Abstract
When phylogenetically close, two competing species may reproductively interfere, and thereby affect their population dynamics. We tested for reproductive interference (RI) between two congeneric haplo-diploid spider mites, Tetranychus evansi and Tetranychus urticae, by investigating their interspecific mating and their population dynamics when they competed on the same plants. They are both pests of tomato, but differ in the host plant defences that they suppress or induce. To reduce the effect of plant-mediated interaction, we used a mutant tomato plant lacking jasmonate-mediated anti-herbivore defences in the competition experiment. In addition, to manipulate the effect of RI, we introduced founder females already mated with conspecific males in mild RI treatments or founder, virgin females in strong RI treatments (in either case together with heterospecific and conspecific males). As females show first-male sperm precedence, RI should occur especially in the founder generation under strong RI treatments. We found that T. urticae outcompeted T. evansi in mild, but not in strong RI treatments. Thus, T. evansi interfered reproductively with T. urticae. This result was supported by crossing experiments showing frequent interspecific copulations, strong postmating reproductive isolation and a preference of T. evansi males to mate with T. urticae (instead of conspecific) females, whereas T. urticae males preferred conspecific females. We conclude that interspecific mating comes at a cost due to asymmetric mate preferences of males. Because RI by T. evansi can improve its competitiveness to T. urticae, we propose that RI partly explains why T. evansi became invasive in Europe where T. urticae is endemic.
Introduction
In some animal species, males readily mate with heterospecific females, supposedly because they are unable to discriminate between the species (Gro¨ning and Hochkirch, 2008), and they may even prefer to mate with heterospecific females instead of conspecific females (for example, Ludden et al., 2004; Hochkirch et al., 2008). This may come at a cost to the females because it can hamper or even block their reproduction and it may even cause populations of their species to be outcompeted. Thus, reproductive interference may alter the species composition in a community (Kuno, 1992), but such an effect can be brought also about by other competitive processes. For example, communities of plant-inhabiting arthropods are not only determined by direct competition, but also by indirect modes of competition, such as plant-mediated competition (Denno et al., 1995; Ohgushi, 2005; Denno and Kaplan, 2007) and predator-mediated competition (Holt, 1977; Holt and Lawton, 1994; van Veen et al., 2006). Hence, to test for the occurrence of reproductive interference (RI) between species and its effect on the outcome of the interspecific competition, it is necessary not only to perform crossing experiments between (closely related) species and to compare the population dynamics in presence and absence of other species in the field (Gro¨ning and Hochkirch, 2008), but also to carefully design population experiments in which other competitive processes, such as plant-mediated and predator-mediated competition, are excluded and in which the effect of RI is manipulated (for example, Kishi et al., 2009).
To assess the role of RI, we studied two congeneric haplo-diploid species of spider mites, Tetranychus evansi Baker & Pritchard (Acari: Tetranychidae) and Tetranychus urticae (Koch) (Acari: Tetranychidae) (Figure 1). Both species are important pests of solanaceous crops, especially of tomato plants (Solanum lycopersicum L.), the first species being a host plant specialist and the second being a generalist (Helle and Sabelis, 1985; Navajas et al., 2012). Each of the two species spins a protective web on the leaves of their host plant, and under such webs groups of spider mites feed, mate, reproduce and develop. Before mating males guard the last moulting stage of the females and mate directly upon emergence of the female from the exuvium. Proximity to the emerging female is an important determinant of male mating success (for example, Potter et al. 1976). Tetranychus evansi from South America has become an invasive species first in Africa and then in Europe (Boubou et al., 2012; Navajas et al., 2012). Nowadays, its distribution overlaps with that of the closely related species T. urticae in these regions (Helle and Sabelis, 1985; Navajas et al., 2012). Hence, these two species are expected to interact with each other when sharing host plants in the field. Both species belong to the same genus Tetranychus, and in spider mites, RI has been observed between species from the same or even of different genera (Fujimoto et al., 1996; Takafuji et al., 1997; Navajas et al., 2000; Ben-David et al., 2009). RI is, therefore, likely to affect the outcome of competition between T. evansi and T. urticae.
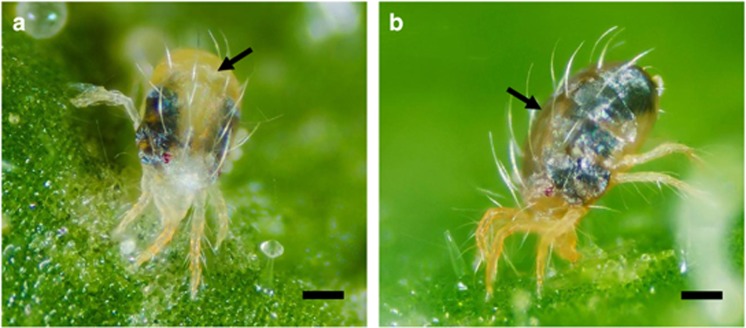
Figure 1.
Photos of two species of spider mites, (a) Tetranychus urticae and (b) Tetranychus evansi, that we used in the experiments. Both species are pest of tomato plants but there is a difference in the colour of the soma (thorax, plus abdomen): green or whitish for T. urticae and red for T. evansi. These colours stem from pigment in the haemolymph and internal tissues (not the cuticle or the internal gut lumen).
Using these two species of spider mites provides us with advantages in designing a competition experiment that allows testing for the effect of RI on spider mite dynamics. First, spider mites show strong first-male sperm precedence (Boudreaux, 1963; Helle, 1967), indicating that secondary matings are less effective and that females do not exhibit cryptic male choice by selecting among sperm of various males for fertilisation of their eggs. This allowed us to manipulate the founder females that were introduced to the competition experiment as follows. In one experiment, we created a mild RI treatment in the founder generation by releasing heterospecific and conspecific males with females that had previously mated with conspecific males. These females can, therefore, reproduce irrespective of secondary matings with heterospecific males. In a parallel experiment, we created a strong RI treatment in the founder generation by releasing heterospecific and conspecific males with virgin females, which can then mate with conspecific and heterospecific males, the latter of which can potentially result in decreased reproduction. A second advantage of using the two species of spider mites is that there is much knowledge of the impact they have on host plant defences: within leaves of tomato plants many strains of T. urticae induce the jasmonate(JA)-mediated plant defences (Li et al., 2002; Ament et al., 2004; Kant et al., 2004, 2008), whereas T. evansi downregulates these defences, even below household levels (Sarmento et al., 2011a), thereby making the host plant more profitable for both T. evansi and T. urticae (Sarmento et al., 2011b). By using mutant def-1 tomato plants that are deficient in mounting JA-mediated plant defences (Howe et al., 1996; Li et al., 2002; Ament et al., 2004; Kant et al., 2008), we were able to reduce the effect of JA-mediated plant defences in our competition experiments, thereby gaining more analytic power to detect RI. Also, we were able to exclude predator-mediated competition by introducing only the two spider mite species on the plants in an isolated environment (climate room).
Hence, by taking advantage of these features from our experimental system, we studied the effect of RI on the competition between T. evansi and T. urticae. This was done by comparing the population growth of the two species between experiments initiated by founder females differing in reproductive status (virgin or mated with conspecific males) on mutant def-1 tomato plants and in absence of natural enemies of the spider mites. We also investigated their mating behaviour, their reproductive compatibility, and male mate choice when males were offered females of these two species simultaneously, in order to analyse the results from our competition experiments.
Materials and methods
Plants
Tomato seeds (S. lycopersicum cv. Castlemart) were sown in 12-cm pots and watered two times per week. After maintaining the plants in a greenhouse for 3 weeks, they were transferred to a climate chamber (25 °C; 60% relative humidity (RH); 16:8 h light:dark photoperiod). When the tomato plants were 4–5-week-old, leaves were detached and then used to maintain the colonies. The interspecific competition experiments were carried on def-1 mutant tomato plants that have a Castlemart genetic background (Howe et al., 1996). These def-1 plants were also grown from seeds and maintained in the same way as the Castlemart plants. We used this plant genotype because the def-1 mutation impairs the accumulation of JA and thereby also the JA-mediated plant defence mechanisms induced after wounding by herbivorous arthropods (Howe et al., 1996). In this way, we reduced the effect of plant-mediated competition between the two phytophagous species.
Mites
Tetranychus urticae and T. evansi were collected from S. nigrum L. in Málaga (N36°34′29″, W5°57′35″), Spain, August 2010. Harvested plant material was placed in separate zip-bags and transported to Amsterdam. All adult females died during transport, however, there were still mites in different developmental stages on the sampled leaves. Subsequently the plant material was transferred to separate trays with tomato leaves and incubated in a quarantine climate box to allow mites to complete the development (25 °C; 60% RH; 16:8 h light:dark photoperiod). These quarantine cultures were checked every 2 days and adult females were transferred to clean tomato leaves and moved to a climate room providing the same conditions. When the colonies were established, 10 males per colony were sampled and placed in 70% ethanol. The species identity of both strains was confirmed on the basis of the aedeagus (the male reproductive organ) morphology (Gutierrez and Etienne, 1986). Whereas T. evansi was reared on detached tomato leaves (cv. Castlemart), T. urticae was reared on detached common bean leaves (Phaseolus vulgaris L.) on wet cotton wool in a plastic box under constant climatic conditions (25 °C; 60% RH; 16:8 h light:dark photoperiod). At least 1 month before use in the experiments, T. urticae was moved to detached tomato leaves under the same climatic conditions.
Females of the two species to be used for the experiments were collected in the teleiochrysalis stage (i.e. moulting stage preceding adult phase; thus definitely before mating occurs immediately after adult emergence) so that they were virgins after their last moult. Hence, we could always exactly infer their mating status from subsequent exposure to males. Males of the two species were obtained from eggs laid by virgin females. Because spider mites reproduce by arrhenotokous parthenogenesis, virgin females produce haploid eggs that develop into males (Helle and Sabelis, 1985).
Crossing experiments
Leaf discs (1.5 cm in diameter), punched from tomato plants (4–5 week-old), were placed on wet cotton wool in a Petri dish, and used as an arena for either mating or rearing of offspring. To observe premating behaviour of spider mites, a single teleiochrysalis female was placed onto a leaf disc where it moulted into the adult phase within 24 h. Subsequently, three virgin males, 1–7-day-old since their last moult, were introduced for mating. The occurrence and duration of copulations was recorded for 0.5 h using a camera (Leica IC80 HD, Leica Microsystems GmbH, Germany) connected to a binocular microscope and a computer with adequate software (LAS EZ software version 2.0.0, Leica Microsystems GmbH). In an independent experiment, we assessed reproductive compatibility between females and males in exactly the same set-up (one female, three males, leaf disc). They were allowed to copulate and oviposit for 5 days. Egg and juvenile survival was checked every 48 h, and gender was checked when they developed into adults. The leaf discs were replaced with fresh ones every 4 days. Using this set-up, we carried out mating and crossing experiments between T. urticae and T. evansi, and also within each of the two species as a control. In addition, we assessed reproduction by virgin females of each of the two species. We evaluated the compatibility of T. urticae and T. evansi by comparing the number of eggs deposited, the offspring survival and the sex ratio (% females) between intraspecific and interspecific crosses. Prezygotic reproductive barriers were inferred from differences in sex ratios, whereas postzygotic barriers were inferred from juvenile survival. The crossing experiments were all performed under constant climatic conditions (25 °C; 60% RH; 16:8 h light:dark photoperiod).
Male mate choice
In spider mites, females have a preference when given a choice between males differing in relatedness and bacterial infection status (Vala et al., 2004; Tien et al., 2011). However, females usually cannot express this preference because males guarding females in the teleiochrysalis stage immediately mate upon female emergence (Potter et al., 1976). We, therefore, assumed that male mate choice prevails. A teleiochrysalis female of T. evansi and one of T. urticae were introduced on a leaf disc (1.5 cm). After the two females moulted to the adult phase, one T. evansi male or one T. urticae male, moulted 2 days earlier, were released on the leaf arena. Male behaviour was observed until copulation occurred. The pairs in which copulation did not occur within 0.5 h after male introduction were excluded from further analysis. This experiment was carried out under constant climatic conditions (25 °C; 60% RH; 16: 8 h light:dark photoperiod).
Interspecific competition on plants
To investigate the competitive relation between the two species, we observed population growth of the mites over a period of 4 weeks (2–3 generations) for the case where two species were introduced together on a tomato plant and for the case where each of the two species mites were introduced separately on a tomato plant. To detect the effect of RI, we modified the reproductive status of founder females in two treatments: they were either virgin or mated with conspecific males. The experimental design is summarized in Table 1. As the spider mites increase exponentially and as they, by doing so, cause death of the tomato plant within a few weeks, we introduced a small number of mites (four females and four males) on a tomato plant as founders. To reduce the effect of JA-mediated plant defence, we used an intact def-1 tomato plant as the host plant. Details of the process of the experiment were as follows.
Table 1. Design of the interspecific competition experiment between T. urticae and T. evansi on tomato plants.
| Treatment | Strong RI | Mild RI | Control1 | Control 2 | Control 3 | Control 4 |
|---|---|---|---|---|---|---|
| Founders | Mix, virgin | Mix, mated | T. urticae virgin | T. evansi virgin | T. urticae mated | T. evansi mated |
| Female | ||||||
| T. urticae | 2 virgin | 2 mated | 4 virgin | — | 4 mated | — |
| T. evansi | 2 virgin | 2 mated | — | 4 virgin | — | 4 mated |
| Male | ||||||
| T. urticae | 2 virgin | 2 virgin | 4 virgin | — | 4 virgin | — |
| T. evansi | 2 virgin | 2 virgin | — | 4 virgin | — | 4 virgin |
Abbreviation: RI, reproductive interference.
Six treatments, each replicated six times, were done with various combinations of virgin or mated females and virgin males with the two different species (T. urticae or T. evansi) at the start of the experiments. Numbers in the body of the table refer to the number of individuals released.
Four females and four males were released together on a leaflet of the third or fourth leaf from an intact def-1 tomato plant (3-week-old). Their establishment on the leaflet was checked for 3 days after mite introduction. When mites were missing during these first 3 days (for example, by accidental death or by falling down), they were replaced to ensure a fixed number of founder mites (thereby reducing the probability that small initial differences in numbers were magnified through exponential growth).
We carried out six treatments, differing in species composition (‘T. urticae', ‘T. evansi' or ‘mix') and the mating status of founder females (‘virgin' or ‘mated') (Table 1). In the first treatment (mix, virgin), two females and two males of each species were released, of which the females were virgin. This served to assess competition under conditions where RI could operate strongly from the start of the experiment (strong RI treatment). To obtain weak RI, we released two mated females and two males of each species (mix, mated; mild RI treatment). Here, RI could mainly arise among the offspring of the mated females. A further four treatments served as controls and consisted of either four mated or four virgin females and four males of each species separately (T. urticae virgin, T. urticae mated, T. evansi virgin and T. evansi mated). In all treatments, females and males were 2-day-old since they reached adulthood. To obtain mated females, 1-day-old virgin females were introduced on leaf arenas with males of the same species, and they were kept together for 1 day to give them sufficient opportunity to copulate. The ratio of males and females on each mating arena was 1:2.
After releasing the mites on the plant, the adult females of each species were counted once per week over a period of four weeks. Females of the two species were differentiated based on their body colour (resulting from pigments in the haemolymph and internal tissue, not in the cuticle or the gut) (Figure 1). The experiments were carried out under constant climatic conditions (25 °C; 60% RH; 16:8 h light:dark photoperiod).
Statistical analysis
The number of pairs where copulation occurred was compared between intra- and interspecific crossings for each of the two species of males, using Fisher's exact test for count data. To test for effects of the species of the male (T. urticae or T. evansi) and crossing type (intra- or interspecific) on the duration of copulation (s), we used a two-way analysis of variance (two-way ANOVA). To test for effects of female species (T. urticae or T. evansi) and crossing type (intra- or interspecific) on the number of eggs, we also used a two-way ANOVA. Juvenile survival rate and offspring sex ratio were analysed using generalised linear models with a binomial error distribution using the species of the female (T. urticae or T. evansi) and mating type (intra- or interspecific) as factors. The effect of each factor was assessed with likelihood ratio tests, comparing the saturated model with the model without the factor.
Male mate choice was analysed using exact binomial tests for each species, taking the probability to choose a conspecific female equal to 0.5 under the null hypothesis.
For the analysis of interspecific competition on plants, generalised linear mixed-effects models (with a Poisson error distribution, ‘glmer' in package ‘lme4' from the statistical package R) were used because repeated measurements were taken over time. To compare the growth curve (change of the number of females over time on a plant) among the control treatments (i.e. in the absence of interspecific competition), a model was constructed with species (‘T. urticae' or ‘T. evansi'), the reproductive status (‘virgin' or ‘mated'), time (weeks after mite introduction) and their interactions as fixed factors and with replicate as random factor. To compare the growth curve between strong RI treatment (mix, virgin) and mild RI treatment (mix, mated), a model was constructed with species (‘T. urticae' or ‘T. evansi'), the reproductive status (‘virgin' or ‘mated'), time (weeks after mite introduction) and their interactions as fixed factors and with replicate as random factor. As we want to know if RI affects the competition (the relation of growth curves) between T. urticae and T. evansi, we tested the effect of higher-order interaction (species × time × reproductive status) on the number of female mites in the latter model. If the competition between T. urticae and T. evansi changes depending on the reproductive status (virgin or mated), the higher-order interaction (species × time × reproductive status) should be significant. To confirm if simply the reproductive status of the founder females affects the relation of growth curve between these two species, we tested the effect of the higher-order interaction in the former model. In the tests, we compared the saturated model with the model without higher-order interaction by a likelihood ratio test. We followed the procedure described by Faraway (2006) in constructing these models. The analyses were performed with the statistical package R ver. 2.14.2 (R Development Core Team (2011)).
Results
Crossing experiment
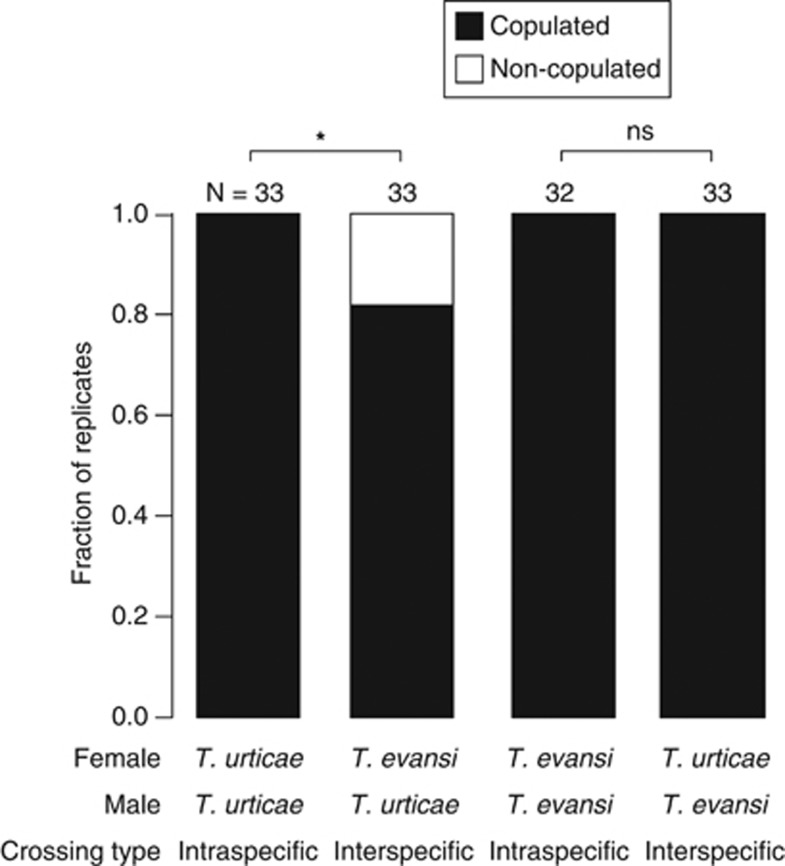
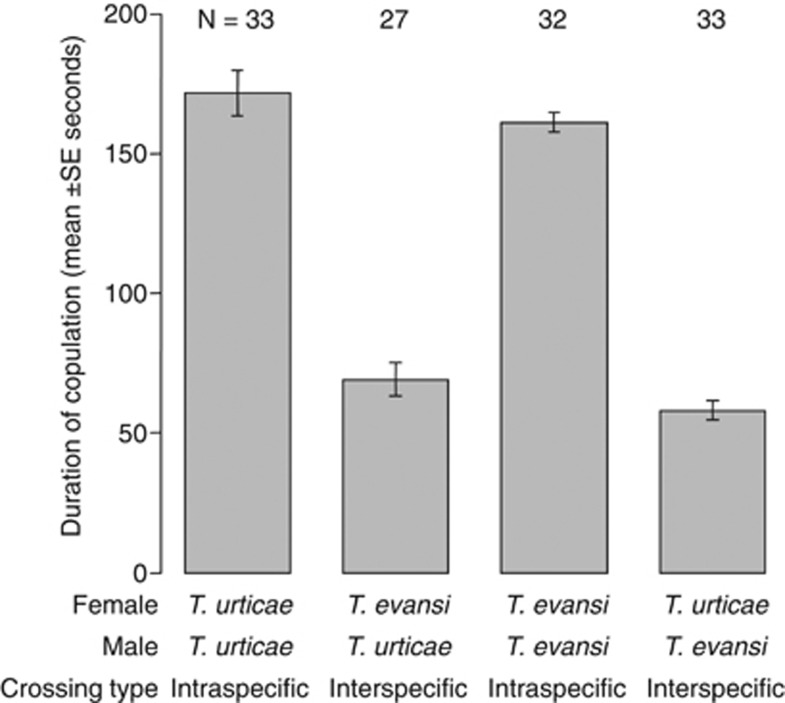
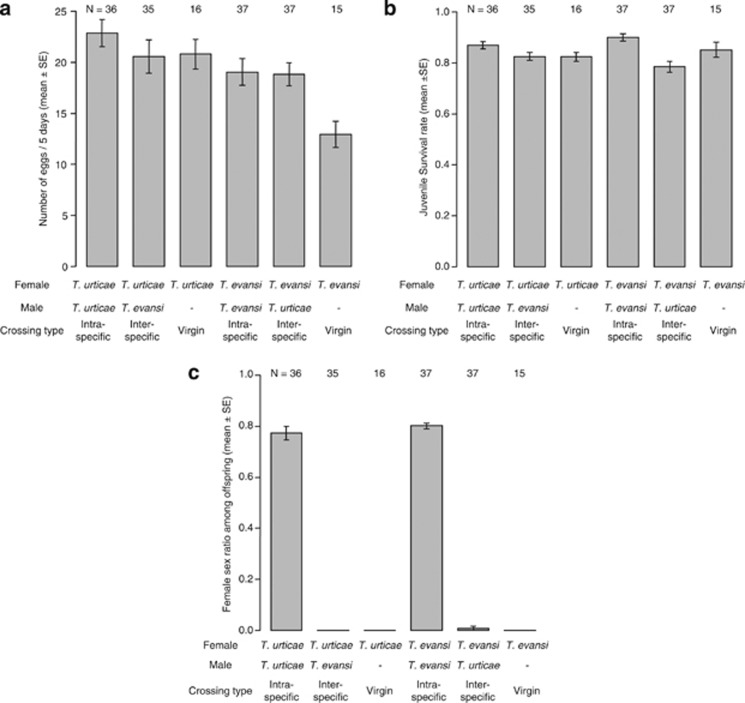
Interspecific copulation occurred between females of T. urticae and males of T. evansi as well as between males and females of the respective species, although the frequency of copulation of T. urticae males with T. evansi females was significantly lower than that of intraspecific copulations in T. urticae (Fisher's exact test, T. urticae (♀) × T. urticae (♂) vs T. evansi (♀) × T. urticae (♂): P=0.024, T. evansi (♀) × T. evansi (♂) vs T. urticae (♀) × T. evansi (♂): P=1.000; Figure 2). The duration of the copulation in interspecific combinations was 2.5 times shorter than that in intraspecific pairs and this difference was significant (two-way ANOVA, crossing type: F1,122=340.44, P<0.001, male species: F1,122=3.65, P=0.06; Figure 3). The number of eggs produced after interspecific crossings did not differ significantly from that after intraspecific crossings, although the number of eggs laid by T. urticae females was significantly larger than that by T. evansi (two-way ANOVA, crossing type: F1,142=0.83, P=0.36, female species: F1,142=4.33, P=0.039; Figure 4a). The juvenile survival rate of offspring from interspecific crossings was lower than that from intraspecific crosses, but this effect was bordering significance (likelihood ratio test, χ2=3.68, P=0.06; Figure 4b). The effect of female species on the juvenile survival rate of offspring was not significant (likelihood ratio test, χ2=0.003, P=0.96; Figure 3b). Unmated females and females of interspecific crossings produced predominantly male offspring, but females from intraspecific crosses produced female-biased offspring (likelihood ratio test, cross type: χ2=983.19, P<0.001, female species: χ2=0.16, P=0.69; Figure 4c). Only a single replicate of the crossing between T. evansi females and T. urticae males yielded three females, which represented genuine hybrids (Figure 4c).
Figure 2.
The fraction of replicates in which intra- or interspecific copulations occurred within 0.5 h after introduction of a virgin female and three virgin males onto a tomato leaf arena. Asterisks indicate statistically significant differences (Fisher's exact test, *P<0.05).
Figure 3.
Bar plots of the duration of intra- or interspecific copulations. Number of replicates are given above the column bars expressing the means, whereas the s.e.m are given as vertical line bars. The effect of crossing type on the duration of copulations was significant, and the effect of species of male was bordering significance (two-way ANOVA, crossing type: F1,122=340.44, P<0.001, male species: F1,122=3.65, P=0.06).
Figure 4.
Bar plots of (a) the number of eggs, (b) the juvenile survival rate of offspring and (c) the female sex ratio among offspring produced by females mated with conspecific or heterospecific males or by virgin females. Column bars express means and vertical line bars represent standard errors. The effect of crossing type on the number of eggs was not significant (two-way ANOVA, crossing type: F1,142=0.83, P=0.36, female species: F1,142=4.33, P=0.039). However, the effect of crossing type on the juvenile survival rate of offspring was bordering significance (likelihood ratio test, cross type: χ2=3.68, P=0.06, female species: χ2=0.003, P=0.96), and the effect of crossing type on the sex ratio of offspring was significant (likelihood ratio test, cross type: χ2=983.19, P<0.001, female species: χ2=0.16, P=0.69).
Male mate choice
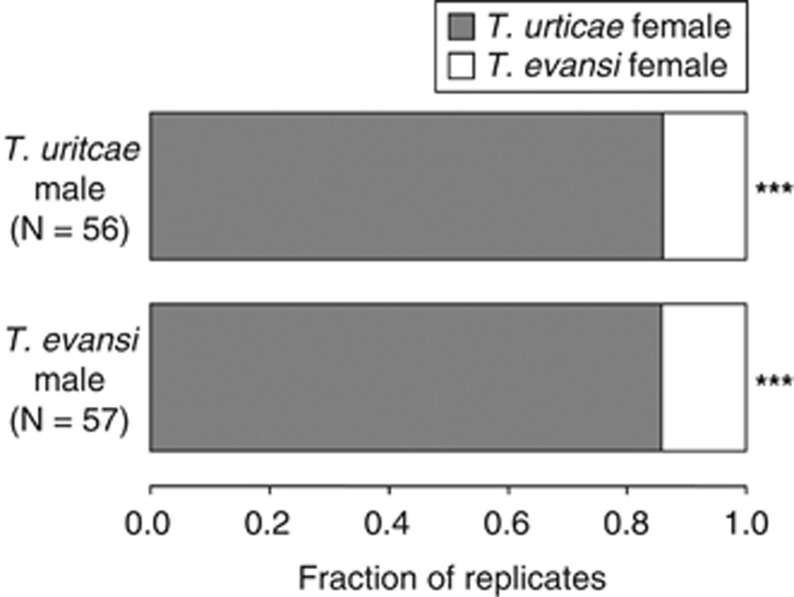
Most T. urticae males first copulated with conspecific females (exact binomial test, P<0.001; Figure 5). However, most T. evansi males first copulated with T. urticae females rather than with conspecific females (exact binomial test, P<0.001; Figure 5).
Figure 5.
The fraction of T. urticae (gray bar) or T. evansi (white bar) females that T. urticae (upper bar) and T. evansi (lower bar) males selected for the first mating after being introduced onto a leaf arena with one T. urticae and one T. evansi female together (exact binomial test, ***P<0.001).
Competition on plants
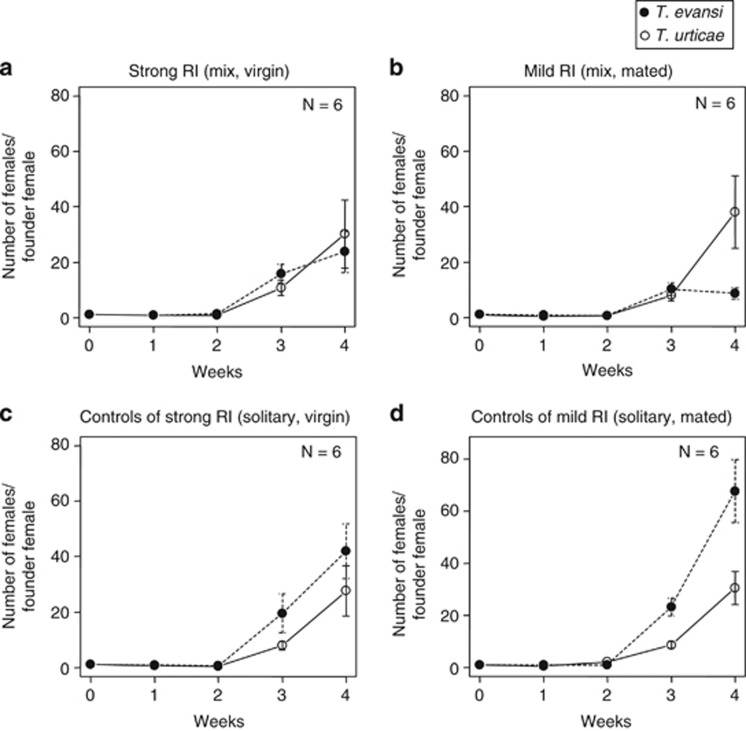
In treatments where each of the two species was introduced separately on a def-1 tomato plant (controls; Table 1), the number of females increased with time and the growth rate of T. evansi was higher than that of T. urticae. The difference between growth curves of T. urticae and T. evansi did not depend on the reproductive status, as the interaction between species, time and reproductive status did not have a significant effect (likelihood ratio test, species × reproductive status × time: χ2=0.645, df=1, P=0.422; Figures 6c and d).
Figure 6.
Population dynamics of T. urticae and T. evansi on tomato plants (def-1) over a period of 4 weeks after mite introduction in strong RI treatment (a), mild RI treatment (b), controls of strong RI treatment (controls 1 and 2) (c), and controls of mild RI treatment (controls 3 and 4) (d). For the mite release design, see Table 1. Filled circles indicate the mean number of T. evansi adult females per founder female, and open circles indicate the mean number of T. urticae adult females per founder female. Vertical bars indicate s.e.m. The number of replicates per treatment is six. When both species were released on a tomato plant together (panels a and b), the interaction between species, reproductive status and time had a significant effect on the number of female mites (likelihood ratio test, species × reproductive status × time: χ2=19.261, df=1, P<0.001). However, when these species were released on a tomato plant separately (panels c and d), the effect of the interaction was not significant (likelihood ratio test, species × reproductive status × time: χ2=0.645, df=1, P=0.422).
When the two species were introduced together on the same plants (treatments of strong RI and mild RI; Table 1), there was a significant effect in the interaction between species, reproductive status and time (likelihood ratio test, species × reproductive status × time: χ2=19.261, df=1, P<0.001; Figures 6a and b). This means that the competitive relation between T. urticae and T. evansi (the difference between the growth curves of T. urticae and T. evansi) significantly differs between strong and mild RI treatments (Figures 6a and b).
Discussion
We found that under strong RI treatment, the colony growth of T. evansi was similar to that of T. urticae (Figure 6a), whereas under mild RI treatment, T. evansi was inferior to T. urticae (Figure 6b). These results suggest that T. evansi counteracted T. urticae by interfering with the reproduction of this competitor. This inference is supported by the results of crossing experiments and male mate choice experiments: interspecific copulation readily occurred between these two species (Figures 2 and 3), they are reproductively incompatible (postmating, prezygotic reproductive barriers; Figure 4) and T. evansi males preferred T. urticae females to conspecific females, whereas T. urticae males preferred conspecific females to T. evansi females (Figure 5).
Given that T. evansi suppresses and T. urticae induces host plant defences, the infestation of the plants by T. evansi makes the host plant more profitable for T. urticae (Sarmento et al., 2011b). Indeed, earlier experiments showed that the oviposition rate of T. urticae increased on tomato leaves previously infested by T. evansi, whereas T. evansi showed a decrease in oviposition on leaves previously infested by T. urticae (Sarmento et al., 2011a). Thus, their co-occurrence on tomato plants may benefit T. urticae but comes at a cost to T. evansi. Nevertheless, T. evansi was found to outcompete T. urticae (Sarmento et al., 2011b). Moreover, field observations in Spain showed that T. evansi invasion replaced native Tetranychus species including T. urticae (Ferragut et al., 2013). Hence, given that T. evansi suppresses tomato plant defence, the question arises how T. evansi prevents T. urticae to profit from this. One explanation for the successful invasion of T. evansi into Europe is that it can prevent T. urticae from taking advantage of the suppressed plant defences by covering the exploited leaf area with a more dense web than that of T. urticae and by producing even more web in response to T. urticae, thereby reducing access of this competitor to the leaf covered by T. evansi web (Sarmento et al., 2011b). However, the web produced by T. evansi may be more effective when populations are large (note that both species produce web and live under the web) and it may still be questioned to what extent it has a role at low mite densities. We suggest that other processes, such as RI, shown in this article, may explain why T. evansi prevents T. urticae from profiting from the T. evansi-induced suppression of plant defences and also why T. evansi succeeded in invading and displacing other Tetranychus species already established. RI likely works against invasive species, because colonisation of invasive species typically starts from small number of founders (Sakai et al., 2001), especially when the introduction occurs accidentally by human activity as in the case of T. evansi (Boubou et al., 2012). RI is frequency dependent and the majority has an advantage when inter-sexual interactions between species are reciprocal (Kuno, 1992). However, most of the inter-sexual interactions between species are asymmetric (Gro¨ning and Hochkirch, 2008), and this can allow invasive species to overcome the disadvantage of small colony size. Recently, biotype B of the whitefly, Bemisia tabaci, invaded and displaced indigenous biotypes of the species in Zhejiang (China; indigenous biotype is ZHJ1) and Queensland (Australia; indigenous biotype is AN; Liu et al., 2007). Behavioural observations revealed asymmetric mating interactions between invasive and indigenous biotypes: when the proportion of males in the offspring increased by adding males of the same or different biotype to a pair, the pair of biotype B increased the number of copulations and consequently increased the proportion of female progeny regardless of the biotype of additional males, however, the pair of indigenous biotypes (ZHJ1 and AN) decreased the number of copulations and the proportion of female progeny when additional males were biotype B (Liu et al., 2007). This asymmetric mating interaction and the capacity to adjust the sex ratio in favour of the population increase in biotype B are expected to be key features of biotype B driving its widespread invasions and displacement, and this expectation is supported by long-term field surveys and population experiments in cages (Liu et al., 2007). In our study, T. evansi males showed mating preference to T. urticae females, whereas T. urticae males prefer conspecific females. It is unknown why T. evansi males prefer females of T. urticae. We hypothesize that T. urticae females produce more of a similar sex pheromone to attract males near emergence from the last moulting stage. Whatever the explanation may be, the preference of T. evansi males to copulate with T. urticae females is possibly one of the key features of T. evansi driving its invasion and displacement in areas endemic to T. urticae.
To exclude or reduce indirect plant-mediated interactions between the two mite species via mite-induced plant defences, we used a def-1 tomato mutant that is deficient in JA accumulation after wounding and hence lacks the expression of genes in response to increased JA. In doing so, we expected that T. urticae could not benefit from the suppressed plant defences induced by T. evansi on the same leaves and that T. evansi would not incur a cost by the plant defences induced by T. urticae. However, the results did not completely meet our expectations. Comparing the population dynamics of T. evansi under mild RI treatment (Figure 6b) and the control (T. evansi, mated; Figure 6d), it can be seen that the population growth of T. evansi was hampered by the presence of T. urticae. In contrast, the same comparison for T. urticae shows that colony development of T. urticae was promoted by the presence of T. evansi (mild RI: Figure 6b; T. urticae, mated: Figure 6d). This suggests that T. urticae induces plant defences that hamper the reproductive performance of other spider mites, not only through JA signalling. Previous studies showed higher reproductive performance of spider mites on a def-1 tomato mutant compared with a wild tomato (Li et al., 2002; Ament et al., 2004), but recent studies show circumstantial evidence for anti-herbivore defences mediated by salicylic acid, another signal transducer mediating the expression of plant defence genes (Farouk and Osman, 2011, but see Kawazu et al., 2011). Although we recognize the possibility of a role for plant defences that are not mediated by JA, we emphasize that this does not change our conclusion regarding the impact of RI. We expect RI to be much more common than shown so far, if future experiments are based on experimental designs that exclude other factors (such as plant defence and shared natural enemies).
Data archiving
Data available from the Dryad Digital Repository: 10.5061/dryad.2n85d.
Acknowledgments
We thank Dr Merijn R Kant, Dr Lívia Silva Ataíde and Mrs Dan Li from the University of Amsterdam for their useful suggestions on the study of interspecific competition on plants and Dr Michiel van Wijk for his help in setting up the video recording equipment. Dr Arne Janssen and Dr Sara Magalhães provided many useful comments on a first draft of the manuscript. Jan van Arkel made the excellent photos of the two mite species shown in Figure 1. JMA was funded as a postdoc via NWO Earth and Life Sciences (ALW) TOP (854.11.005) and YS was funded as a postdoc via the budget of MWS for his Royal Academy of Sciences (KNAW) professorship (selected in 2006 for 5 years and prolonged from 2012 to 2015).
The authors declare no conflict of interest.
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David T, Gerson U, Morin S. Asymmetric reproductive interference between two closely related spider mites: Tetranychus urticae and T. turkestani (Acari: Tetranychidae) Exp Appl Acarol. 2009;48:213–227. doi: 10.1007/s10493-008-9228-9. [DOI] [PubMed] [Google Scholar]
- Boubou A, Migeon A, Roderick GK, Auger P, Cornuet J-M, Magalhães S, et al. Test of colonisation scenarios reveals complex invasion history of the red tomato spider mite Tetranychus evansi. PLoS ONE. 2012;7:35601. doi: 10.1371/journal.pone.0035601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreaux HB. Biological aspects of some phytophagous mites. Ann Rev Entomol. 1963;8:137–154. [Google Scholar]
- Denno RF, McClure MS, Ott JR. Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Ann Rev Entomol. 1995;40:297–331. [Google Scholar]
- Denno RF, Kaplan I.2007Plant-mediated interactions in herbivorous insects: mechanisms, symmetry and challenging the paradigms of competition pastIn: Ohgushi T, Craig TP, Price PW (eds)Ecological Communities: Plant Mediation in Indirect Interaction Webs Cambridge University Press: New York; 19–50. [Google Scholar]
- Faraway JJ. Texts in Statistical Science, Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. Chapman & Hall/CRC: Boca Raton; 2006. [Google Scholar]
- Farouk S, Osman MA. The effect of plant defense elicitors on common bean (Phaseolus vulgaris L.) growth and yield in absence or presence of spider mite (Tetranychus urticae Koch) infestation. J Stress Phys Biochem. 2011;7:5–22. [Google Scholar]
- Ferragut F, Garzón-Luque E, Pekas A. The invasive spider mite Tetranychus evansi (Acari: Tetranychidae) alters community composition and host-plant use of native relatives. Exp Appl Acarol. 2013;60:321–341. doi: 10.1007/s10493-012-9645-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Hiramatsu T, Takafuji A. Reproductive interference between Panonychus mori Yokoyama and P. citri (McGregor) (Acari: Tetranychidae) in peach orchards. Appl Entomol Zool. 1996;31:59–65. [Google Scholar]
- Gro¨ning J, Hochkirch A. Reproductive interference between animal species. Q Rev Biol. 2008;83:257–282. doi: 10.1086/590510. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Etienne J. Les Tetranychidae de l'^ıle de la Réunion et quelques-uns de leurs prédateurs. Agronomie Tropicale. 1986;41:84–91. [Google Scholar]
- Helle W. Fertilization in the two-spotted spider mite (Tetranychus urticae: Acari) Entomol Exp Appl. 1967;10:103–110. [Google Scholar]
- Helle W, Sabelis MW.1985Spider Mites Their Biology, Natural Enemies and ControlVol. 1A,Elsevier: Amsterdam [Google Scholar]
- Hochkirch A, Bücker A, Gro¨ning J. Reproductive interference between the common ground-hopper Tetrix undulata and the slender ground-hopper Tetrix subulata (Orthoptera, Tetrigidae) Bull Entomol Res. 2008;98:605–612. doi: 10.1017/S0007485308005907. [DOI] [PubMed] [Google Scholar]
- Holt RD. Predation, apparent competition, and the structure of prey communities. Theor Popul Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Holt RD, Lawton JH. The ecological consequences of shared natural enemies. Annu Rev Ecol Syst. 1994;25:495–520. [Google Scholar]
- Howe GA, Lightner J, Browse J, Clarence A, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004;135:483–495. doi: 10.1104/pp.103.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Sabelis MW, Haring MA, Schuurink RC. Intraspecific variation in a generalist herbivore accounts for differential induction and impact of host-plant defences. Proc R Soc Lond B. 2008;275:443–452. doi: 10.1098/rspb.2007.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu K, Mochizuki A, Sato Y, Sugeno W, Murata M, Seo S, et al. Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod Plant Int. 2011;6:221–230. [Google Scholar]
- Kishi K, Nishida T, Tsubaki Y. Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol. 2009;78:1043–1049. doi: 10.1111/j.1365-2656.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- Kuno E. Competitive exclusion through reproductive interference. Res Popul Ecol. 1992;34:275–284. [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002;130:494–503. doi: 10.1104/pp.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-S, De Barro PJ, Xu J, Luan J-B, Zang L-S, Ruan Y-M. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 2007;318:1769–1772. doi: 10.1126/science.1149887. [DOI] [PubMed] [Google Scholar]
- Ludden ST, Collins SSA, Brools MA, Winter M. Some males are choosier than others: species recognition in blue waxbills. Behaviour. 2004;141:1021–1039. [Google Scholar]
- Navajas M, Tsagkarakov A, Lagnel J, Perrot-Minnot M-J. Genetic differentiation in Tetranychus urticae (Acari: Tetranychidae): polymorphism, host races or sibling species. Exp Appl Acarol. 2000;24:365–376. doi: 10.1023/a:1006432604611. [DOI] [PubMed] [Google Scholar]
- Navajas M, de Moraes GJ, Auger P, Migeon A. Review of the invasion of Tetranychus evansi: biology, colonization pathways, potential expansion and prospects for biological control. Exp Appl Acarol. 2012;59:43–65. doi: 10.1007/s10493-012-9590-5. [DOI] [PubMed] [Google Scholar]
- Ohgushi T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst. 2005;36:81–105. [Google Scholar]
- Potter DA, Wrensch DL, Johnston DE. Aggression and mating success in male spider mites. Science. 1976;193:160–161. doi: 10.1126/science.193.4248.160. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2011R: A Language and Environment for Statistical ComputingR Foundation for Statistical Computing: Vienna, Austria. http://www.R-project.org/ .
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, et al. The population biology of invasive species. Annu Rev Ecol Evol Syst. 2001;32:305–332. [Google Scholar]
- Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MGA, et al. A herbivore that manipulates plant defence. Ecol Lett. 2011;14:229–236. doi: 10.1111/j.1461-0248.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento RA, Lemos F, Dias CR, Kikuchi WT, Rodrigues JCP, Pallini A, et al. A herbivorous mite down-regulates plant defence and produces web to exclude competitors. PLoS ONE. 2011;6:23757. doi: 10.1371/journal.pone.0023757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takafuji A, Kuno E, Fujimoto H. Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp Appl Acarol. 1997;21:379–391. [Google Scholar]
- Tien NSH, Massourakis G, Sabelis MW, Egas M. Mate choice promotes inbreeding avoidance in Tetranychus urticae. Exp Appl Acarol. 2011;54:119–124. doi: 10.1007/s10493-011-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen FJ, Morris RJ, Godfray HCJ. Apparent competition, quantitative food webs and the structure of phytophagous insect communities. Annu Rev Entomol. 2006;51:187–208. doi: 10.1146/annurev.ento.51.110104.151120. [DOI] [PubMed] [Google Scholar]
- Vala F, Egas M, Breeuwer JAJ, Sabelis MW. Wolbachia affects oviposition and mating behaviour of its spider mite host. J Evol Biol. 2004;17:692–700. doi: 10.1046/j.1420-9101.2003.00679.x. [DOI] [PubMed] [Google Scholar]