Abstract
OBJECTIVES
To test the hypothesis that acidosis at birth is associated with the combined primary outcome of death or neurodevelopmental impairment (NDI) in extremely low birth weight (ELBW) infants, and to develop a predictive model of death/NDI exploring perinatal acidosis as a predictor variable.
STUDY DESIGN
The study population consisted of ELBW infants born between 2002-2007 at NICHD Neonatal Research Network hospitals. Infants with cord blood gas data and documentation of either mortality prior to discharge or 18-22 month neurodevelopmental outcomes were included. Multiple logistic regression analysis was used to determine the contribution of perinatal acidosis, defined as a cord blood gas with a pH<7 or base excess (BE)<-12, to death/NDI in ELBW infants. In addition, a multivariable model predicting death/NDI was developed.
RESULTS
3979 patients were identified of whom 249 had a cord gas pH<7 or BE<-12 mEq/L. 2124 patients (53%) had the primary outcome of death/NDI. After adjustment for confounding variables, pH<7 and BE<-12 mEq/L were each significantly associated with death/NDI (OR=2.5[1.6,4.2]; and OR=1.5[1.1,2.0], respectively). However, inclusion of pH or BE did not improve the ability of the multivariable model to predict death/NDI.
CONCLUSIONS
Perinatal acidosis is significantly associated with death/NDI in ELBW infants. Perinatal acidosis is infrequent in ELBW infants, however, and other factors are more important in predicting death/NDI.
Keywords: Cord blood gas, Premature infant, Preterm infant, Neurodevelopmental impairment
INTRODUCTION
Neurodevelopmental impairment (NDI) remains a major morbidity for infants born prematurely, particularly for extremely low birth weight (ELBW) infants (≤1000g).(1, 2) While many of these infants fare relatively well, data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) and the Vermont Oxford Network indicate that approximately one-third of ELBW infants will die and approximately one-third of survivors will suffer NDI.(3-6)
Accurate prediction of death/NDI in preterm infants based on clinical information readily available early in an infant's hospital course would be invaluable for counseling parents and making clinical decisions to continue or withhold intensive care support. However, such prediction is notoriously difficult.(7) Early variables known to correlate with the combined outcome of death/NDI in ELBW infants include gestational age, birth weight, race/ethnicity, antenatal steroids, multiple gestation, and 5-minute Apgar scores.(4, 8, 9)
The relative importance of perinatal acidosis in predicting poor outcomes in extremely preterm infants has not been well defined. Severe acidosis at birth is one of the strongest predictors of death/NDI in full term infants with suspected hypoxic/ischemic encephalopathy for whom there are relatively few other major risk factors for poor outcomes.(10) In contrast, in preterm infants numerous antecedent variables and clinical events may be associated with death or NDI including developmental immaturity of organ systems, intraventricular hemorrhage, infection, hypoxia due to poor lung function, and inadequate nutrition.(11) Against this complex backdrop of physiologic derangements, the relative importance of perinatal acidosis as a predictor of outcomes in preterm infants is much less clear with various small studies reporting conflicting findings.(12-18)
A better understanding of the role of perinatal acidosis in ELBW infants is important both to aid in predicting outcomes and to help elucidate mechanisms of neural injury in this population. Using data from a large cohort of ELBW infants in the NICHD NRN Generic Database, we examined the hypothesis that perinatal acidosis is associated with the combined outcome of death/NDI in ELBW infants. In addition, we developed a model incorporating perinatal acidosis as well as other early predictor variables, such as birth weight (BW), gestational age (GA), antenatal steroids, and sex, to assist in predicting outcomes.
METHODS
Study population
The NICHD NRN Generic Database (GDB) is a registry of very low birth weight infants (401-1500g) cared for at the academic centers of the NRN. The database includes prospectively collected maternal data recorded at the time of birth and infant outcome data collected from birth until death, transfer, discharge or 120 days. For the current study, we included live-born infants 401-1000g BW delivered in NRN hospitals from 2002-2007, including those who did not survive beyond the delivery room. Infants born with major congenital anomalies including congenital heart defects, chromosomal anomalies and central nervous system abnormalities were excluded. Each NRN center's institutional review board approved the data collection for the GDB.
Statistical analyses and definitions
Cord blood gas pH and base excess (BE) were treated as primary predictor variables and were analyzed both as continuous variables and with the pre-specified cut-offs of pH<7.0 and BE<-12 mEq/L. These cut-offs were chosen as they were deemed to represent clinically significant but not overwhelming degrees of acidosis. When data were available for both umbilical venous and arterial blood samples, the arterial value was used as this is more reflective of the baby's condition. Potential confounders included GA, BW, small for gestational age (SGA), sex, race/ethnicity, biological mother's insurance status, multiple gestation, antenatal steroids (ANS), NRN center at birth, and maternal hypertension, hemorrhage and antibiotics. GA was defined as the best obstetrical estimate. SGA was defined as birth weight <10th percentile for sex and GA.(19) ANS were defined as any corticosteroid given during the pregnancy to accelerate fetal lung maturity. Maternal hypertension was defined as systolic blood pressure greater than 140 mm Hg or a diastolic pressure greater than 90 mm Hg recorded on at least 2 occasions during the pregnancy. Maternal hemorrhage included placenta previa, placental abruption, or threatened abortion resulting in bleeding after 20 weeks of pregnancy. Maternal antibiotics were defined as antibiotics given during the delivery hospitalization.
The primary outcome variable was death/NDI considered together, as they are competing outcomes. NDI was assessed in surviving infants at 18-22 months by standardized history, neuromotor exam, and the Bayley Scales of Infant Development.(20) The Bayley II exam was used for follow-up evaluations performed before mid-2007 when it was replaced with the Bayley III exam. NDI was defined as moderate to severe CP, Bayley II Mental Developmental Index (MDI) or Psychomotor Developmental Index (PDI)<70 or Bayley III cognitive<85 or Gross Motor Function Classification System (GMFCS) level greater than 2, bilateral vision loss worse than 20/200, and bilateral hearing loss requiring amplification or, beginning in 2006, complete deafness. Secondary outcome variables analyzed were death and NDI considered as separate outcomes, grade III/IV intraventricular hemorrhage (IVH) defined by Papile's criteria, seizures, respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), proven necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP).(21) RDS was defined as having physical signs such grunting, flaring, and retracting and/or a supplemental oxygen requirement in the first 24 hours. BPD was defined as requiring supplemental oxygen at 36 weeks post-menstrual age. Proven NEC included NEC of Bell's staging criteria IIA through IIIB.(22) ROP included plus disease or stage 3 or greater ROP in either eye.
Statistical analyses were performed at the Data Coordinating Center at RTI International, Research Triangle Park, NC, using SAS 9.2. Each analysis was conducted using available data without imputation for missing data. For comparisons between infants included and excluded from the analysis, chi-square tests for categorical data, t-test for normally distributed data, and Wilcoxon rank sum tests for skewed data were used. To assess the relationship between acidosis and each outcome, logistic regression models were constructed with and without adjusting for potential confounders. Separate analyses were performed treating cord pH and BE as continuous or as categorical variables, and goodness of fit was compared using Akaike's information criterion (AIC). Sensitivity analyses were conducted using multiple imputation methods to account for missing data. For development of a prediction model based on earliest available clinical data, the data were randomly split evenly into training and validation sets. Using the training dataset, a multivariable logistic regression model was constructed with an outcome of NDI/death. Pre-specified variables evaluated for inclusion in the prediction model were cord pH, BE, GA, BW, 5-minute Apgar, SGA, multiple gestation, race/ethnicity, biological mother's insurance status, sex, maternal hypertension, maternal hemorrhage, maternal antibiotics, and ANS. The final model was identified using backwards selection where predictors remained in the model if p-value<0.05. The model adjusted for Bayley version and center. The odds ratios for each final predictor variable were normalized by dividing the odds ratios by the minimum odds ratio for that predictor variable to generate a weighted scoring system.(10) Receiver operator curve (ROC) analysis was used to determine the optimum cut-off value for the score in order to predict death/NDI. The predictive ability of the scoring system was tested using the validation dataset. Spearman rank correlation was used to determine association of cord BE and pH with 5-minute Apgar.
RESULTS
Incidence of acidosis in ELBW infants
Among 9324 ELBW infants born from 2002-2007 in the database, cord blood pH and BE were available for 4807 infants (Figure1). Of these, primary outcome follow-up information was available for 3979 infants. Acidosis, defined as a cord pH<7 or a BE<-12, was uncommon, occurring in only 249 infants (6.3%) in spite of the various fetal or maternal conditions that led to preterm birth. BE<-12 (N=239) occurred more often than pH<7 (N=101) but there was considerable overlap between the two groups (Figure 1). Mothers of infants with acidosis at birth were significantly less likely to be Non-Hispanic White, to have private insurance, or to have received antenatal steroids or antibiotics (Table 1). They were also more likely to have experienced a hemorrhage. Acidotic infants were smaller than those without acidosis and had lower median 5-minute Apgar scores.
Figure 1.
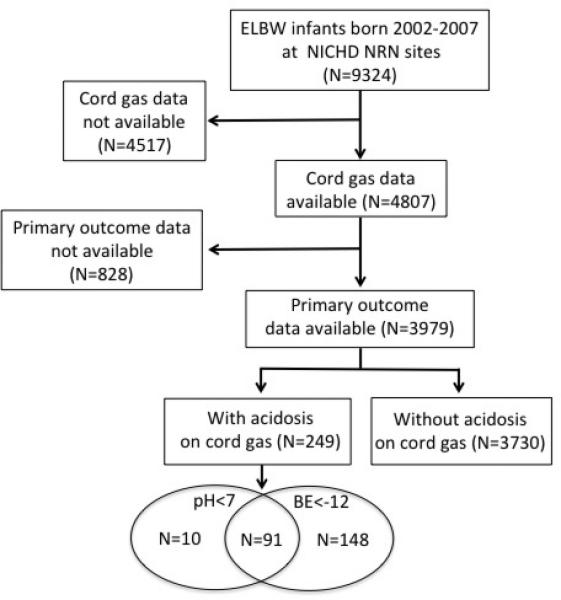
Flow diagram of patients available for study.
Table 1.
Subject Characteristics by Acidosis Status
| Variable | Without Acidosis N = 3730 | With Acidosis N=249 | p-value |
|---|---|---|---|
| Race/ethnicity | 0.004 | ||
| Hispanic/Latino (%) | 20.1 | 21.0 | |
| Non-Hispanic Black (%) | 38.3 | 48.4 | |
| Non-Hispanic White (%) | 37.6 | 27.0 | |
| Other (%) | 4.0 | 3.6 | |
| Private insurance (%) | 39.9 | 31.1 | 0.006 |
| Antenatal steroid (%) | 85.2 | 70.3 | <0.001 |
| Maternal antibiotics (%) | 67.1 | 56.9 | <0.001 |
| Maternal hemorrhage (%) | 17.2 | 29.8 | <0.001 |
| Birth weight (mean+/−SD grams) | 758+/−146 | 730+/−142 | 0.003 |
| 5-minute Apgar (median(5th, 95th percentile)) | 7(2,9) | 5(1,9) | <0.001 |
| Small for gestational age (%) | 17.0 | 16.9 | 0.97 |
| Multiple gestation (%) | 22.5 | 18.5 | 0.14 |
| Male (%) | 51.5 | 51.4 | 0.97 |
| Maternal hypertension (%) | 31.0 | 36.7 | 0.06 |
| Gestational age (mean+/−SD weeks) | 26+/−1.9 | 26+/−2.0 | 0.27 |
Notes: p-values based on Cochran-Mantel-Haenszel chi-square test statistic for categorical outcomes, t-tests for continuous outcomes, and Wilcoxon rank-sum test statistics for ordinal data.
Association of Acidosis with Outcomes
Acidosis at birth was associated with high rates of the primary outcome of death/NDI (OR=1.8, p<0.001), and the secondary outcomes of death alone (OR=2.1, p<0.001), grade III/IV IVH (OR=1.9, p<0.001), and seizures (OR=1.6, p<0.05) in unadjusted analysis.(Table 2) Of 249 infants with acidosis, 165 had the primary outcome of death/NDI (115 patients died, 50 survived with NDI). After adjusting for potentially confounding variables, acidosis remained significantly associated with death/NDI (OR=1.5, p<0.05), death alone (OR=1.9, p<0.001), and grade III/IV IVH (OR=1.8, p<0.05).
Table 2.
Outcomes for Acidotic (Cord pH Values <7 and/or BE <-12) vs. Non-Acidotic ELBW Infants†
| Outcome Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)†† |
|---|---|---|
| Death/NDI | 1.8 (1.4,2.3)** | 1.5 (1.1,2.1)* |
| Death alone | 2.1(1.6, 2.8)** | 1.9 (1.4, 2.6)** |
| NDI alone | 1.2 (0.8, 1.7) | 1.1 (0.8,1.7) |
| Grade III/IV IVH | 1.9 (1.4,2.5)** | 1.8 (1.3,2.5)* |
| Seizures | 1.6 (1.1, 2.4)* | 1.3 (0.9,2.1) |
| Respiratory distress | 1.2 (0.5,2.6) | x |
| BPD | 1.0 (0.7,1.4) | 1.1 (0.7,1.6) |
| Proven NEC | 0.8 (0.5, 1.2) | 0.7 (0.5,1.2) |
| ROP | 0.8 (0.6, 1.3) | 1.0 (0.6,1.6) |
all values adjusted for Bayley version
It adjusted for GA, BW, SGA, multiple gestation, race/ethnicity, sex, ANS, center, and maternal hypertension, hemorrhage, and antenatal antibiotics
p < 0.05
p < 0.001
unable to calculate
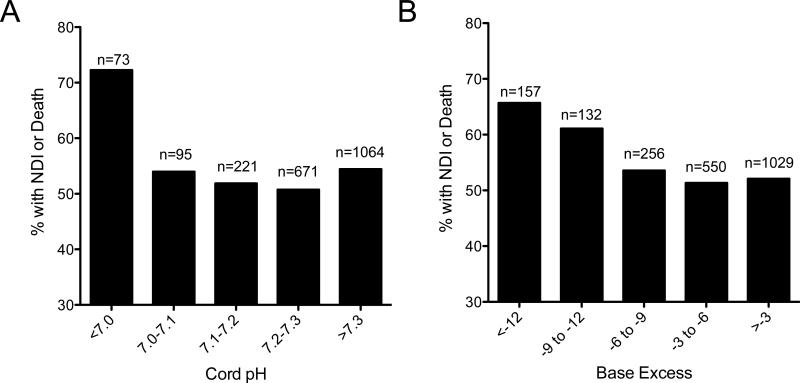
Figures 2A and 2B show the unadjusted rates of death/NDI as a function of pH and BE, respectively. The relationship between acidosis and NDI/death proved to be nonlinear, so pH and BE were treated as categorical variables with predefined cutoffs of pH<7 or BE<-12 after AIC goodness of fit comparisons confirmed better correlations than with linear fit models using pH and BE as continuous variables.
Figure 2.
Unadjusted rates of NDI/death plotted as a function of cord pH (A) or cord base excess (B) are shown for the 3979 infants born at NICHD NRN hospitals from 2002-2007 for whom cord blood gas information and complete neurodevelopmental follow-up information were available.
By category of acidosis, death/NDI occurred in 73 of 101 infants with cord blood gas pH<7 (OR=2.3, p<0.001 compared to infants with pH≥7). Death/NDI occurred in 157 of 239 infants with BE<-12 mEq/L (OR=1.7, p<0.001 compared to infants with BE≥-12).(Table 3) After adjustment for potentially confounding variables, pH<7 remained significantly associated with death/NDI (OR=2.5, p<0.001), death alone (OR=2.3, p<0.001), NDI alone (OR=1.9, p<0.05), and grade III/IV IVH (OR=1.7, p<0.05). BE<-12 mEq/L remained significantly associated with death/NDI (OR=1.5, p<0.05), death alone (OR=1.9, p<0.001), and grade III/IV IVH (OR=1.6, p<0.05). Additional sensitivity analyses using multiple imputation to allow for inclusion of subjects with missing data for predictors or outcome data were conducted and were consistent with the primary findings of significant associations of pH<7 or BE<-12 with NDI/death.
Table 3.
Outcomes for Acidotic vs. Non-Acidotic ELBW Infants by Category of Acidosis†
| Cord pH Values <7 vs. ≥7 | BE values <-12 vs. ≥-12 | |||
|---|---|---|---|---|
| Outcome Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)†† | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)†† |
| Death/NDI | 2.3 (1.5,3.6)** | 2.5 (1.6,4.2)** | 1.7 (1.3,2.3)** | 1.5 (1.1,2.0)* |
| Death alone | 2.1(1.4, 3.1)** | 2.3 (1.5, 3.7)** | 2.1 (1.6, 2.7)** | 1.9 (1.4, 2.6)** |
| NDI alone | 1.9 (1.1, 3.2)* | 1.9 (1.0,3.4)* | 1.1 (0.8, 1.7) | 1.1 (0.7, 1.6) |
| Grade III/IV IVH | 1.9 (1.2,3.0)* | 1.7 (1.0,2.9)* | 1.8 (1.3,2.4)** | 1.6 (1.1,2.3)* |
| Seizures | 1.4 (0.8, 2.6) | 1.4 (0.7,2.6) | 1.7 (1.2,2.5)* | 1.4 (.9,2.2) |
| Respiratory distress | 1.1 (0.4,3.6) | X | 1.1 (0.5,2.5) | X |
| BPD | 1.2 (0.7,2.0) | 1.5 (0.8,2.7) | 1.0 (0.7,1.4) | 1.0 (0.7,1.5) |
| Proven NEC | 0.7 (0.4, 1.6) | 0.8 (0.4,1.8) | 0.8 (0.5,1.3) | 0.8 (0.5,1.3) |
| ROP | 0.7 (0.4, 1.4) | X | ||
all values adjusted for Bayley version
It adjusted for GA, BW, sGa, multiple gestation, race/ethnicity, sex, ANS, center, and maternal hypertension, hemorrhage, and antenatal antibiotics
p < 0.05
p < 0.001
x unable to calculate
Predictive Model Development
The final prediction model of death/NDI included the variables GA, BW, 5-minute Apgar, multiple gestation, sex, biological mother's insurance, maternal hypertension and ANS but not pH or BE. pH and BE dropped out of the final prediction model regardless of whether pH or BE were considered independently or together. Of the predictors, low BW, low GA, low 5-minute Apgar scores, and male sex were most strongly associated with increased predicted likelihood of death/NDI (p<0.001). One explanation for pH and BE dropping out of the predictive model when they were previously found to be significantly associated with the primary outcome is that perinatal acidosis might be reflected in a low 5-minute Apgar score. Indeed a Spearman rank correlation test identified a significant association between both pH and BE and 5-minute Apgar (rs=0.146 for pH, rs=0.183 for BE, p-values<0.001). For the remaining variables, odds ratios were calculated and then each variable was assigned a score with the sum of the scores corresponding to an individual infant's risk of death/NDI (Table 4). ROC curve analysis was used to determine the optimum cut-off value for the score in order to predict death/NDI. In predicting death/NDI, the highest sensitivity and specificity achieved for the training dataset with or without pH and BE included as variables were 0.75 and 0.64 (corresponding to a score cut-off of 10.7). The model performed similarly with the validation dataset, correctly classifying about 70% of the infants (sensitivity and specificity of 0.71 and 0.64). Alternative models, including non-linear models and alternative categorizations of pH and BE, were also considered and none of the models were significantly improved over the results presented using binomial categorization of pH and BE. Forward and stepwise selection of variables also produced the same final model. As collinearity of gestational age, birth weight, and SGA could potentially destabilize the multivariable model, we also reran the adjusted models for NDI/death including just one of these variables at a time and again obtained similar results. Similar results were also obtained when the dataset was limited to umbilical artery samples with the exclusion of umbilical venous samples or when the dataset was limited to singletons.
Table 4.
Assigned Scores for Predictor Variables in Death/NDI Model
| Variable | Score* | P-value |
|---|---|---|
| Birth Weight (g) | ||
| 1000 | 1 | <0.001 |
| 900 | 1.16 | |
| 800 | 1.35 | |
| 700 | 2.25 | |
| 600 | 3.06 | |
| 500 | 6.91 | |
| 400 | 27.7 | |
| Gestational Age (wks) | ||
| > 27 | 1 | <0.001 |
| ≥ 25 and ≤ 27 | 1.43 | |
| < 25 | 2.55 | |
| 5 Minute Apgar | ||
| > 6 | 1 | <0.001 |
| > 3 and ≤ 6 | 1.81 | |
| ≤ 3 | 2.89 | |
| Sex | ||
| Female | 1 | <0.001 |
| Male | 2.10 | |
| Antenatal Steroids | ||
| Yes | 1 | 0.014 |
| No | 1.48 | |
| Insurance | ||
| Private | 1 | <0.001 |
| Other | 1.54 | |
| Maternal Hypertension | ||
| Yes | 1 | 0.031 |
| No | 1.32 | |
| Multiple Gestation | ||
| No | 1 | 0.009 |
| Yes | 1.42 | |
Scores based on normalized odds ratios with a higher score indicating an increased risk of death/NDI.
DISCUSSION
We utilized a large dataset of patients derived from the NICHD NRN preterm infant registry and follow-up studies to study the associations of perinatal acidosis with outcomes in ELBW infants. We found that cord blood gas values of pH<7 or BE<-12 are both significantly associated with death/NDI and severe IVH in ELBW infants after adjustment for confounding variables (Tables 2 and 3). However, acidosis is relatively infrequent in ELBW infants (occurring in only 6.3% of patients) and thus contributes to death and disability in only a small fraction of ELBW infants. While this rate of acidosis seems low given the expected high rates of placental insufficiency, chorioamnionitis, and other pathological conditions that might lead to preterm delivery, it is higher than published rates of acidosis in term infants and is consistent with other published studies in preterm infants.(12, 23) Inclusion of acid/base status into a model with predictor variables including birth weight, gestational age, 5 minute Apgar score, and other variables, does not improve the model's predictive ability perhaps because of the infrequency of acidosis and because acid/base status and 5 minute Apgar score are strongly associated co-variates.
A number of smaller studies have previously attempted to define the importance of acid/base status in ELBW infants. In studies of 219 ELBW infants from 1979-1989, Gaudier et al found that acid-base status was associated with adverse neurologic outcomes but not survival.(14, 15) Victory et al found an association of acidosis with IVH/PVL but did not evaluate NDI or death.(13) In a retrospective case controlled study, Lavrijsen et al studied 44 acidotic preterm infants <32 weeks GA compared to 67 nonacidotic controls. Acidotic preterm infants had higher rates of seizures and IVH, but a significant correlation of acidosis with longer term outcomes was not found.(16) Beeby et al studied 623 infants less than 32 weeks GA and found that acidosis was not associated with adverse outcomes at 1 year after adjusting for confounding variables.(12) These results are in contrast to ours perhaps because we studied a much larger cohort of younger infants with later follow-up.
Although we had hoped accounting for acid/base status would significantly improve our ability to predict adverse outcomes, our final model had only modest predictive ability, consistent with other studies.(7) Acuity scores such as the CRIB-II and SNAP scores, other models based on early data, and even caregiver intuition have been evaluated for their predictive ability in order to assist parents and caregivers of ELBW infants with difficult decisions regarding the discontinuation of intensive care.(8, 24-26) However, even with sophisticated statistical techniques such as neural networks and classification tree analysis, models to date cannot predict adverse outcomes with high accuracy.(8, 27) In our case, consideration of acid/base status helped little; neither pH nor BE remained in the final model likely because they were relatively rare and were covariate with the 5-minute Apgar score. As acidotic infants would be expected to be ill-appearing, the correlation of acid/base status with Apgar scores is not surprising.
Our study has several strengths that distinguish it from previous work investigating the relationship of perinatal acidosis with outcomes in preterm infants. Most previous studies were small or moderately sized, single center studies. By contrast, the NICHD NRN includes multiple sites with diverse patient populations and standardized data collection and follow-up. With 3979 infants studied, this is by far the largest study of its kind with enough subjects to analyze multiple secondary outcomes and to account for multiple confounding variables. In developing our prediction model, we were able to divide the large dataset into development and a test subset such that we were able to validate our model on a different set of infants. Finally, overall outcomes have improved since some of the older studies were published, and the relative importance of perinatal acidosis may have changed as morbidity from other factors declines.
A limitation of our study is the large number of infants with missing cord blood gas data, largely due to variations in practice at the different NRN sites. Cord blood gas measurement is not mandated by the NRN and the frequency of cord blood gas collection varied between sites from 0% to 82%. Infants excluded due to lack of cord gas data were younger on average (24.9 vs. 25.8 weeks), smaller (691g vs 757g), had lower Apgars (5 vs. 7), were less likely to have received antenatal steroids (68% vs 84%) and antenatal antibiotics (62% vs. 67%), than those with cord gas information available (p<0.001 for all). Compared to infants included in the study, infants missing cord gas data had higher mortality rates (44% vs 30%, p<0.001) and small but statistically significant differences in NDI rates in survivors (37% vs. 34%, p<0.01). These differences are a potential source of bias if trying to apply our predictive model to all ELBW infants. A second limitation is that in the last years of the study, neurodevelopmental assessments were performed using the Bayley III exam which has been reported to underestimate disability.(28) For this reason we used a Bayley III cognitive score cutoff of 85 such that the rates of death/NDI were similar in the cohorts evaluated with Bayley II or III (63% vs. 62%). We also controlled for the Bayley exam version in all analyses.
ELBW infants continue to experience high rates of death/NDI due to physiologic derangements associated with extreme prematurity. Our study indicates that, if present in a given infant at birth, acidosis is highly associated with poor outcomes. However, since perinatal acidosis is infrequent in ELBW infants as a population, other factors may be more important for medical prediction and as targets of future research. In addition our study adds to previous work indicating that outcomes of ELBW infants remain difficult to predict from early clinical data making decisions about the withdrawal of care based solely on such data problematic. Algorithms relying on serial assessments of an infant's physiologic status after birth may prove more valuable in predicting the ultimate clinical outcome.(29, 30)
What is known about this topic: ELBW infants are at high risk of death or neurodevelopmental impairment from intracranial hemorrhage, infection, and other complications of prematurity. The incidence of perinatal acidosis and its potential associations with death, neurodevelopmental impairment, or other outcomes have not been well defined for ELBW infants.
What this study adds: A cord blood pH<7 or BE<-12 was found in 6% of ELBW infants. Perinatal acidosis was significantly associated with death or neurodevelopmental impairment, but other factors are likely more important in predicting long term outcomes.
ACKNOWLEDGEMENTS
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network's Generic Database Study.
Data collected at NICHD Neonatal Research Network (NRN) sites were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Dr. Tracy Nolen (DCC Statistician) had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003-2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2007).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; William Oh, MD; Angelita M. Hensman, RN BSN.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Nancy S. Newman, RN.
Cincinnati Children's Hospital Medical Center, University of Cincinnati Hospital, , and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Kathy J. Auten, MSHS; Melody B. Lohmeyer, RN MSN.
Emory University, Children's Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, M01 RR39) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC; Ann M. Blackwelder, RNC BS MS.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; James A. Lemons, MD; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie Dawn Wilson, BSN CCRC.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Betty K. Hastings; Jeanette O'Donnell Auman, BS; Carolyn Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS CCRC, Melinda S. Proud, RCP; Andrew W. Palmquist, RN.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN.
University of Alabama at Birmingham Health System and Children's Hospital of Alabama (U10 HD34216, M01 RR32) – Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Paul R. Wozniak, MD;
Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT.
University of Iowa Children's Hospital (U10 HD53109, M01 RR59) – John A. Widness, MD; Karen J. Johnson, RN BSN; Nancy J. Krutzfield, RN MA.
University of Miami, Holtz Children's Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Ruth Everett-Thomas, RN MSN.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Lu-Ann Papile, MD; Conra Backstrom Lacy, RN.
University of Rochester, Golisano Children's Hospital (U10 HD40521, M01 RR44, UL1 RR24160) – Dale L. Phelps, MD; Linda J. Reubens, RN CCRC; Mary Rowan, RN; Erica Burnell, RN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; Alicia Guzman; Susie Madison, RN.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Beverly Foley Harris, RN BSN; Claudia I. Franco, RNC MSN; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Maegan C. Simmons, RN; Patti Pierce Tate, RCP.
University of Utah, University Hospital, LDS Hospital, and Primary Children's Medical Center (U10 HD53124, UL1 RR25764, M01 RR64) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Jennifer J. Jensen, RN BSN; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN BSN.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children's Hospital (U10 HD40498, M01 RR7122) – T. Michael O'shea, MD MPH; Nancy J. Peters, RN CCRP.
Wayne State University, Hutzel Women's Hospital and Children's Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN.
Yale University, Yale-New Haven Children's Hospital and Bridgeport Hospital (U10 HD27871, UL1 RR24139, MO1 RR125, M01 RR6022) – Richard A. Ehrenkranz, MD; Harris C. Jacobs, MD; Patricia Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN.
FUNDING:
This work was supported by the UAB Department of Pediatrics and by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) grant 5U10HD034216.
Abbreviations
- ANS
Antenatal Steroids
- CP
Cerebral Palsy
- BE
Base Excess
- BPD
Bronchopulmonary Dysplasia
- BW
Birth Weight
- ELBW
Extremely Low Birth Weight
- GA
Gestational Age
- GMFCS
Gross Motor Function Classification System
- IVH
Intraventricular Hemorrhage
- MDI
Mental Developmental Index
- NEC
Necrotizing Enterocolitis
- NDI
Neurodevelopmental Impairment
- NRN
Neonatal Research Network
- PDI
Psychomotor Developmental Index
- RDS
Respiratory Distress Syndrome
- ROP
Retinopathy of Prematurity
Footnotes
COMPETING INTERESTS:
The authors have no known competing interests.
REFERENCES
- 1.Wilson-Costello D. Is there evidence that long-term outcomes have improved with intensive care? Semin Fetal Neonatal Med. 2007;12(5):344–54. doi: 10.1016/j.siny.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Poole WK, et al. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–43. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 4.Tyson JE, Parikh NA, Langer J, et al. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008;358(16):1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercier CE, Dunn MS, Ferrelli KR, et al. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 97(4):329–38. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacKendrick W. Understanding neurodevelopment in premature infants: applied chaos theory. J Pediatr. 2006;148(4):427–9. doi: 10.1016/j.jpeds.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Ambalavanan N, Baibergenova A, Carlo WA, et al. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. J Pediatr. 2006;148(4):438–44. doi: 10.1016/j.jpeds.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Broitman E, Ambalavanan N, Higgins RD, et al. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 2007;151(5):500–5. 5, e1–2. doi: 10.1016/j.jpeds.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambalavanan N, Carlo WA, Shankaran S, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118(5):2084–93. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 11.Arpino C, D'Argenzio L, Ticconi C, et al. Brain damage in preterm infants: etiological pathways. Ann Ist Super Sanita. 2005;41(2):229–37. [PubMed] [Google Scholar]
- 12.Beeby PJ, Elliott EJ, Henderson-Smart DJ, et al. Predictive value of umbilical artery pH in preterm infants. Arch Dis Child. 1994;71(2):F93–6. doi: 10.1136/fn.71.2.f93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victory R, Penava D, da Silva O, et al. Umbilical cord pH and base excess values in relation to neonatal morbidity for infants delivered preterm. Am J Obstet Gynecol. 2003;189(3):803–7. doi: 10.1067/s0002-9378(03)00974-8. [DOI] [PubMed] [Google Scholar]
- 14.Gaudier FL, Goldenberg RL, Nelson KG, et al. Acid-base status at birth and subsequent neurosensory impairment in surviving 500 to 1000 gm infants. Am J Obstet Gynecol. 1994;170(1 Pt 1):48–53. doi: 10.1016/s0002-9378(94)70383-3. [DOI] [PubMed] [Google Scholar]
- 15.Gaudier FL, Goldenberg RL, Nelson KG, et al. Influence of acid-base status at birth and Apgar scores on survival in 500-1000-g infants. Obstet Gynecol. 1996;87(2):175–80. doi: 10.1016/0029-7844(95)00407-6. [DOI] [PubMed] [Google Scholar]
- 16.Lavrijsen SW, Uiterwaal CS, Stigter RH, et al. Severe umbilical cord acidemia and neurological outcome in preterm and full-term neonates. Biol Neonate. 2005;88(1):27–34. doi: 10.1159/000084096. [DOI] [PubMed] [Google Scholar]
- 17.Fee SC, Malee K, Deddish R, et al. Severe acidosis and subsequent neurologic status. Am J Obstet Gynecol. 1990;162(3):802–6. doi: 10.1016/0002-9378(90)91014-4. [DOI] [PubMed] [Google Scholar]
- 18.Hibbard JU, Hibbard MC, Whalen MP. Umbilical cord blood gases and mortality and morbidity in the very low birth weight infant. Obstet Gynecol. 1991;78(5 Pt 1):768–73. [PubMed] [Google Scholar]
- 19.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N. Bayley Scales of Infant Development. 2nd edition ed. Psychological Corp; New York, NY: 1993. [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of surgery. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorp JA, Rushing RS. Umbilical cord blood gas analysis. Obstet Gynecol Clin North Am. 1999;26(4):695–709. doi: 10.1016/s0889-8545(05)70107-8. [DOI] [PubMed] [Google Scholar]
- 24.Lodha A, Sauve R, Chen S, et al. Clinical Risk Index for Babies score for the prediction of neurodevelopmental outcomes at 3 years of age in infants of very low birthweight. Dev Med Child Neurol. 2009;51(11):895–900. doi: 10.1111/j.1469-8749.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 25.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361(9371):1789–91. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 26.Meadow W, Frain L, Ren Y, et al. Serial assessment of mortality in the neonatal intensive care unit by algorithm and intuition: certainty, uncertainty, and informed consent. Pediatrics. 2002;109(5):878–86. doi: 10.1542/peds.109.5.878. [DOI] [PubMed] [Google Scholar]
- 27.Ambalavanan N, Carlo WA, Bobashev G, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–73. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PJ, De Luca CR, Hutchinson E, et al. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–6. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 29.Saria S, Rajani AK, Gould J, et al. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2(48):48ra65. doi: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambalavanan N, Carlo WA, Tyson JE, et al. Outcome trajectories in extremely preterm infants. Pediatrics. 2012;130(1):e115–25. doi: 10.1542/peds.2011-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]