstract
Human brucellosis can be acquired from infected animal tissues by ingestion, inhalation, or contamination of conjunctiva or traumatized skin by infected animal products. In addition, Brucella is recognized as a biowarfare threat agent. Although a vaccine to protect humans from natural or deliberate infection could be useful, vaccines presently used in animals are unsuitable for human use. We tested orally administered live, attenuated, purine auxotrophic B. melitensis WR201 bacteria for their ability to elicit cellular and humoral immune responses and to protect mice against intranasal challenge with B. melitensis 16M bacteria. Immunized mice made serum antibody to lipopolysaccharide and non-O-polysaccharide antigens. Splenocytes from immunized animals released interleukin-2 and gamma interferon when grown in cultures with Brucella antigens. Immunization led to protection from disseminated infection and enhanced clearance of the challenge inoculum from the lungs. Optimal protection required administration of live bacteria, was related to immunizing dose, and was enhanced by booster immunization. These results establish the usefulness of oral vaccination against respiratory challenge with virulent Brucella and suggest that WR201 should be further investigated as a vaccine to prevent human brucellosis.
Human brucellosis, caused mostly by Brucella abortus, B. melitensis, and B. suis, can be acquired by ingestion, inhalation, or contamination of conjunctiva or traumatized skin by infected animal products (3). Bacteria spread, presumably via lymphatics and blood (8), from the site of entry to the mononuclear phagocyte system. Although generalized symptoms of fever, sweating, and fatigue are nearly universal for patients with acute brucellosis, onset can be insidious and many patients present with or develop localized foci of infection, especially in the bones and joints (24). Control of brucellosis in domestic food animals has markedly reduced the incidence of human brucellosis in the United States, but the disease represents an important cause of morbidity worldwide. A human vaccine would be valuable for individuals who may be occupationally exposed to brucellae and for persons who consume unpasteurized dairy products from areas in which brucellae are endemic. In addition, Brucella species are recognized as biowarfare or bioterror threat agents by the Center for Disease Control, further supporting the need to develop effective medical protective measures against them.
We have previously reported that levels of B. melitensis WR201, a purEK deletion mutant of B. melitensis 16M, are attenuated for growth in mononuclear phagocytes (5) and in mice (4) after intraperitoneal (i.p.) inoculation relative to parent strain results. Mice inoculated i.p. with strain WR201 make antibody directed against lipopolysaccharide (LPS) and Brucella protein, and their splenocytes produce gamma interferon (IFN-γ) and interleukin-2 (IL-2) when grown in cultures with Brucella antigens (11). In addition, immunization of mice by i.p. inoculation of WR201 reduces the intensity of spleen infection after i.p. challenge with strain 16M and prevents dissemination of bacteria to spleen and liver after intranasal (i.n.) challenge. Immunization with strain WR201 via the i.p. route also modestly accelerates the clearance of strain 16M from the lung after i.n. challenge (11). While these data are encouraging for demonstrating attenuation, immunogenicity, and efficacy, the i.p. route of immunization is unlikely to be popular for a human vaccine. Moreover, administration of partially attenuated Brucella vaccines to humans via subcutaneous inoculation or scarification leads to substantial local reactivity (22). For these reasons, we examined the utility of oral vaccination with strain WR201. Oral vaccination would be more convenient, would reduce potential adverse effects occasioned by parenteral vaccination, and might also provide additional protection by stimulating the common mucosal immune system as well as inducing systemic immunity.
In the present report, we show that levels of strain WR201 are attenuated relative to strain 16M levels when administered orally and induces cellular, humoral, and mucosal immune responses. Oral immunization leads to protection against systemic spread of bacteria and enhanced clearance of bacteria from the lungs following i.n. challenge with strain 16M. Comparisons of inoculum doses, single or booster immunizations, and use of live or killed bacteria indicated the feasibility of an oral live, attenuated vaccine approach and suggest that purine auxotrophy is an attractive attenuating strategy for further vaccine development.
MATERIALS AND METHODS
Bacterial strains.
B. melitensis 16M was obtained from Gerhardt Shurig (Virginia Polytechnic Institute, Blacksburg, Va.). Strain WR201, a purine auxotroph, was derived from strain 16M (5). Storage and preparation of primary and secondary cultures have been described previously (12).
Animals.
Female BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). All animals were fed food and water ad libitum and maintained in laminar flow racks under conditions of 12 h of light and 12 h of darkness in BSL-3 facilities. Experiments were conducted with 8- to 10-week-old age-matched animals.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition.
Immunization and challenge of mice.
For oral inoculation, secondary stock was grown overnight in shaker flasks in brucella broth at 37°C. A total of 3 to 4 ml of this broth culture was plated on multiple 15-cm-diameter petri dishes containing brucella agar. After 3 days of incubation at 37°C with 5% CO2, plates were harvested with saline. Brucella bacteria were pelleted, washed twice, and standardized to 5 × 1011 cells/ml by measurement of optical density (OD). The actual viable inoculum was confirmed by dilution and plating on brucella agar and ranged from 6.4 ×1010 to 1.7 ×1011 CFU. A total of 0.2 ml of this suspension was given to mice 15 min after oral administration of 0.2 ml of sterile 2.5% sodium bicarbonate via a 20-gauge disposable feeding needle attached to a 1-ml syringe. Animals were not anesthetized during immunization. In designated experiments, bacteria were further diluted to provide an inoculum of 1010 or 109 CFU. In one experiment, strain WR201 was killed by treatment overnight at room temperature with 0.8% (vol/vol) formaldehyde prior to administration to mice.
For virulent strain 16M i.n. challenge, 30 μl of bacterial suspension adjusted to contain 104 CFU of bacteria by reading the OD at 600 nm (OD600) was administered with a micropipette dropwise into the external nares to mice that were anesthetized with xylazine and ketamine. Before i.n. challenge, three to five mice from each immunized and nonimmunized group were euthanized by CO2 inhalation. Sera were collected for determining Brucella-specific antibody, and spleens were grown in cultures for the detection of any surviving vaccine strain.
Serologic methods.
To measure anti-Brucella-specific antibody in sera, blood was taken at different time points and serum samples were harvested and stored at −20°C. Levels of Brucella anti-LPS and antiprotein antibody in serum were measured by enzyme-linked immunosorbent assay (ELISA) performed as described previously (11). Antibody titers were calculated using the dilution of serum that gave an A410 reading nearest to 0.5 (which falls within the linear part of the OD dilution curve). The titer (expressed as OD units) was obtained by multiplying the reciprocal dilution of the serum by the actual A410 at that dilution. To measure salivary immunoglobulin A (IgA), 50 to 100 μl of saliva samples from five mice in each group was collected with a micropipette after i.p. injection of 70 to 100 μg of pilocarpine (Sigma, St. Louis, Mo.) to induce salivation (9). Total salivary Brucella LPS-specific IgA levels were measured by ELISA using the same method used for analysis of serum antibody levels.
Determination of splenocyte cytokine production.
In selected experiments, individual spleens from five naïve control mice or animals immunized with strain WR201 8 weeks earlier were grown in cultures as described previously (11). A crude bacterial lysate (RFBL) was prepared from a 48-h culture of B. melitensis WRR51, a rough mutant made from strain 16M by deletion of wboA, a gene that encodes glycosyltransferase, required for synthesis of the O-polysaccharide component of LPS. After the bacteria were washed in 0.9% NaCl, the pellet was lysed in 0.5% sodium dodecyl sulfate and dialyzed (molecular mass cutoff, 12 to 14 kDa) with saline. The protein content of the remaining lysate was determined at 1.8 mg/ml by a Bio-Rad protein assay. Cells from both immunized and saline-treated mice were grown in cultures with 200 ng of bacterial lysate/ml for 24 h. After 24 h, culture supernatant fluids were filter sterilized, frozen, and analyzed by ELISA using monoclonal antibody pairs, cytokine standards, and protocols obtained from Pharmingen (San Diego, Calif.) for interleukin-2 (IL-2) and/or gamma interferon (IFN-γ) levels. Cytokine assays were performed in duplicate for each sample, and the concentration of each cytokine in the original sample of supernatant fluid was expressed in picograms per milliliter by reference to the standard curve.
Quantitation of brucellae in lungs, livers, and spleens.
At various times after oral immunization with strain WR201 or challenge with strain 16M, mice were euthanized by CO2 inhalation, serum was collected, and spleen, lungs, and livers were removed. Organs were suspended in 1 ml of sterile 0.9% NaCl and individually homogenized in tissue grinders. A total of 0.5 ml of undiluted homogenates and 10 μl of serial 10-fold saline dilutions of homogenates was grown in cultures on brucella agar containing bacitracin (25 U/ml) and polymyxin B (5 U/ml). After incubation for 3 to 5 days at 37°C, the Brucella colonies were enumerated and the number of CFU per organ was calculated from the dilutions. Organs resulting in no colonies on the first plate were considered noninfected but were assigned a log value of 0 for calculation purposes.
Fecal culture.
Four fresh fecal pellets from five animals were collected in a tube containing 1 ml of 0.9% NaCl and vortexed. The fecal suspension was then plated on brucella agar plates containing bacitracin (25 U/ml), polymyxin B (5 U/ml), and kanamycin (50 μg/ml) to select for strain WR201, which is kanamycin resistant (5). The intensity of bacterial growth was graded from 1+ to 4+, since even with this selective medium the presence of multiple contaminants limited our ability to quantitate the number of fecal brucellae accurately.
Statistical methods.
Quantitative culture data (intensity of infection) at each time point were expressed as mean log CFU ± standard deviation (SD) for each group. Cytokine content of culture supernatant fluids was expressed as mean cytokine concentration ± SD for each group of five mice. The significance of differences between groups was determined by Student's t test or by analysis of variance (ANOVA) with Tukey's multiple pairwise comparisons when more than two groups were analyzed simultaneously. For cytokine data, values were log normalized (log of 1 + cytokine content) prior to calculation to improve normality. Frequency of organ infection was expressed as the fraction of infected organs versus total organs, and the significance of differences between groups was determined by Fisher's exact test. Statistical analyses were performed using Minitab software. A P value of <0.05 was regarded as significant.
RESULTS
Selection of optimal immunizing dose.
In preliminary studies, oral administration of 107, 108, or 109 CFU of strain 16M resulted in dose-related infection of spleens and livers (data not shown). Only a minority of animals inoculated with the lower doses became infected. In contrast, all animals (five per time point) inoculated orally with 109 CFU had infected spleens at 2, 4, and 8 weeks (2.3 [± 0.4], 4.6 [± 0.1], and 4.6 [± 0.3] log CFU, respectively). These same animals all had infected livers at 4 and 8 weeks. On the basis of this information and previous studies demonstrating attenuation of strain WR201 levels when given i.p. (4), we administered 109, 1010, or 1011 CFU of WR201 orally to groups of 20 mice. Administration of a single oral dose of 1010 or 1011 CFU of WR201 led to infection of lungs, livers, and/or spleens in all animals (Table 1 and Fig. 1). Spleens were consistently infected beginning 1 week after oral inoculation. Recovery of bacteria from lungs was variable over the entire 4-week period. Livers were infected early but cleared by 4 weeks. At a dose of 109 CFU, infection in all organs was variable (Table 1) and was markedly reduced in spleens compared to the results seen with doses of either 1010 or 1011 CFU (Fig. 1). Doses of 1010 or 1011 CFU resulted in similar intensities of spleen infection (Fig. 1). Mice appeared healthy throughout the course of the experiments.
TABLE 1.
Frequency of organ infection at various time points after oral administration of different doses of strain WR201 bacteriaa
| Day | No. of organs infected at indicated dose (CFU)/total no.
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung
|
Liver
|
Spleen
|
|||||||
| 109 | 1010 | 1011 | 109 | 1010 | 1011 | 109 | 1010 | 1011 | |
| 3 | 1/5 | 1/4b | 2/4b | 1/5 | 1/5 | 4/4b | 1/5 | 4/5 | 5/5 |
| 7 | 2/5 | 3/4b | 1/5 | 1/5 | 4/5 | 1/5 | 4/5 | 5/5 | 5/5 |
| 14 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 1/5 | 5/5 | 5/5 | 5/5 |
| 28 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 1/5 | 5/5 | 5/5 |
Groups of five mice were immunized orally with 109, 1010, or 1011 CFU of strain WR201. At the indicated days thereafter, mice were sacrificed and the number of bacterial CFU in organ homogenates was determined by serial dilution and plating on brucella agar. Data represent the number of organs infected as a fraction of the number of organs cultured. Limit of detection was 2 CFU/organ.
One organ from group could not be evaluated for infection due to culture plate contamination.
FIG. 1.
Course of oral infection in BALB/c mice with different doses of B. melitensis WR201. Animals were sacrificed at different time points after oral infection, and spleens were harvested and homogenized. The numbers of CFU per organ were determined by serial dilutions and plating on brucella agar. The limit of detection is 2 CFU/organ.
Sera from mice euthanized at the 4-week time point were analyzed for levels of antibodies directed against LPS and RFBL (Table 2). Mice immunized with 109 CFU tended to have lower antibody titers than those given either of the two higher doses, but the differences were not statistically significant. To insure that all animals immunized in subsequent experiments received an adequate number of organisms, we chose an oral dose of 1011 CFU as a standard inoculum. In these additional experiments, we examined the immunogenicity and protective efficacy of live versus dead organisms, vaccination with multiple versus single doses, and administration of various CFU levels of vaccine strain. In addition to the groups of mice required in each experiment to address these specific issues, a control group of mice sham vaccinated with saline and one group that was orally vaccinated with a single dose of 1011 CFU of strain WR201 and challenged i.n. 8 weeks later with strain 16M were always included. This strategy allowed us to demonstrate the overall reproducibility of the oral vaccination-i.n. challenge model across all seven studies (experiments I to VII) (Table 3 and Table 4). Groups of five mice were euthanized at various time points early after challenge (day 1 to week 2) to determine intensity of infection in the lungs (Table 3). Similarly, in all experiments groups of at least 15 mice were euthanized 8 weeks after challenge to determine infection in lungs, livers, and spleens (Table 4).
TABLE 2.
Production of Brucella-specific serum antibody in mice inoculated orally with strain WR201 bacteriaa
| WR201 dose(CFU) | Antibody level (OD ± SD)
|
|||
|---|---|---|---|---|
| Anti-LPS
|
Anti-RFBL
|
|||
| IgG | IgM | IgG | IgM | |
| 109 | 913 ± 2,008 | 31 ± 47 | 49 ± 102 | 1.2 ± 0.45 |
| 1010 | 3,069 ± 3,863 | 65 ± 78 | 47 ± 90 | 1 ± 0 |
| 1011 | 3,686 ± 2,959 | 87 ± 82 | 62 ± 87 | 1 ± 0 |
Mice were inoculated with 109, 1010, or 1011 CFU of WR201, and blood was collected 4 weeks later for determination of serum anti-LPS or anti-RFBL antibody levels by ELISA. OD units represent the dilution of serum required to give an A410 of 0.5.
TABLE 3.
Lung infection at early time points in mice immunized orally with 1011 CFU of strain WR201 bacteria and challenged i.n. 8 weeks later with strain 16M bacteriaa
| Expt and time pointb | Infection (Log CFU [mean ± SD]) in mice immunized with:
|
|
|---|---|---|
| Saline | WR201 | |
| I | ||
| Day 1 | 3.31 ± 0.21 | 2.62 ± 0.32c |
| Wk 1 | 2.9 ± 0.37 | 1.49 ± 0.22c |
| Wk 2 | 2.46 ± 0.88 | 0.71 ± 1.02c |
| II | ||
| Day 1 | 3.34 ± 0.55 | 2.76 ± 0.59 |
| Wk 1 | 2.37 ± 0.42 | 2.74 ± 0.41 |
| III | ||
| Day 1 | 3.35 ± 0.26 | 2.76 ± 0.24c |
| Wk 1 | 2.33 ± 0.65 | 2.33 ± 0.17 |
| Wk 2 | 1.72 ± 1.24 | 1.65 ± 0.67 |
| IV | ||
| Day 1 | 3.15 ± 0.49 | 2.86 ± 0.37 |
| Wk 1 | 2.67 ± 0.72 | 1.79 ± 1.00 |
| V | ||
| Day 1 | 2.86 ± 0.29 | 2.33 ± 0.48 |
| VI | ||
| Day 1 | 3.51 ± 0.54 | 3.49 ± 0.23 |
| VII | ||
| Day 1 | 3.4 ± 0.11 | 2.71 ± 0.57c |
| Wk 1 | 2.34 ± 0.33 | 1.35 ± 0.83c |
| Alle | ||
| Day 1 | 3.27 ± 0.21 | 2.79 ± 0.35c |
| Wk 1 | 2.52 ± 0.25 | 1.94 ± 0.58d |
Mice were immunized with 1011 CFU of WR201 or sham-immunized with saline. Eight weeks later, all were challenged i.n. with 104 CFU of 16M. Animal were euthanized at the times indicated after challenge and the number of bacterial CFU in the lungs was determined by serial dilution and plating on Brucella agar. Data for experiments I to VII are expressed as mean ± SD log CFU for groups of 5 mice.
Experiment numbers are the same throughout the text.
Significantly different from saline group (P < 0.05).
P < 0.08 versus saline group by Student's t test; P < 0.05 by Mann-Whitney U test).
Results represent means ± SD of all seven experiments (day 1) or five experiments (Week 1).
TABLE 4.
Organ infection in mice immunized orally with 1011 CFU of strain WR201 bacteria and challenged i.n. 8 weeks later with strain 16M bacteriaa
| Expt and group | Result for:
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
Lung
|
Liver
|
||||
| No. infected/total no. | Mean log (CFU ± SD) | No. infected/total no. | Mean log (CFU ± SD) | No. infected/total no. | Mean log (CFU ± SD) | |
| I | ||||||
| Saline | 14/14 | 4.05 ± 1.33 | 11/16 | 1.42 ± 1.16 | 12/16 | 1.88 ± 1.28 |
| WR201 | 4/14b | 0.42 ± 0.87c | 8/15 | 0.92 ± 1.00 | 5/15b | 0.54 ± 0.99c |
| II | ||||||
| Saline | 16/16 | 3.83 ± 1.25 | 14/17 | 1.76 ± 1.17 | 8/11 | 1.82 ± 1.45 |
| WR201 | 6/18b | 0.79 ± 1.27c | 8/15 | 1.1 ± 1.08 | 2/12b | 0.31 ± 0.74c |
| III | ||||||
| Saline | 17/17 | 3.85 ± 1.18 | 13/16 | 1.74 ± 1.12 | 12/16 | 1.82 ± 1.27 |
| WR201 | 6/15b | 0.94 ± 1.19c | 6/15b | 0.67 ± 0.95c | 2/13b | 0.29 ± 0.75c |
| IV | ||||||
| Saline | 16/16 | 3.04 ± 0.97 | 8/15 | 1.21 ± 1.32 | 6/16 | 0.43 ± 0.71 |
| WR201 | 7/18b | 1.01 ± 1.45c | 9/18 | 0.77 ± 0.86 | 4/18 | 0.27 ± 0.57 |
| V | ||||||
| Saline | 33/36 | 3.02 ± 1.53 | 14/37 | 0.70 ± 1.21 | 12/37 | 0.80 ± 1.27 |
| WR201 | 8/37b | 0.62 ± 1.18c | 6/37b | 0.29 ± 0.67 | 4/37b | 0.21 ± 0.62c |
| VI | ||||||
| Saline | 17/17 | 3.84 ± 0.95 | 12/14 | 1.59 ± 0.96 | 10/12 | 1.39 ± 0.94 |
| WR201 | 9/17b | 1.38 ± 1.53c | 9/14 | 1.32 ± 1.22 | 5/16b | 0.54 ± 0.92c |
| VII | ||||||
| Saline | 14/15 | 2.70 ± 1.45 | 10/15 | 0.95 ± 0.90 | 11/15 | 1.53 ± 1.05 |
| WR201 | 3/15b | 0.41 ± 0.88c | 6/15 | 0.63 ± 0.80 | 3/15b | 0.14 ± 0.32c |
| All | ||||||
| Saline | 127/131 | 3.48 ± 0.56 | 82/130 | 1.34 ± 0.40 | 71/123 | 1.38 ± 0.56 |
| WR201 | 43/134b | 0.79 ± 0.35c | 52/129b | 0.81 ± 0.33c | 25/126b | 0.32 ± 0.15c |
Animals were euthanized 8 weeks after challenge, and the number of bacterial CFU in organs was determined by serial dilution and plating of tissue homogenates. Although all organs from all animals were harvested in each experiment, culture results from some organs could not be interpreted due to plate contamination.
Significantly different from sham-immunized (saline) group by Fisher's exact test.
Significantly different from sham-immunized (saline) group by Student's t test.
Clearance of immunizing inoculum.
In all seven subsequent studies, clearance of strain WR201 from spleens was determined for three to five animals per group at 8 weeks after the final vaccine dose prior to challenge with strain 16M. No bacteria were recovered from the spleens of 54 mice at these time points. In addition, inguinal lymph nodes from 15 animals gave uniformly negative results. Fecal culture experiments were performed on 57 mice receiving 1011 CFU of strain WR201. WR201 was present (3+) in feces of all 10 mice sampled at day 1, 7 of 9 mice sampled at day 2, and 6 of 15 mice sampled at day 3. In one study, in which feces were sampled for up to 10 days, two of five mice gave positive results at days 7 and 9 and one of five still gave positive results at day 10, although only a few colonies were present on the plates from day 7 onward. These data indicated that strain WR201 was progressively lost from the feces following oral administration.
Immune response to orally administered live and dead strain WR201 bacteria.
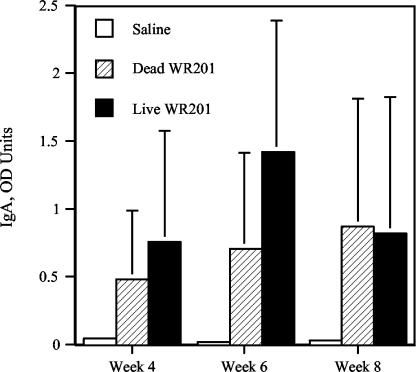
In an initial study to determine the subtype of anti-Brucella antibody response to oral immunization, we collected sera from mice 8 weeks after administration of 1011 live strain WR201 bacteria and determined anti-LPS IgG levels by ELISA. IgG anti-LPS titers were 1,061 (± 1,985) OD units, while control animals inoculated orally with saline had titers of ≤50 OD units. Analysis of subclass titers demonstrated that the great majority of anti-LPS was IgG3 (2,144 [± 4,499] OD units), with little IgG1 (211 [± 392] OD units), IgG2a (58 [± 60] OD units), or IgG2b (33 [± 69] OD units). To determine whether immunization with live organisms was required to induce anti-LPS antibody and cellular responses to Brucella antigens, animals were treated orally with 1011 live or dead strain WR201 brucellae or saline. Serum obtained 8 weeks after immunization from animals given live organisms contained 6,049 (± 4,077) OD units of anti-LPS antibody versus 445 (± 642) OD units for animals given dead bacteria (P < 0.02). No antibody (<10 OD units) was detected in animals given saline. The Brucella LPS-specific salivary IgA response was also measured in pilocarpine-induced saliva at 4, 6, and 8 weeks after oral immunization (Fig. 2). Live and dead brucellae evoked significant or near-significant mucosal antibody titers at all three time points. In contrast to results with serum antibody, there was no difference in levels of anti-LPS IgA content of saliva between mice immunized with live or dead bacteria at any time point. No anti-LPS IgA was detected in saliva from animals sham immunized with saline (Fig. 2). These data indicated that live bacteria were not required to elicit either serum or mucosal antibody response to LPS but that live bacteria were more effective than dead in eliciting serum anti-LPS.
FIG. 2.
IgA anti-Brucella LPS antibodies in saliva obtained at three different time points from mice immunized orally with 1011 strain WR201 bacteria. Saliva was collected with a micropipette from five mice in each group after i.p. injection of 70 to 100 μg of pilocarpine. Total salivary Brucella-specific IgA levels were measured by ELISA (P = 0.0598 for dead and P = 0.01 for live WR201).
To determine the response of immune cells to Brucella antigens, we prepared spleen cells 8 weeks after oral administration of saline or live or dead brucellae. One-half of each spleen was grown in cultures for bacterial CFU determination; all spleens were found to be sterile (limit of detection, 4 CFU/spleen). Cells from the other spleen half were grown in cultures for 48 h with RFBL or medium control. IL-2 content and IFN-γ content were then measured in culture supernatant fluids. The IL-2 content of fluids derived from RFBL-stimulated cells from live strain WR201-vaccinated animals was at least 50-fold greater than the IL-2 content from sham-immunized mice and 25-fold greater than the IL-2 content from mice immunized with killed organisms (Table 5). These differences were highly significant (P < 0.001). The IFN-γ content in fluids from stimulated, live-vaccinated animals was 6- to 8-fold greater than the IFN-γ content in fluids from sham- or dead bacterium-vaccinated groups. These differences approached significance (P = 0.07 and P = 0.06, respectively). The supernatant fluid cytokine content of RFBL-stimulated cells from animals immunized with dead bacteria was not significantly different from that of cells from sham-immunized mice (P = 0.47 for IL-2 and 0.99 for IFN-γ). Medium-treated cells from sham-immunized and both bacterium-immunized groups of animals also did not differ in their levels of cytokine production.
TABLE 5.
Production of IL-2 and IFN-γ from mice immunized with live or dead strain WR201 bacteriaa
| Inoculum | IL-2 (pg/ml ± SD) from cells cultured with:
|
IFN-γ pg/ml ± SD from cells cultured with:
|
||
|---|---|---|---|---|
| Medium | RFBL | Medium | RFBL | |
| Saline | 5 ± 4 | 4 ± 4 | 94 ± 94 | 208 ± 200 |
| Dead WR201 | 3 ± 3 | 8 ± 9 | 276 ± 328 | 276 ± 333 |
| Live WR201 | 4 ± 2 | 219 ± 180b | 147 ± 131 | 1,607 ± 1,466c |
Mice (n = 5/group) were sham immunized with saline or with dead or live WR201. Eight weeks later, spleen cells from individual mice were cultured with medium or RFBL. Supernatant fluids were collected 48 h later and assayed by ELISA for cytokine content. Data are expressed as means ± SD.
Significantly different from both dead and saline inoculum groups by ANOVA on log-normalized data with Tukey's simultaneous pairwise comparisons.
P = 0.056 versus dead inoculum group and P = 0.071 versus saline inoculum group by ANOVA on log-normalized data with Tukey's simultaneous pairwise comparisons.
Vaccine efficacy of orally administered live and dead strain WR201 brucellae.
To determine the effectiveness of immunization with live or dead bacteria, we challenged immunized and sham-immunized mice i.n. with strain 16M 8 weeks after immunization and cultured homogenates of lungs, livers, and spleens for Brucella bacteria at intervals up to 8 weeks after challenge. Animals immunized with live WR201 had reduced frequencies of infected spleens, lungs, and livers compared to animals sham immunized with saline (experiment III; Table 4) or immunized with dead bacteria (Fig. 3). Animals immunized with killed organisms also showed a trend toward reduced frequency of infection in these organs, but the reduction was significant only for spleen infection (Fig. 3). Similar effects were observed for the number of CFU/organ. Again, the intensity of infection in animals immunized with live strain WR201 brucellae was significantly less than that in sham-immunized mice (experiment III; Table 4). Animals immunized with dead organisms had trends for reduced intensity of infection, but these were not significant.
FIG. 3.
Percentages of organs infected 8 weeks after i.n. challenge of mice with strain 16M. Animals were orally immunized eight weeks before i.n. challenge with 1011 live or dead strain WR201 bacteria. Spleens, lungs, and livers from 15 mice were harvested 8 weeks after i.n. challenge.
Although all organs from all animals were harvested in each experiment, some culture results from some animals could not be interpreted due to contamination.
Assessment of protective immunity against brucellosis by use of live strain WR201 bacteria at a single dose of 1011 CFU.
We previously observed that i.p. immunization with live strain WR201 both accelerated clearance of i.n.-administered strain 16M from the lung and prevented bacterial dissemination to the liver and spleen (11). We examined both these features of anti-Brucella immunity in the present study. As described above, in the course of testing different immunization schedules we performed seven separate experiments in which mice were immunized orally with 1011 live WR201 and challenged i.n. 8 weeks later with 104 virulent strain 16M bacteria. In all of these experiments, organs were grown in cultures for Brucella bacteria for 1 day and 8 weeks postchallenge; in some experiments, organs were also grown in cultures for 1 and 2 weeks. Table 4 shows the log CFU in lungs at these early time points following strain 16M challenge of immunized mice. At 1 day postchallenge, all the lungs were infected with an average of 3.27 (± 0.35) log CFU in sham-immunized mice. In four of seven experiments, the number of brucellae in the lungs of immunized animals was significantly less than in the lungs of animals sham immunized with saline. Over all seven experiments, the mean log CFU/lung at 1 day was 3.27 ± 0.21 for sham-immunized and 2.79 ± 0.35 for immunized mice (P = 0.009). At weeks 1 and 2, all lungs were still infected but the number of organisms, even in sham-immunized mice, had decreased. A significant relative reduction in lung CFU in immunized mice at 1 week postchallenge was seen in two of five experiments (experiments I and VII; Table 3). In experiment II, the number of lung CFU was slightly but not significantly higher in immunized mice; in two experiments, the numbers were identical in immunized and sham-immunized mice (experiment III) or insignificantly lower in immunized mice (experiment IV). Overall, mean log CFU/lung at 1 week was 2.52 ± 0.25 in sham-immunized and 1.94 ± 0.58 in immunized mice (P = 0.075). Only two experiments examined lungs at 2 weeks postchallenge. In both, there was a tendency toward reduced CFU in immunized compared to sham-immunized animals. These data indicated that oral immunization with live strain WR201 modestly reduced the number of bacteria in the lung at early time points.
Table 4 summarizes the results from seven experiments 8 weeks after the strain 16M challenge (results are from groups that were given saline or 1011 strain WR201 bacteria once). At 8 weeks, bacteria were present in the spleens of 127 of 131 (97%) sham-immunized but only 43 of 134 (32%) immunized mice (P < 0.001), a protective efficacy of 67%. Mean log CFU/spleen was 3.48 ± 0.54 in sham-immunized and 0.79 ± 0.35 in immunized mice (P < 0.001). Immunization also reduced the frequency and intensity of infection in the liver. Bacteria were present in the livers of 71 of 123 (58%) sham-immunized and only 25 of 126 (20%) immunized mice (P < 0.001), and mean log CFU/liver was reduced from 1.38 ± 0.56 to 0.33 ± 0.15, respectively (P < 0.001). Interestingly, the modest effect of immunization on lung infection noted at early time periods was more clearly apparent at 8 weeks. A trend toward reduced frequency and intensity of lung infection in immunized compared to sham-immunized animals was observed in all seven experiments. Overall, brucellae were present in lungs of 82 of 130 (63%) sham-immunized but in only 52 of 129 (40%) immunized mice (P < 0.01), and the intensity of infection was reduced from 1.34 (± 0.40) log CFU in sham-immunized mice to 0.81 (± 0.33) log CFU in immunized mice (P = 0.02). These studies showed that immunization with a single oral dose of 1011 CFU of live strain WR201 bacteria significantly reduced both pulmonary and disseminated infection after i.n. challenge with strain 16M.
Assessment of protective immunity against brucellosis by use of multiple doses of strain WR201.
Since a single dose of orally administered strain WR01 protected mice against i.n. challenge with virulent strain 16M, we asked whether boosting with this mutant would enhance protection. Age-matched mice were given one, two, or three doses of 1011 CFU of WR201 8 weeks apart. Immunizations were scheduled so all animals were challenged at the same time (8 weeks after their last vaccine dose) with the same inoculum. A single dose of strain WR201 (experiment V; Table 3 and Table 4) protected 76% of mice from dissemination of infection to the spleen (Fig. 4). Two doses protected 88% and three doses protected 95% of animals compared to the results seen with controls sham immunized with saline. Differences in the frequencies of spleen infection between sham immunization and any of the immunization schedules were all highly significant (P < 0.001). The frequency of spleen infection was significantly (P = 0.035) more reduced with three doses of strain WR201 compared to the results seen with one dose. Differences between one and two doses and two and three doses were not significant. The intensity of spleen infection was also reduced as the number of WR201 doses increased. Interestingly, increasing the number of doses of WR201 did not further reduce the already low frequency of infection or intensity of infection in lung and liver (Fig. 4) induced by immunization with a single dose.
FIG. 4.
Percentages of organs infected 8 weeks after i.n. challenge of mice with B. melitensis 16M. These mice were inoculated orally with 1011 strain WR201 bacteria one, two, or three times and 8 weeks after the last immunization were challenged i.n. with 104 strain 16M. A total of 36 animals per time point were used.
In this same study, splenocytes were examined for antigen-stimulated ex vivo cytokine production (Table 6). Spleen cells obtained from five unchallenged animals from each group 8 weeks after the last administration of vaccine were grown in cultures with medium or RFBL. Culture supernatant fluids were collected 24 h later and assayed for IL-2. Culture fluids from cells of all groups grown in cultures in medium alone and from cells from sham-immunized animals contained ≤2 pg of IL-2/ml, while fluids from antigen-stimulated cells from all immunized groups had detectable IL-2 levels (Table 6). The IL-2 content for animals immunized twice or three times was significantly (P < 0.01) greater than that for animals immunized once or not at all. However, there was no difference between the results seen with animals immunized twice or three times.
TABLE 6.
IL-2 and anti-LPS IgG responses in animals immunized once, twice, or three times with strain WR201 bacteriaa
| Inoculum | IL-2 (pg/ml ± SD) | Anti-LPS IgG (OD ± SD) |
|---|---|---|
| Saline | 0 ± 0 | 2 ± 0 |
| WR201 | ||
| 1 × | 33 ± 16b | 1,448 ± 1,330b |
| 2 × | 205 ± 148b,c | 7,898 ± 4,572b |
| 3 × | 188 ± 114b,c | 3,302 ± 1,680b |
Groups of five animals were orally immunized with 1011 CFU of WR201 once, twice, or three times at 8-week intervals. Sham-immunized control animals received saline three times. Serum and spleen cells were obtained 8 weeks after the last dose of vaccine. Serum anti-LPS IgG levels were determined by ELISA. IL-2 content levels were measured by ELISA in supernatant fluids from spleen cells cultured for 24 h with RFBL. Data are expressed as means ± SD. The significance of differences between groups was determined by ANOVA with Tukey's simultaneous pairwise comparisons on log-normalized data.
Significantly different from sham-immunized (saline) group results.
Significantly different results from those seen with mice immunized once.
Serum antibody titers obtained at the same time as spleen cells also demonstrated the presence of anti-LPS IgG in immunized animals. Although there were trends for increased anti-LPS in multiply immunized mice compared to the results seen with animals immunized once, these differences were not statistically significant (Table 6).
Assessment of protective immunity with different immunizing doses of strain WR201.
As described above, preliminary studies over a 4-week immunization period indicated that animals immunized with 1010 or 1011 CFU of WR201 had longer persistence of vaccine strain in their spleens and higher antibody titers than animals immunized with 109 CFU. In a single experiment (experiment VI; Table 3 and Table 4), mice were immunized with 109, 1010, or 1011 CFU of strain WR201 bacteria and challenged 8 weeks later with strain 16M. Protection was assessed by culture of tissues 8 weeks after challenge. Immunization with 109 CFU did not protect animals from the presence of strain 16M in lungs, livers, or spleens (Table 7), although there were trends toward reduced infection in each of these organs. In contrast, immunization with either of the higher doses significantly reduced the frequency of strain 16M-infected spleens and livers and significantly reduced the intensity of infection in spleens compared to the results seen with sham-immunized animals (Table 7). A strong trend was also observed for reduced intensity of infection in liver in animals immunized with the higher doses. Interestingly, there was no consistent trend toward better protection with 1011 versus 1010 CFU. Even though the higher doses significantly protected animals from strain 16M and 109 CFU did not, there was no difference among the immunized groups for any parameter when they were compared by ANOVA.
TABLE 7.
Organ infection in mice immunized orally with different doses of strain WR201 bacteria and challenged i.n. 8 weeks later with strain 16M bacteriaa
| Inoculum | Result for:
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
Lung
|
Liver
|
||||
| No. infected/total no. | Mean log (CFU ± SD) | No. infected/total no. | Mean log (CFU ± SD) | No. infected/total no. | Mean log (CFU ± SD) | |
| Saline | 17/17 | 3.84 ± 0.95 | 12/14 | 1.59 ± 0.96 | 10/12 | 1.39 ± 0.94 |
| WR201 (CFU) | ||||||
| 109 | 16/17 | 3.17 ± 1.50 | 11/15 | 1.41 ± 0.92 | 9/17 | 0.90 ± 1.08 |
| 1010 | 10/17b | 1.63 ± 1.68c | 11/17 | 1.10 ± 1.00 | 6/17b | 0.48 ± 0.81d |
| 1011 | 9/17b | 1.38 ± 1.53c | 9/14 | 1.32 ± 1.22 | 5/16b | 0.54 ± 0.92e |
Animals were immunized with the indicated number of CFU of WR201, challenged i.n. with 104 16M, and euthanized 8 weeks after challenge. The number of bacterial CFU in organs was determined by serial dilution and plating of tissue homogenates.
Significantly different from sham-immunized (saline) group by Fisher's exact test.
Significantly different from sham-immunized (saline) group and from 109 CFU group by ANOVA with Tukey's simultaneous pairwise comparisons.
P = 0.064 versus saline group by ANOVA with Tukey's simultaneous pairwise comparisons.
P = 0.096 versus saline group by ANOVA with Tukey's simultaneous pairwise comparisons.
In summary, these experiments demonstrated that oral administration of strain WR201 enhanced clearance of virulent brucellae from the lung and protected animals against disseminated infection in spleens and livers following i.n. challenge with virulent strain 16M. Protective efficacy required immunization with live bacteria and was related to the size of the immunizing dose and the number of doses administered. Immunization resulted in anti-LPS antibody in serum and saliva, anti-RFBL antibody in serum, and induction of splenocytes that produced IFN-γ and IL-2 when grown in cultures with Brucella antigen. These experiments suggest that oral immunization with live vaccines attenuated by deletion of genes required for purine synthesis is an attractive strategy to protect animals or humans against infection by inhalation of virulent B. melitensis bacteria.
DISCUSSION
A number of recent reports have documented the efficacy of live, attenuated vaccines for protection against challenge with virulent Brucella bacteria in mice (2, 6, 10, 13, 16, 18, 21, 23). With the exception of one group (18), the researchers reporting these studies have all given vaccine strains parenterally. Moreover, except for work with strain WR201 reported previously by Hoover et al. (11) and work reported by Pasquali et al. (18), other workers have challenged mice either i.p. or i.v. and defined protection as reduction in spleen CFU. We have chosen a more rigorous main criterion of protection, namely, the percentage of animals that do not have Brucella in their spleens, although we also observe reduction in spleen CFU at multiple time points after i.n. challenge. Our use of a pathophysiologically relevant (i.e., mucosal) route of challenge allows determination of the effect of adaptive immunity both on elimination of bacteria from the site of inoculation and on systemic infection.
The results of these experiments, in which mice were immunized orally with strain WR201, were similar in most respects to those of studies in which mice were immunized i.p. with the same strain and challenged i.n. with strain 16M (11). Comparison of our preliminary dose-ranging experiment using strain 16M to our studies with strain WR201 indicated that WR201 levels were markedly attenuated. All animals given 109 CFU of 16M still had infected spleens at 8 weeks, while no animals given 109 to 1011 CFU of WR201 had splenic infection at that time point. These results of bacterial clearance are similar to those of studies in which the courses of infection were compared in mice given WR201 or 16M i.p. (4).
Immunization with strain WR201 by either route led to similar levels of protection against dissemination from lungs to spleen 8 weeks after challenge (50 to 70% efficacy after i.p. challenge [11]; 67% after oral challenge). Although the two routes were similarly effective in reducing the number of bacteria in lung in the first few weeks after challenge, we cannot directly compare their overall levels of efficacy on late clearance, as we did not examine 8-week data in the previous study. We had anticipated that oral immunization, which should lead to production of anti-LPS antibody in secretions (as we noted with respect to saliva in the present study), might be more effective in protection against a lung challenge than i.p. immunization, which may not induce secretory antibody. Our failure in the present study to observe a clear protective advantage of oral over i.p. immunization is consistent with a previous report by Bhattacharjee et al. (1) that i.n. immunization with a complex (LPS-GBOMP) of B. melitensis LPS and Neisseria meningitidis group B outer membrane protein elicits high levels of pulmonary anti-LPS IgG, IgM, and IgA as well as systemic antibody and inhibits dissemination to liver and spleen but does not prevent or reduce lung infection. Interestingly, oral immunization with strain WR201 in the present study induced more IgG3 than IgG1 antibody whereas i.n. immunization with LPS-GBOMP induced predominantly IgG1. Elzer et al. (7) reported a similar predominance of IgG3 anti-LPS antibodies in late serum samples from mice infected i.p. with live, smooth strains of B. abortus.
These data suggest that immunization with live brucellae (B. melitensis or B. abortus) by either mucosal or nonmucosal routes evokes an anti-LPS serum IgG response similar to that of immunization with LPS alone (14) and qualitatively different from immunization with LPS-GBOMP (1). The serum anti-LPS IgG titers measured in animals immunized orally once with live strain WR201 were similar to those obtained after i.p. immunization (11) and approximately the same as those obtained with a single i.n. dose of LPS-GBOMP (1). Boosting with i.n. GBOMP-LPS increases serum anti-LPS IgG approximately 20-fold (1), but repeated immunization with live WR201 led only to a statistically insignificant increase. In the present study, immunization with live or dead bacteria elicited similar salivary anti-LPS IgA, but the level of serum anti-LPS IgG antibody induced by dead bacteria was only about one-fourth of that induced with live organisms. Salivary IgA reflects stimulation of the common mucosal immune system (15), which probably occurred equivalently when either dead or live organisms contacted the gastrointestinal mucosa, while serum antibody titers reflect persistence of bacteria in systemic locations. In contrast, immunization with dead organisms induced no production of IL-2 and IFN-γ by antigen-stimulated spleen cells. Dead bacteria were markedly less effective than live organisms for protection against either disseminated or local, pulmonary infection, although immunization with dead organisms did afford significant protection against dissemination to spleens.
Taken together, these data suggest that systemic or mucosal antibodies may not play an important role in protection against local lung infection but that systemic antibodies are important for protection against bacterial dissemination. Systemic antibody-mediated protection against disseminated infection may reflect containment of organisms in lymph nodes, as noted for studies of guinea pig and murine models (17, 19, 20). Induction of T-cell-mediated immunity, on the other hand, may promote enhanced clearance of bacteria from the lung. Whether this effect also requires participation of systemic antibodies is unknown. This concept would suggest that use of an oral route for immunization against B. melitensis with live vaccines may have advantages for ease of delivery and reduced side effects but may not confer an advantage for efficacy against lung challenge. Interestingly, oral immunization with B. abortus RB51 (18) is protective against oral but not i.p. challenge with B. abortus 2308, suggesting a role for mucosal host defenses against brucellosis acquired by ingestion of foodstuffs, especially unpasteurized dairy products. While we have not tested the relative efficacy levels of i.p. versus oral immunization with strain WR201 against oral challenge infection, the data obtained with strain RB51 suggest that use of WR201 by the oral route may also be beneficial for protection against foodborne brucellosis caused by virulent B. melitensis.
Our studies also demonstrated an effect of dose and booster immunization on protective efficacy and on potential immune correlates of protection. Administration of ≥1010 CFU of vaccine or administration of more than one dose resulted in increased protection. This increase was significantly associated with increased serum anti-LPS IgG titers in animals given higher doses and a trend toward higher titers in animals given more than one vaccine dose. Similarly, antigen-stimulated splenocytes of mice given 1010 or 1011 CFU of vaccine produced more IL-2 than those of mice given only 109 CFU. Interestingly, administration of a third dose had no advantage over administration of two doses for protection against dissemination of challenge bacteria to the spleen. In addition, no benefit of two or three doses versus one dose was demonstrable for protection from dissemination to the liver or clearance from the lung. In this particular experiment, however, the efficacy of a single dose was relatively high (76% protection in spleens). This anomaly may have limited our ability to observe differences in efficacy between the two- and three-dose regimens in this organ and to find statistically significantly enhanced efficacy of booster immunization in lungs and livers. Enhanced efficacy with booster immunization is encouraging for vaccine development. It may allow use of fewer organisms per dose, which may in turn reduce the potential for vaccine-induced symptomatic infection.
These studies confirm and extend previous observations that deletion of the purEK operon leads to significant attenuation of B. melitensis levels (4, 5) but allows development of a protective immune response (11) in a respiratory challenge model in mice. The clearance of orally administered strain WR201 from lungs, livers, and spleens by 8 weeks after immunization and the ability of the organism to induce a protective response against i.n. challenge with strain 16M suggest that induction of purine auxotrophy in B. melitensis has promise as a strategy for the development of a safe, convenient, and effective human vaccine. As with all live vaccines, further demonstration of safety in additional animal models will be important to determine whether this strategy alone will attenuate B. melitensis sufficiently to allow testing in volunteers.
Acknowledgments
We thank Joseph Thompson, Lynnette Young, Adrien Ravizee, Hugh Wylie, and Peter Chen for their superb technical assistance in conducting these experiments.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, Department of the Air Force, or the Department of Defense.
Editor: F. C. Fang
REFERENCES
- 1.Bhattacharjee, A. K., L. Van de Verg, M. J. Izadjoo, L. Yuan, T. L. Hadfield, W. D. Zollinger, and D. L. Hoover. 2002. Protection of mice against brucellosis by intranasal immunization with Brucella melitensis lipopolysaccharide as a noncovalent complex with Neisseria meningitidis group B outer membrane protein. Infect. Immun. 70:3324-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boschiroli, M. L., S. L. Cravero, A. I. Arese, E. Campos, and O. L. Rossetti. 1997. Protection against infection in mice vaccinated with a Brucella abortus mutant. Infect. Immun. 65:798-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan, T. M., S. L. Hendricks, C. M. Patton, and R. A. Feldman. 1974. Brucellosis in the United States, 1960-1972; an abattoir-associated disease. Part III. Epidemiology and evidence for acquired immunity. Medicine (Baltimore) 53:427-439. [PubMed] [Google Scholar]
- 4.Crawford, R. M., L. Van De Verg, L. Yuan, T. L. Hadfield, R. L. Warren, E. S. Drazek, H. H. Houng, C. Hammack, K. Sasala, T. Polsinelli, J. Thompson, and D. L. Hoover. 1996. Deletion of purE attenuates Brucella melitensis infection in mice. Infect. Immun. 64:2188-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazek, E. S., H. S. Houng, R. M. Crawford, T. L. Hadfield, D. L. Hoover, and R. L. Warren. 1995. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect. Immun. 63:3297-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmonds, M. D., A. Cloeckaert, and P. H. Elzer. 2002. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet. Microbiol. 88:205-221. [DOI] [PubMed] [Google Scholar]
- 7.Elzer, P. H., R. H. Jacobson, S. M. Jones, K. H. Nielsen, J. T. Douglas, and A. J. Winter. 1994. Antibody-mediated protection against Brucella abortus in BALB/c mice at successive periods after infection: variation between virulent strain 2308 and attenuated vaccine strain 19. Immunology 82:651-658. [PMC free article] [PubMed] [Google Scholar]
- 8.Enright, F. M. 1990. The pathogenesis and pathobiology of Brucella infection in domestic animals, p. 301-320. In K. Nielsen and J. R. Duncan (ed.), Animal brucellosis. CRC Press, Inc., Boca Raton, Fla.
- 9.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdy, M. E., S. M. El-Gibaly, and A. M. Montasser. 2002. Comparison between immune responses and resistance induced in BALB/c mice vaccinated with RB51 and Rev. 1 vaccines and challenged with Brucella melitensis bv. 3. Vet. Microbiol. 88:85-94. [DOI] [PubMed] [Google Scholar]
- 11.Hoover, D. L., R. M. Crawford, L. L. Van De Verg, M. J. Izadjoo, A. K. Bhattacharjee, C. M. Paranavitana, R. L. Warren, M. P. Nikolich, and T. L. Hadfield. 1999. Protection of mice against brucellosis by vaccination with Brucella melitensis WR201(16MDeltapurEK). Infect. Immun. 67:5877-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 68:5314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez de Bagues, M. P., P. H. Elzer, S. M. Jones, J. M. Blasco, F. M. Enright, G. G. Schurig, and A. J. Winter. 1994. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect. Immun. 62:4990-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz, R. S., and D. T. Berman. 1986. Influence of endotoxin-protein in immunoglobulin G isotype responses of mice to Brucella abortus lipopolysaccharide. Infect. Immun. 54:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestecky, J. 1993. Saliva as a manifestation of the common mucosal immune system. Ann. N. Y. Acad. Sci. 694:184-194. [DOI] [PubMed] [Google Scholar]
- 16.Monreal, D., M. J. Grillo, D. Gonzalez, C. M. Marin, M. J. De Miguel, I. Lopez-Goni, J. M. Blasco, A. Cloeckaert, and I. Moriyon. 2003. Characterization of Brucella abortus O-polysaccharide and core lipopolysaccharide mutants and demonstration that a complete core is required for rough vaccines to be efficient against Brucella abortus and Brucella ovis in the mouse model. Infect. Immun. 71:3261-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardon, P., and J. Marly. 1978. Resistance of normal or immunized guinea pigs against a subcutaneous challenge of Brucella abortus. Ann. Rech. Vet. 9:419-425. [PubMed] [Google Scholar]
- 18.Pasquali, P., A. Rosanna, C. Pistoia, P. Petrucci, and F. Ciuchini. 2003. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect. Immun. 71:2326-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plommet, M., A. Serre, and R. Fensterbank. 1987. Vaccines, vaccination in brucellosis. Ann. Inst. Pasteur Microbiol. 138:117-121. [DOI] [PubMed] [Google Scholar]
- 20.Sulitzeanu, D. 1959. The fate of killed, radioiodinated Brucella abortus injected into mice. J. Immunol. 82:304-312. [PubMed] [Google Scholar]
- 21.Vemulapalli, R., Y. He, S. Cravero, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2000. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun. 68:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vershilova, P. A. 1961. The use of live vaccine for vaccination of human beings against brucellosis in the USSR. Bull. W. H. O. 24:85-89. [PMC free article] [PubMed] [Google Scholar]
- 23.Winter, A. J., G. G. Schurig, S. M. Boyle, N. Sriranganathan, J. S. Bevins, F. M. Enright, P. H. Elzer, and J. D. Kopec. 1996. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57:677-683. [PubMed] [Google Scholar]
- 24.Young, E. J. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283-289. [DOI] [PubMed] [Google Scholar]