SUMMARY
Most simian immunodeficiency viruses use their Nef protein to antagonize the host restriction factor tetherin. A deletion in human tetherin confers Nef resistance, representing a hurdle to successful zoonotic transmission. HIV-1 group M evolved to utilize the viral protein U (Vpu) to counteract tetherin. Although HIV-1 group O has spread epidemically in humans, it has not evolved a Vpu-based tetherin antagonism. Here we show that HIV-1 group O Nef targets a region adjacent to this deletion to inhibit transport of human tetherin to the cell surface, enhances virion release, and increases viral resistance to inhibition by interferon-α. The Nef protein of the inferred common ancestor of group O viruses is also active against human tetherin. Thus, Nef-mediated antagonism of human tetherin evolved prior to the spread of HIV-1 group O and likely facilitated secondary virus transmission. Our results may explain the epidemic spread of HIV-1 group O.
INTRODUCTION
Human immunodeficiency viruses (HIVs) are the consequence of numerous zoonotic transmissions of primate lentiviruses to humans (Sharp and Hahn, 2011). Both HIV-1 and HIV-2 are classified into multiple groups, each of which arose from an independent transmission of a simian immunodeficiency virus (SIV). The four groups of HIV-1 (M, N, O, and P) originated from SIVs infecting chimpanzees and gorillas, whereas an SIV from sooty mangabeys is the direct precursor of at least nine groups of HIV-2. However, the viruses resulting from these transmissions have spread with very different efficiency in the human population. The AIDS pandemic resulted from a single transmission of a chimpanzee virus (SIVcpz) that led to the emergence of HIV-1 group M strains. In contrast, HIV-1 group N strains, which are also of chimpanzee origin, have been detected in fewer than 20 individuals (Delaugerre et al., 2011). The other two groups of HIV-1 are both more closely related to SIVgor infecting gorillas, but again, the two transmissions have had very different outcomes. HIV-1 group P has only been found in two Cameroonian individuals (Plantier et al., 2009; Vallari et al., 2011), whereas group O viruses account for 1%–2% of all HIV-1 infections in Cameroon and neighboring countries in west-central Africa (Vessiére et al., 2010). Overall it is estimated that HIV-1 group O has infected about 100,000 individuals (Mourez et al., 2013).
Differences in adaptation to the new human host are likely one reason for the differential spread of the four groups of HIV-1 (Sauter et al., 2010; Sharp and Hahn, 2011). In particular, the host restriction factor tetherin seems to represent a significant obstacle for successful cross-species transmissions of primate lentiviruses. Tetherin blocks the release of virions from infected cells (Neil et al., 2008; Van Damme et al., 2008) and thus contributes to the control of viral replication in vivo (Liberatore and Bieniasz, 2011). Most SIVs, including the precursors of HIV-1 and HIV-2, encode Nef proteins, which antagonize tetherin in their respective primate hosts (Jia et al., 2009; Sauter et al., 2009; Zhang et al., 2009). However, the human tetherin gene contains a deletion that removes five amino acids from its cytoplasmic domain and confers resistance to SIV Nef proteins. It is currently believed that this presents a barrier to successful spread of SIV among humans, which can only be overcome by switching from Nef to other viral antagonists (Sauter et al., 2009; Yang et al., 2010). Indeed, during adaptation to humans, HIV-1 group M and (less effectively) N viruses evolved the ability to utilize another viral protein (Vpu) to counteract tetherin (Sauter et al., 2009, 2012). However, previous studies suggested that HIV-1 group O and P viruses failed to evolve an effective antagonist of human tetherin (Sauter et al., 2009, 2011; Petit et al., 2011; Vigan and Neil, 2011; Yang et al., 2011). Thus, it has remained a mystery why HIV-1 group O viruses have been capable of infecting tens of thousands of people.
To explore this conundrum, we performed functional analyses of group O Nef proteins, including their inferred most recent common ancestor (MRCA). In agreement with previous results (Sauter et al., 2009; Yang et al., 2010, 2011), group O Nefs had only modest effects on virus release in transient transfection assays. Unexpectedly, however, they efficiently downmodulated human tetherin from the cell surface by targeting a region immediately adjacent to the deletion. This tetherin downmodulation function enhanced virus release from primary CD4+ T cells and increased viral resistance to inhibition by interferon-α (IFNα). Notably, the MRCA of group O Nefs was also active against human tetherin, suggesting that this function was acquired prior to the spread of contemporary HIV-1 O strains. Our results demonstrate that the five amino acid deletion in human tetherin does not confer resistance to primate lentiviral Nef proteins and suggest that gain of antitetherin activity by Nef facilitated the spread of HIV-1 group O among humans.
RESULTS
HIV-1 Group O Nefs Suppress Cell Surface Expression of Human Tetherin
Previous studies performed in transfected 293T cells suggested that HIV-1 group O viruses failed to evolve an effective antagonist of human tetherin (Sauter et al., 2009; Yang et al., 2011). We noted, however, that some HIV-1 O Nefs (O-Nefs) increased virus release slightly in the presence of human tetherin (Sauter et al., 2009). To examine whether these modest effects were an indication of antitetherin activity, we tested the effect of eight contemporary O-Nefs on virus release. All O-Nefs were expressed at detectable levels (Figure S1A available online) and exhibited substantially lower potency than the control HIV-1 M NL4-3 Vpu in the virus release assay (Figure 1A, left). However, while group M- and N-Nefs were entirely inactive, some O-Nefs enhanced infectious virus and p24 release above background in transfected 293T cells (Figure 1A, right).
Figure 1. Antitetherin Activity of HIV-1 Group O Nef Proteins.
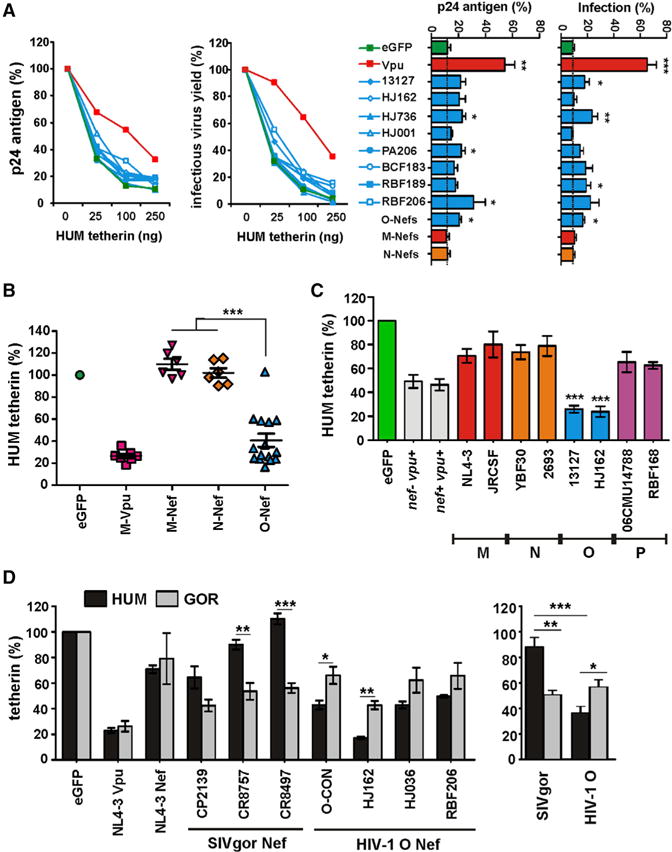
(A) Virus release from 293T cells cotransfected with an HIV-1 NL4-3 ΔvpuΔnef construct and vectors expressing the indicated nef alleles or human (HUM) tetherin. Virus yield was determined by p24 ELISA (left) or infection of TZM-bl cells (right). Data show mean percentages (± SEM) relative to those detected in the absence of tetherin (100%) obtained in three independent experiments. The right panel shows the levels of p24 antigen and infectious virus release in the presence of 100 ng of HUM tetherin expression construct. For comparison, results obtained for 18 M- and 9 N-Vpus are shown in the bar diagram. Stars refer to the difference from the EGFP control panel. *p < 0.05, **p < 0.01, ***p < 0.001.
(B) Effect of HIV-1 Nefs on surface expression of human tetherin. Shown are the levels of tetherin cell surface expression in the presence of HIV-1 group M Vpus or M-, N-, and O-Nefs relative to those measured in 293T cells transfected with the EGFP control vector and tetherin expression plasmid alone (100%). Each symbol represents one individual nef or vpu allele. Shown are average values derived from four to six independent experiments.
(C) PHA-activated PBMCs were transduced with VSV-G-pseudotyped vpu-defective HIV-1 NL4-3 constructs expressing the indicated nef alleles and examined for tetherin surface expression 3 days later. Shown are average levels (± SEM) of surface expression in virally infected (EGFP+) cells relative to uninfected cells (100%). *** indicates that O-Nefs are significantly (p < 0.001) more active than M-, N-, or P-Nefs.
(D) Nef-dependent reduction of HUM and GOR tetherin surface expression in 293T cells. Shown are the levels of tetherin cell surface expression in the presence of various Nefs relative to those measured in cells transfected with the control vector expressing only EGFP. The right panel shows the average levels of HUM and GOR tetherin surface expression in the presence of the three SIVgor and HIV-1 O Nefs (see also Figure S1).
To determine whether these minor effects on virus release were associated with Nef-mediated reduction of cell surface expression of human tetherin, we cotransfected 293T cells with vectors coexpressing Nef or Vpu and EGFP (or EGFP alone for control) together with a construct expressing the human tetherin ortholog. HIV-1 M Vpus reduced human tetherin cell surface expression by about 75%, whereas HIV-1 M and N Nefs had no significant effects (Figure 1B). Surprisingly, however, coexpression of each of 15 HIV-1 O Nef proteins clearly reduced cell surface expression of human tetherin (Figures 1B and S1B). The only inactive O-Nef was derived from an infectious molecular clone of HIV-1 group O (pCMO2.5) (Tebit et al., 2004), which had also failed to exhibit antitetherin activity in a previous study (Yang et al., 2011). Since the CMO2.5 nef allele was also poorly active in other assays for Nef function (data not shown), we excluded it from further analyses. Overall, O-Nefs were almost as active as M-Vpus in downmodulating human tetherin. Since earlier studies had mainly examined virus release (Sauter et al., 2009; Yang et al., 2011) or focused only on group O Vpu proteins (Vigan and Neil, 2011), this group O Nef function has previously gone unrecognized.
To confirm the effects of O-Nefs on human tetherin in primary target cells of HIV-1, we cloned several group O nef alleles into a vpu-defective HIV-1 NL4-3 IRES-EGFP proviral reporter construct (Schindler et al., 2006). Flow cytometric analyses showed that HIV-1 group O Nefs decreased cell surface expression of tetherin substantially in HIV-1-infected human PBMCs, whereas Nef proteins from HIV-1 group M, N, and P strains showed only modest effects (Figure 1C). In contrast, the efficiency of Nef-mediated downmodulation of CD4 and MHC-I was similar in all groups of HIV-1 as well as SIVgor (Figure S1C).
To visualize the effects of Nef on the subcellular localization of tetherin, we cotransfected 293T cells with constructs expressing AU1-tagged Nefs or HA-tagged human tetherin and examined them by laser-scanning confocal microscopy. In the absence of Nef or in the presence of the group M NL4-3 Nef, tetherin was localized at the cell surface as well as intracellularly (Figures S1D and S1E). In contrast, tetherin was detected almost exclusively in intracellular compartments in the presence of the HJ162 O-Nef (Figure S1F). However, in the presence of the SIVgor CP2139 Nef, a significant portion of tetherin remained at the cell surface (Figure S1G). Thus, HIV-1 group O Nefs evolved the ability to decrease surface levels of tetherin and sequester it to intracellular perinuclear compartments.
To examine whether O-Nefs specifically adapted to humans, we analyzed nef alleles from three contemporary SIVgor and HIV-1 group O strains for their ability to counteract human as well as gorilla tetherin. Predictably, SIVgor Nefs decreased surface expression levels of gorilla tetherin more efficiently than those of human tetherin (Figure 1D). In contrast, HIV-1 group O Nefs were significantly more active against the human tetherin ortholog (Figure 1D). Thus, HIV-1 O Nefs clearly gained activity against human tetherin during adaptation to the human host.
The Nef Protein of the MRCA of HIV-1 Group O Counteracts Human Tetherin
To assess whether the ability of O-Nefs to suppress cell surface expression of human tetherin evolved prior to the spread of HIV-1 group O in the human population, we inferred the Nef sequence encoded by the MRCA of group O viruses using maximum-likelihood methods. For comparison, we also inferred the Nef sequences of the group M- and N-MRCAs; we synthesized two M-MRCA Nefs, varying in one residue, to reflect a difference in the sequences inferred by different reconstruction methods (Figure S2A). All ancestral Nef proteins were expressed (Figure S2B) and downmodulated CD4 and MHC-I as efficiently as Nefs from contemporary group M, N, and O strains (Figure S2C).
The O-MRCA Nef significantly increased the release of infectious HIV-1 in 293T cells transfected with different doses of tetherin expression plasmids (Figures 2A and S2D). Notably, the O-MRCA Nef counteracted both human and gorilla tetherin, whereas SIVgor Nef was only active against the latter, and M- and N-MRCA Nefs were inactive (Figure 2B). In agreement with results from virus release assays, M- and N-MRCA Nefs affected surface expression of human and gorilla tetherin only marginally, whereas the O-MRCA Nef reduced expression of both by 70%–80% in transfected 293T cells (Figure 2C), and of human tetherin by about 70% in HIV-1-infected PBMCs (Figure 2D).
Figure 2. Functional Analysis of MRCA HIV-1 Nef Proteins.
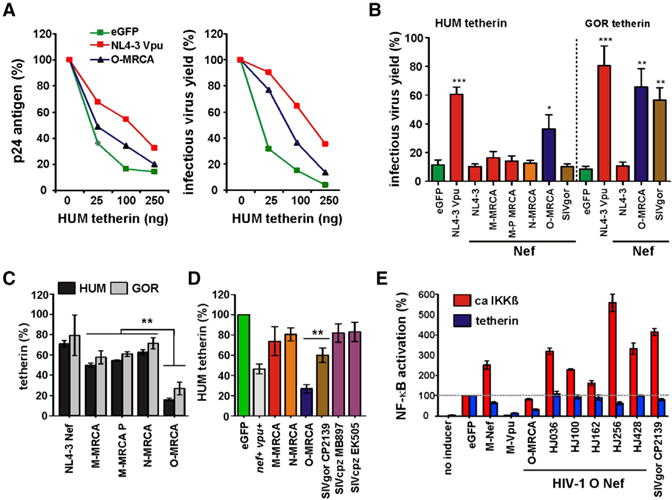
(A) p24 antigen (left) and infectious virus (right) release from 293T cells cotransfected with an HIV-1 NL4-3 ΔvpuΔnef construct and vectors expressing the NL4-3 Vpu or the O-MRCA Nef and the indicated amounts of a vector expressing HUM tetherin. Shown are percentages relative to those detected in the absence of tetherin (100%). Values are means from three experiments.
(B) Infectious virus yield from 293T cells cotransfected with an HIV-1 NL4-3 ΔvpuΔnef construct and vectors expressing the indicated nef alleles in combination with plasmids expressing HUM or GOR tetherin. Shown are average values derived from triplicate infections of TZM-bl indicator cells relative to those obtained in the absence of tetherin expression vector (100%).
(C) Levels of tetherin cell surface expression in the presence of HIV-1 group M, N, and O MRCA Nefs relative to those measured in 293T cells transfected with the EGFP control vector (100%). The M-MRCA and M-MRCA P Nefs differ by a single E to P substitution at their N terminus (Figure S2A). Values in all panels are means (± SEM) from at least three experiments.
(D) PHA-activated PBMCs were transduced with VSV-G-pseudotyped vpu-defective HIV-1 NL4-3 constructs expressing the indicated nef alleles and examined for tetherin surface expression three days later. The results show average levels (± SEM) in virally infected (EGFP+) cells relative to uninfected cells (100%).
(E) Effect of various Nef and Vpu proteins on NF-κB activation. Activation of NF-κB-dependent firefly luciferase expression in 293T cells cotransfected with tetherin or constitutively active IKKβ, NF-κB-dependent (three NF-κB binding sites) firefly luciferase reporter constructs, a plasmid expressing Gaussia luciferase under the control of a minimal promoter, and the indicated nef alleles. Shown are average values derived from three experiments (see also Figure S2).
Recently, it has been shown that tetherin also acts as an immune sensor of HIV-1 that induces NF-κB-dependent proinflammatory responses (Galão et al., 2012). To examine possible effects of O-Nefs on the signaling activity of human tetherin, we cotransfected 293T cells with a tetherin expression plasmid, an NF-κB-dependent firefly luciferase reporter construct, and a plasmid expressing Gaussia luciferase under the control of a minimal promoter. Dual-luciferase assays were performed, and the firefly luciferase signals were normalized to the corresponding Gaussia luciferase signals to compensate for differences in transfection efficiencies and cell numbers. We found that the O-MRCA Nef suppressed NF-κB activation almost as efficiently as the control NL4-3 Vpu, whereas contemporary HIV-1 O nef alleles did not suppress tetherin-mediated NF-κB activation (Figure 2E). It has previously been reported that M-Nefs enhance NF-κB activity (Fortin et al., 2004). To determine whether O-Nefs share this function, we stimulated NF-κB activation by coexpression of a constitutively active mutant of IKKβ in the presence of Vpu and various Nef proteins. Interestingly, all contemporary O-Nefs boosted NF-κB activation, whereas the O-MRCA Nef had no enhancing effect (Figure 2E). Thus, downstream activating effects of contemporary O-Nefs may mask possible inhibitory effects on NF-κB activity through downmodulation of human tetherin.
HIV-1 Group O Nefs Target a Distinct Region in Human Tetherin
Nef proteins of primate lentiviruses, including those encoded by SIVcpz and SIVgor, target the precise region of five amino acids in tetherin that is deleted in the human ortholog (Jia et al., 2009; Sauter et al., 2009; Zhang et al., 2009). To determine whether O-Nefs target a different region, we utilized a “repaired” version of human tetherin (HUM-rep) in which the deleted residues had been reintroduced (Sauter et al., 2009) and analyzed the effect of alanine scanning mutations in the cytoplasmic domain on its susceptibility to SIVgor and HIV-1 O Nef proteins. As expected (Sauter et al., 2009), alterations in amino acid residues 14–18 (DDIWK) that are missing in human tetherin disrupted its susceptibility to SIVgor Nef (Figure 3). However, these alterations did not affect the susceptibility of tetherin to O-MRCA or HJ162 Nefs. In contrast, changes in residues 5–8 (SYDY) impaired the ability of these O-Nefs to downmodulate tetherin (Figure 3). Analysis of single alanine scanning mutants confirmed that the YDY residues in human tetherin play a role in downmodulation by O-Nefs (Figure S3A). Notably, the O-MRCA Nef tolerated changes at the N terminus of human tetherin better than the HJ162 Nef (Figures 3 and S3A). All mutant forms of tetherin were efficiently expressed (Figure S3B). These mapping experiments showed that during adaptation to humans HIV-1 O nef alleles evolved the capability to target a region in the cytoplasmic domain of human tetherin that is directly adjacent to the deleted residues.
Figure 3. Mapping of Residues in Human Tetherin Targeted by HIV-1 O Nefs.
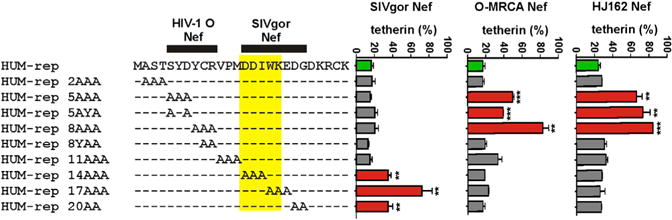
Effect of alanine substitutions in the cytoplasmic part of HUM-rep tetherin on susceptibility to SIVgor, O-MRCA, and HJ162 nef alleles. Shown are the levels of tetherin cell surface expression in the presence of Nef relative to those measured in cells transfected with the EGFP-only control vector (100%). Values are averages (± SEM) derived from three experiments (see also Figure S3).
Complex Determinants of Group O Nef-Mediated Antagonism of Human Tetherin
To determine which amino acid changes in Nef conferred the ability to downmodulate human tetherin, we analyzed a set of 12 chimeras between the active O-MRCA and inactive SIVgor CP2139 Nefs (Figure 4A). We found that most of these Nef chimeras showed functional activities intermediate between the O-MRCA and SIVgor Nefs. Substitution of the N- and C-terminal parts of the O-MRCA Nef by those of SIVgor significantly reduced the effect on cell surface expression of human tetherin (Figure 4A). Western blot analysis confirmed that these functional differences were not due to different Nef expression levels (Figure S3A). Analyses of additional chimeras between the HIV-1 O HJ162 or 13127 Nef proteins and the SIVgor Nef confirmed that residues in the N- and C-terminal region contribute to the ability of O-Nefs to downmodulate human tetherin (Figure 4B). Sequence comparison revealed that active and inactive Nefs differ in at least eight amino acid residues at their C termini (Figure S4A). Whereas mutation of the C-terminal four of these residues had no significant effect, mutation of the N-terminal four residues (K186V, Q188R, S192A, and L195T) significantly reduced O-MRCA and HJ162 Nef-mediated downmodulation of human tetherin (Figure 4B). These four residues are in close proximity of each other and are all exposed at the surface of Nef (Figure 4C). Thus, they may form a distinct interaction site with tetherin. However, none of the mutations in HIV-1 O and SIVgor Nefs fully recapitulated the phenotype of the parental Nef proteins.
Figure 4. Mapping of Residues in HIV-1 O Nefs Involved in Modulation of Human Tetherin.
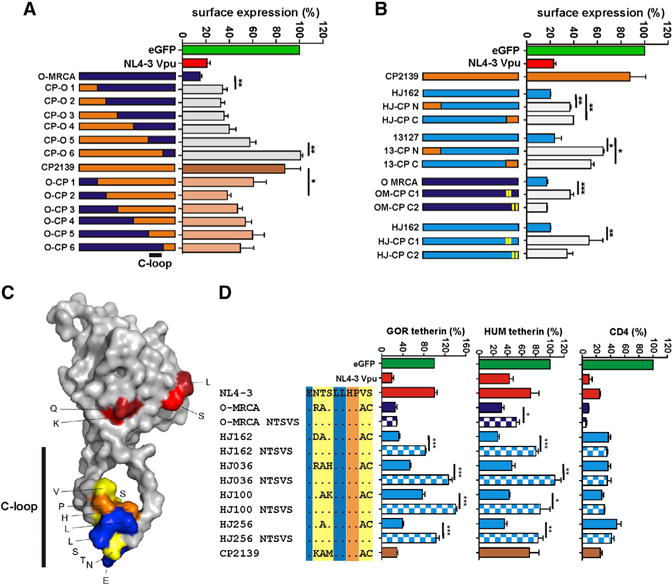
(A and B) Effect of chimeras between HIV-1 O and SIVgor Nefs and mutant O-Nefs on cell surface expression of HUM tetherin. Alterations introduced into the O-MRCA and HJ162 C1 and C2 mutants are shown in Figure S4A.
(C) Localization of amino acid residues involved in antitetherin activity in the HIV-1 Nef structure. The alterations in the C1 region of Nef (red) and the NTS-x4-VS residues (yellow) in NL4-3 Nef critical for the tetherin antagonism, the acidic residue and two leucines (blue) of the dileucine motif involved in the interaction with AP complexes, and two conserved amino acids in between (orange), are highlighted.
(D) Effect of changes in and near the ExxxLL motif of Nef on downmodulation of HUM or GOR tetherin and CD4 cell surface expression. Values represent average expression levels (± SEM; n = 3) relative to those obtained in the presence of the vector expressing only EGFP. Color coding in the alignment corresponds to that in the Nef structure shown in (C). See also Figure S4.
It has been shown that alterations in and near the highly conserved ExxxLL motif in the C-terminal loop of Nef (Figure 4C) were critical for the reacquisition of antitetherin activity of a chimpanzee-adapted HIV-1 strain (Götz et al., 2012). To determine whether these residues are also essential for the antitetherin activity of O-Nefs, we mutated them to the NTS161–163 and VS168/169 residues found in the inactive NL4-3 Nef (Figure 4D). Functional analyses showed that these amino acid substitutions did not affect Nef expression levels (Figure S4B), but impaired the ability of all contemporary HIV-1 O Nefs to reduce the cell surface expression of both human and gorilla tetherin (Figure 4D). Downmodulation by the O-MRCA Nef was also reduced by these changes, albeit less severely and only in the case of human tetherin. These changes in the variable residues of the otherwise highly conserved ExxxLL motif (Figure S4C) also disrupted the modest effect of O-Nefs on virus release in transfected 293T cells (Figure S4D). In agreement with previous results (Götz et al., 2012), these alterations in and adjacent to the ExxxLL adaptor protein interaction site specifically affected tetherin antagonism and had no significant effect on other Nef functions (Figure 4D, data not shown). Thus, these residues are of general relevance for the ability of Nef to counteract tetherin. However, the determinants of Nef-mediated downmodulation of human tetherin seem to be complex and involve multiple regions in O-Nef proteins.
HIV-1 O Nefs Impair Transport of Human Tetherin to the Cell Surface
Next, we explored the mechanism of tetherin downmodulation by HIV-1 O Nefs. It has been reported that SIV Nefs accelerate endocytosis of monkey or ape tetherins (Zhang et al., 2011; Serra-Moreno et al., 2013). To determine whether this is also the case for the effect of O-Nefs on human tetherin, we analyzed the levels of total and internalized tetherin at different time points (Figure 5A, left). We found that the O-MRCA and HJ162 Nefs increased the relative rate of human tetherin internalization after 1hr from 30% to50% (Figure 5A, right). This effect was disrupted by the NTSVS mutations in the HJ162 Nef, but not in the O-MRCA Nef protein. In contrast to Vpu, O-Nefs increased, rather than reduced, the total intracellular levels of human tetherin. We did, however, observe a reduction of a low-molecular-weight form of full-length human tetherin in the presence of the parental and mutant O-MRCA Nefs (Figure S5A).
Figure 5. Mechanism of Downmodulation of Human Tetherin by HIV-1 O Nefs.
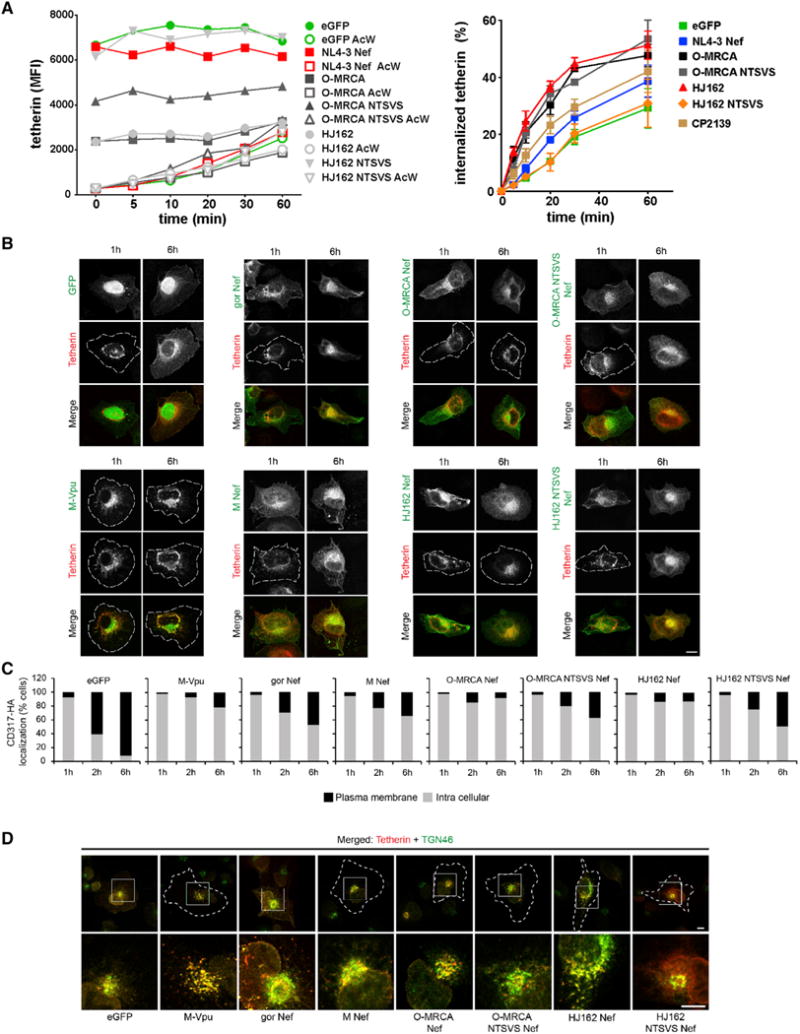
(A) Effects of O-Nefs on cell surface expression and internalization of HUM tetherin. 293T cells were transfected with vectors expressing tetherin and the indicated Nef proteins or only EGFP as control. Cells were stained with antitetherin antibodies, incubated at 37°C for different time points, and analyzed by FACS. The left panel shows the total and intracellular (after acidic wash) MFIs of HUM tetherin expression. The right panel shows the percentage of internalized tetherin in the presence of the indicated Nef proteins or the absence of Nef. Values represent averages derived from three independent experiments.
(B) Effect of O-Nefs on transport of newly synthesized HUM tetherin to the cell surface. The nuclei of HT1080 cells were comicroinjected with a HUM tetherin expression plasmid together with vectors encoding EGFP, NL4-3 Vpu, or the indicated Nef proteins. About 200 cells were microinjected and analyzed. Microphotographs show representative examples. Cell circumferences are indicated by white dashed lines. Scale bar, 10 μM.
(C) Histogram bars depict the relative percentage of cells showing the different categorization (plasma membrane and intracellular versus intracellular only) for each time point from about 200 cells analyzed.
(D) O-Nefs trap newly synthesized HUM tetherin at the trans-Golgi network (TGN). Shown are representative confocal microphotographs of HT1080 cells microinjected as described in (B) that were analyzed for the distribution of the TGN marker 46 at 2 hr postinjection. Depicted are overviews and zoom of the merged tetherin (red) and TGN46 (green) channel. Cell circumferences are indicated by white dashed lines. Scale bar, 10 μM (see also Figure S5).
The microscopy data and western blot analyses suggested that O-Nefs may trap human tetherin in intracellular compartments, where it is protected against proteolytic or lysosomal degradation, but also kept away from the sites of virus budding. To further examine this possibility, we utilized a plasmid microinjection approach that allows monitoring the transport of newly synthesized protein pools (Schmidt et al., 2011). Constructs encoding HA-tagged human tetherin and plasmids encoding EGFP, or AU-1 tagged Vpu or Nef proteins with differing tetherin downmodulating capacity, were comicroinjected into the nucleus of HT1080 cells that do not express endogenous tetherin. As expected (Schmidt et al., 2011), newly synthesized tetherin was rapidly (2 hr) transported to the plasma membrane in EGFP-expressing control cells, whereas M-Vpu trapped tetherin in a perinuclear compartment (Figures 5B and 5C). Anterograde transport of human tetherin was only slightly reduced by the SIVgor and group M Nef proteins, whereas the O-MRCA and HJ162 Nefs inhibited it as efficiently as the M-Vpu (Figures 5B and 5C). At 6 hr postinjection the O-MRCA and HJ162 Nefs reduced the frequency of cells expressing human tetherin at their surface from > 90% to < 10% compared to control cells. In contrast, the O-MRCA-NTSVS and HJ162-NTSVS mutants achieved only 40%–60% reduction, similar to the control SIVgor and HIV-1 M Nefs (Figure 5C). Thus, alterations in and near the ExxxLL motif that impair the tetherin downmodulation activity of O-Nefs also reduce their ability to trap human tetherin in a perinuclear compartment.
Costaining with subcellular markers in cells 2 hr postinjection identified this compartment as the trans-Golgi network (TGN) (Figures 5D and S6A), while overlap with markers for early endosomes or the ER was not observed (data not shown). Similar to group M Vpus (Schmidt et al., 2011), O-Nefs induced potent colocalization of tetherin with the TGN marker TGN46 indicative of tetherin retention during anterograde transport, and HJ162 Nef even disrupted the organization of the TGN (Figures 5D and S6A). These effects were not observed when tetherin interaction-deficient O-Nef mutants were used. Notably, organization of the Golgi apparatus was undisturbed in all cases, but O-Nefs significantly reduced the amounts of tetherin that colocalized with the Golgi marker GM130 (Figure S6B).
These results suggest that O-Nefs slightly increase human tetherin internalization, but also impair the anterograde transport of newly synthesized human tetherin to the cell surface to retain the restriction factor in the TGN. O-Nefs thus affect tetherin anterograde transport at a similar step as M-Vpus, but may employ different mechanisms to prevent efficient plasma membrane delivery of the restriction factor.
HIV-1 O Nefs Do Not Antagonize the Short Isoform of Human Tetherin
Human tetherin is expressed in two isoforms (Cocka and Bates, 2012). The short form is generated by alternative translation initiation from a methionine residue in the cytoplasmic domain and lacks N-terminal serine-threonine and tyrosine motifs, including the region targeted by O-Nefs (Figure 6A). This short form was not significantly degraded by either M-Vpu or O-Nef proteins (Figure 6B). Both isoforms of tetherin were expressed at similar levels at the surface of transfected 293T cells (Figure 6C), and the short isoform was substantially less sensitive to M-Vpu- and O-Nef-mediated downregulation than the full-length form (Figure 6D). The modest effects of O-Nefs on the surface expression of the short isoform were not affected by the mutations in the C-loop of O-Nefs (Figure 6D) and most likely resulted from global effects on cellular trafficking pathways. The envelope glycoprotein of SIV from Tantalus monkeys (SIVtan) and the NL4-3 Vpu protein, which are known to target the extracellular (Gupta et al., 2009) and transmembrane domains of human tetherin, respectively, promoted infectious virus release in the presence of the short isoform, whereas none of the O-Nefs displayed significant enhancing effects (Figure 6E). Thus, in agreement with our finding that O-Nefs target a domain in the N-terminal region of human tetherin that is missing in the short isoform, they are only active against the full-length form.
Figure 6. O-Nefs Do Not Antagonize the Short Isoform of Human Tetherin.
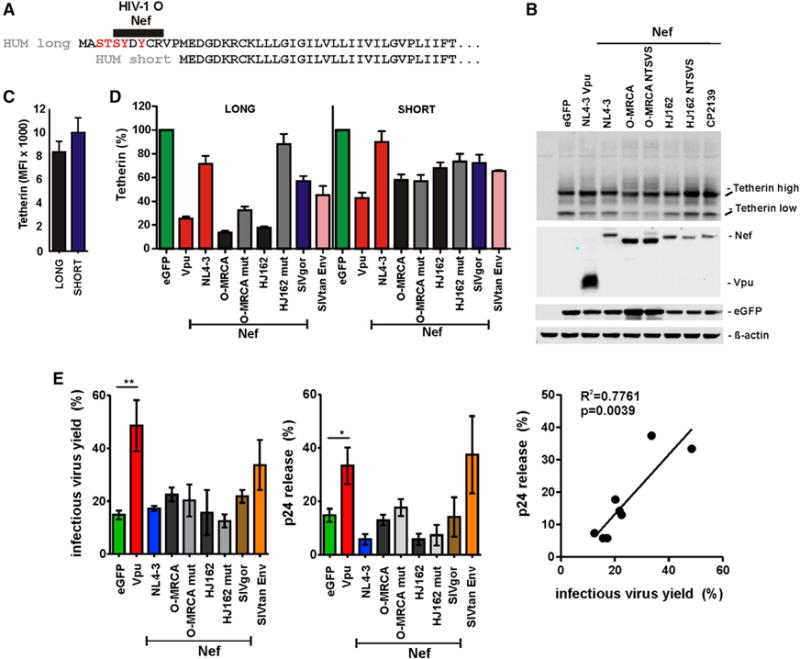
(A) Amino acid alignment of the long and short isoforms of HUM tetherin.
(B) Expression of the short form of human tetherin in the presence of Vpu or the indicated Nef proteins. 293T cells were cotransfected with plasmids expressing the indicated tetherin variants and Vpu or the indicated Nef proteins and examined by western blot 2 days later.
(C) Surface levels of tetherin expressionon 293T cells transfected with constructs expressing the long or short isoforms of human tetherin. Results in (C)–(E) show mean values (± SEM) derived from three to six transfections.
(D) Downmodulation of the two isoforms of human tetherin by the indicated viral proteins. Shown are the levels of cell surface expression relative to those obtained in the presence of the vector expressing only EGFP.
(E) Virus release from 293T cells cotransfected with an HIV-1 NL4-3 ΔvpuΔnef construct and vectors expressing the indicated viral proteins and a plasmid expressing the short isoform of HUM tetherin. Virus yield was determined by p24 ELISA (left) and infection of TZM-bl cells (middle). The right panel shows the correlation between both measurements (see also Figure S6).
Downmodulation of Human Tetherin Reduces IFNα Sensitivity of HIV-1 in Primary T Cells
Although contemporary O-Nefs reduced cell surface expression of human tetherin, they exerted only very subtle effects on virus release. To examine this further, we compared the effects of wild-type (WT) and mutant O-MRCA and HJ162 Nefs on p24 antigen and infectious virus release directly. Western blot analyses showed that in the absence of an antagonist, even low levels of tetherin expression reduced p24 release (Figure S7A, EGFP panel). Reduced levels of p24 release were also observed in the presence of the NL4-3 and mutant O-MRCA and HJ162 Nefs, but not in the presence of Vpu and WT O-MRCA and HJ162 Nefs (Figure S7A). Titration experiments confirmed that the O-MRCA and HJ162 Nefs increased p24 production, and that the NTSVS mutations disrupted this effect (Figures S7B and S7C).
To examine the effect of O-Nef mutations that abrogated tetherin downmodulation on virus release and replication in primary T cells, we generated vpu-defective proviral NL4-3 constructs containing WT and mutant HIV-1 O nef alleles. Notably, the corresponding HIV-1 clones differed only in the three to five amino acids in and near the ExxxLL motif that impaired the ability of O-Nefs to downmodulate human tetherin, but not CD4 and MHC-I. Infection of P4-CCR5 indicator cells showed that these mutations did not affect the infectiousness of progeny virions (Figure 7A). Next, we determined the efficiency of virus release in the presence and absence of IFNα treatment. We found that treatment of primary human T cells with IFNα significantly increased the levels of tetherin surface expression (Figure 7B) and strongly suppressed the release of HIV-1 particles (Figure 7C). Strikingly, however, the tetherin downmodulation function of O-Nefs was associated with levels of p24 release from primary CD4+ T cells that did not differ significantly from the WT vpu containing HIV-1 construct (Figure 7C). On average, the HIV-1 M-Vpu and O-Nef proteins increased p24 production by about 20% in the absence of, and by 40%–50% in the presence of, IFNα treatment (Figure 7D). In contrast, the original NL4-3 M-Nef that is not a tetherin antagonist had no significant effect on virus release in the presence and absence of IFNα, and the mutations in the C-loop of O-Nefs significantly reduced the enhancing activity (Figure 7D). Finally, we analyzed whether the tetherin downmodulation function of O-Nefs affected the sensitivity of HIV-1 replication to inhibition by IFNα. We found that viruses expressing the WT or NTSVS mutant O-Nef proteins replicated with near-identical kinetics (Figure 7E) and produced similar quantities of p24 antigen (Figure 7F) in the absence of IFNα. In cells pretreated with 500 U/ml IFNα, however, HIV-1 constructs expressing WT O-Nefs replicated more effectively and released higher quantities of p24 antigen than those encoding tetherin-inactive Nef proteins. In comparison, complete lack of Nef function reduced viral replication in both the absence and presence of IFNα (Figure 7E). Coinfection experiments confirmed that viruses expressing WT O-Nefs that downmodulate human tetherin outcompeted those containing the mutant nef alleles in IFNα-treated primary T cell cultures (data not shown). Thus, the antitetherin activity of O-Nefs promotes virus release (Figure 7F) and counteracts the antiviral effect of IFNα (Figure 7G) in primary CD4+ T cells.
Figure 7. Role of O-Nef-Mediated Downmodulation of HUM Tetherin in HIV-1 Infectivity and Replication Fitness.
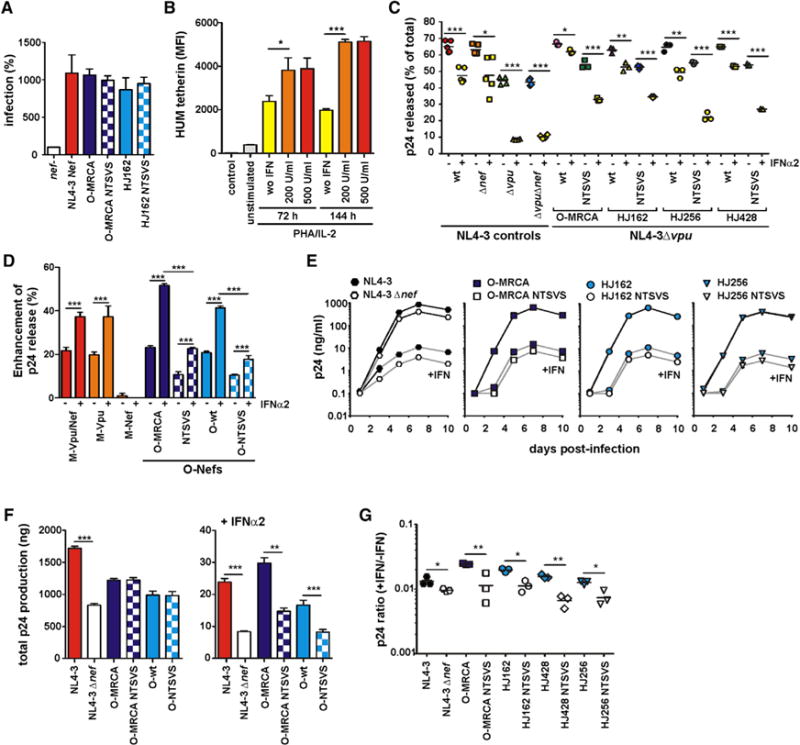
(A) P4-CCR5 indicator cells were infected with vpu-defective HIV-1 NL4-3 IRES-EGFP constructs containing the indicated nef genes or a defective nef allele. Infections were performed in triplicate with virus stocks containing 100 ng of p24 antigen. Shown are average values (± SEM) compared to the infectivity of the nef-defective HIV-1 construct (100%).
(B) Levels of tetherin cell surface expression in uninfected PBMC cultures (n = 3) in the absence and presence of IFNα.
(C) Efficiency of p24 release in CD4+Tcells infected with HIV-1 NL4-3 constructs expressing the indicated nef alleles. Values present percentages of cell-free p24 antigen out of the total p24 detected in the presence and absence of IFNα and were derived from infections of T cells derived from three PBMC donors, each performed in triplicate. Cell-free and cell-associated p24 antigen was quantified by ELISA at 5 days postinfection. The O nef alleles were examined in the context of a vpu-defective NL4-3 construct. Constructs expressing the WT NL4-3 nef or containing disrupted vpu and/or nef alleles were examined for control.
(D) Enhancement of p24 release by HIV-1 group M and N accessory proteins. Data were derived from the experiment shown in (A). Shown are mean values (± SEM; n = 3–9) obtained for the increase of p24 release by the presence of indicated viral genes in the HIV-1 NL4-3 proviral construct compared to the control construct lacking intact vpu and nef genes.
(E) Virus replication in CD4+ T cells in the presence and absence of IFNα. The replication kinetics of HIV-1 NL4-3 constructs expressing the indicated Nef proteins are shown in CD4+ T cells from three donors in the absence (upper lines) and presence (lower lines) of 500 U/ml IFNα. Results show median values of p24 antigen production (± SEM; n = 3). Please note that the upper curves overlay one another.
(F) Cumulative p24 antigen release in the culture supernatant over 10 days of viral replication in the absence (left) and presence (right) of IFNα. Shown are mean values (± SEM) for NL4-3 constructs expressing the O-MRCA and WT O-Nefs (HJ162, HJ428, HJ256) or the respective NTSVS mutant forms.
(G) Ratio of p24 production in the presence and absence of IFNα is plotted for each virus at 5 days postinfection. Symbols are the same as in (B) (see also Figure S7).
DISCUSSION
In the present study, we demonstrate that, in contrast to SIVcpz, SIVgor, and other HIV-1 Nefs, HIV-1 O Nef proteins efficiently reduce cell surface expression of human tetherin. We further show that this antitetherin activity depends on a region in the N-terminal cytoplasmic domain of tetherin located directly adjacent to the deletion in the human protein. Notably, the Nef protein of the inferred common ancestor of contemporary HIV-1 group O strains targets the same region and is an effective antagonist of human tetherin. Thus, the activity of Nef against human tetherin apparently evolved prior to the epidemic spread of HIV-1 group O. This function was previously missed since the only proviral group O clone analyzed (CMO2.5) (Yang et al., 2011) encodes a functionally defective nef allele, and since contemporary O-Nefs are only poorly active in promoting virus release from transfected 293T cells (Figure 1A; Sauter et al., 2009; Yang et al., 2011). In contrast, O-Nefs clearly increased virus release and enhanced virus resistance to inhibition by IFNα in primary CD4+ T cells (Figure 7). It is thus tempting to speculate that Nef-mediated tetherin antagonism facilitated the spread of group O viruses in the human population by counteracting innate immune responses characteristic of acute HIV-1 infection (Parrish et al., 2013; Fenton-May et al., 2013).
Several O-Nefs analyzed in the present study have previously been examined for their antitetherin activity, but only in virus release assays in transfected 293T cells (Sauter et al., 2009; Yang et al., 2011). This assay is commonly used because it allows the testing of restriction factors from different species, and because M-Vpus show a robust phenotype in this experimental setting. HIV-1 O Nefs enhance virus release in transfected 293T cells only marginally (Figure 1A). However, in primary CD4+ T cells, the tetherin downmodulation function of O-Nefs enhanced virus release and reduced the inhibitory effect of IFNα as efficiently as the M-Vpu protein (Figure 7), although they were inactive against the short isoform of human tetherin (Figure 6). Why the antitetherin activity of HIV-1 O Nefs is more evident in primary CD4+ T cells than in transfected 293T cells remains to be determined, but one possible explanation is that contemporary O-Nefs antagonize human tetherin only at physiologically relevant, but not at higher, expression levels. Notably, only the O-MRCA Nef suppressed tetherin-mediated NF-κB activation and lacked the downstream stimulatory effect on NF-κB (Figure 2E). Counteraction of human tetherin without suppression of NF-κB activation by contemporary O-Nefs may thus reflect advanced adaptation to the human host.
The tetherin downmodulation function of O-Nefs increased the efficiency of virus release, but enhanced HIV-1 replication only in CD4+ T cells that were pretreated with IFNα (Figure 7E). Type I interferon levels are particularly high during acute HIV-1 infection, which is characterized by a cytokine storm. To counteract the antiviral effects of this innate immune response, transmitted founder viruses are significantly more resistant to inhibition by IFNα than HIV-1 strains that persist during chronic infection (Parrish et al., 2013; Fenton-May et al., 2013). Thus, the impact of Nef-mediated downmodulation of human tetherin may be most significant during the earliest stages of virus infection.
Evolutionary analyses suggest that HIV-1 group O was transmitted to humans early in the 20th century and thus entered the human population roughly at the same time as group M viruses (Lemey et al., 2004). Group O viruses have infected tens of thousands of people, mostly in Cameroon and neighboring countries. Although group O prevalence is lower than that of pandemic HIV-1 group M, it is substantially higher than that of groups N and P, which have been detected in fewer than 20 individuals. It is thus tempting to speculate that the Nef-mediated antitetherin activity may have facilitated its spread in the human population. Our finding that this specific O-Nef function reduced the sensitivity of HIV-1 to inhibition by IFNα is in agreement with this hypothesis and the previously proposed role of tetherin antagonism in virus transmission (Gupta and Towers, 2009; Sauter et al., 2010). However, the effects of O-Nef on viral replication in CD4+ T lymphocytes (Figure 7E) were weaker than those previously reported for Vpu (Neil et al., 2007). One potential explanation is that the mutant O-Nefs may retain some modest antitetherin activity (Figure 7C). Furthermore, differences in the antitetherin mechanism and efficacy of O-Nefs and M-Vpus and/or in the experimental conditions, such as the concentration and IFN subtypes used, may contribute. In either case, the results of the present study add to existing evidence that the pandemic, epidemic, and rare HIV-1 groups have achieved different degrees of adaptation to the human host. Pandemic group M strains seem to have cleared the tetherin barrier by evolving highly efficient Vpu-mediated counteraction of this restriction factor. Group N strains have evolved modest Vpu-mediated antitetherin activity (Sauter et al., 2009; Yang et al., 2010) and are still in the process of adaptation (Sauter et al., 2012), while group P-derived Vpu and Nef proteins are functionally indistinguishable from their SIVgor counterparts (Sauter et al., 2011; Yang et al., 2011). Our finding that HIV-1 O has adapted to humans and evolved Nef-mediated activity against human tetherin further illustrates that adaptive processes are highly dynamic, and that primate lentiviruses utilize multiple avenues to overcome the restriction factors in a new host.
It has previously been shown that residues in and near the ExxxLL motif in the C-loop are critical for the interaction of SIV and chimpanzee-adapted HIV-1 Nefs with the cytoplasmic domain of tetherin, but not for other Nef functions (Zhang et al., 2011; Götz et al., 2012; Serra-Moreno et al., 2013). We found that these residues are also important for the ability of HIV-1 O Nefs to downmodulate tetherin. They were not responsible, however, for their ability to target a distinct region since they affected downmodulation of both human and gorilla tetherin. The exact alterations that are critical for the ability of O-Nefs to specifically target human tetherin seem to be complex and require further investigation. Previous studies suggest that adaptor protein 2 (AP-2)-dependent endocytosis plays a key role in Nef-mediated downmodulation of tetherin (Zhang et al., 2011; Serra-Moreno et al., 2013). Adaptor protein recruitment seems to be well preserved in O-Nefs since they were fully active in downmodulating CD4, which depends on AP-2-mediated endocytosis. Unexpectedly, however, we found that the O-MRCA and HJ162 Nefs enhanced the rate of internalization of human tetherin only slightly, but clearly inhibited the transport of tetherin to the cell surface. Thus, Nef may exploit different mechanisms to reduce the levels of tetherin at the cell surface. Recently, it has been shown that HIV-1 Vpu hijacks AP-1-depen-dent trafficking pathways to counteract tetherin (Jia et al., 2014). Since AP-1 predominantly mediates vesicular transport from and to the TGN, this interference likely accounts for the reduction of tetherin anterograde transport at the level of the TGN. Our results suggest that O-Nefs may employ a similar mechanism to interfere with transport of tetherin beyond the TGN, which is reminiscent to Nef-mediated downmodulation of MHC-I. The observed effects of O-Nefs on TGN organization and Golgi residence of tetherin also suggest, however, that they have evolved a slightly different molecular mechanism than Vpu to antagonize human tetherin that will be important to dissect in further studies.
In summary, we show that O-Nefs are antagonists of human tetherin and target a region in the cytoplasmic domain that is preserved in the human ortholog of this restriction factor. Thus, the five amino acid deletion in the cytoplasmic domain of human tetherin does not confer complete protection against antagonism by primate lentiviral Nef proteins. This has implications for assessing future zoonotic transmission risks, because tetherin represents an important species barrier, and the great majority of primate lentiviruses use Nef to counteract tetherin in their respective hosts (Sauter et al., 2009; Zhang et al., 2009; Jia et al., 2009). The fact that primate lentiviruses do not necessarily have to switch from Nef to other viral factors to antagonize human tetherin further emphasizes their enormous plasticity to adapt to new host environments.
EXPERIMENTAL PROCEDURES
Generation of MRCA Sequences
Nef MRCA sequences were inferred using both codon and nucleotide evolutionary models as outlined in the Supplemental Experimental Procedures.
Expression Vectors
Bicistronic CMV promoter-based pCG expression vectors coexpressing vpu, nef, CD4, or tetherin and EGFP or DsRed2, respectively, have been described (Sauter et al., 2009). Splice-overlap extension PCR with primers introducing XbaI and MluI restriction sites flanking the reading frames was used to generate chimeric nef alleles as well as tetherin and nef mutants. PCR fragments were purified from agarose gels and inserted into the pCG vector using standard cloning techniques. All PCR-derived inserts were sequenced to confirm their accuracy.
Cell Culture and Transfections
239T and TZM-bl cells were cultured and transfected or infected as described in the Supplemental Experimental Procedures. PBMCs were isolated using lymphocyte separation medium (Biochrom) and cultured as outlined in the Supplemental Experimental Procedures.
Flow Cytometric Analysis
To determine the effect of Vpu and Nef on CD4 and tetherin cell surface expression, 293T cells were transfected by the calcium phosphate method with 1 μg of a CD4 or tetherin expression vector coexpressing EGFP and 5 μg of pCG EGFP/Vpu or Nef constructs expressing EGFP alone or together with Vpu or Nef. Two days posttransfection CD4 or tetherin expression was examined by FACS analysis as described previously (Schindler et al., 2006). See Supplemental Experimental Procedures for details.
Tetherin Antagonism
The capability of Vpu and Nef to antagonize tetherin was determined essentially as described (Sauter et al., 2009). For details, see the Supplemental Experimental Procedures.
Western Blot
Expression of Vpu, Nef, and tetherin was examined as described in the Supplemental Experimental Procedures.
Sequence Analyses
Vpu or Nef amino acid sequences were aligned using multiple sequence alignment with hierarchical clustering (http://multalin.toulouse.inra.fr/multalin). Vpu and Nef sequences were drawn from the HIV Sequence Database (http://www.hiv.lanl.gov/content/index).
Nef Model Building
Structure diagrams based on the SF2 Nef were displayed with PyMOL (http://www.pymol.org; accession number, 3RBB).
Microscopy
Confocal microscopy was performed using an LSM710 confocal microscope (Carl Zeiss) as outlined in the Supplemental Experimental Procedures.
Endocytosis Assay
293T cells were transfected with tetherin and Nef expression constructs as described previously (Götz et al., 2012). Surface tetherin was stained 48 hr posttransfection, and endocytosis was allowed up to 60 min. Endocytosed tetherin was measured by FACS. For details, see the Supplemental Experimental Procedures.
Tetherin Anterograde Biosynthetic Transport Assay
HT1080 cells were microinjected and analyzed by confocal microscopy as reported previously (Schmidt et al., 2011). See the Supplemental Experimental Procedures for details.
Analysis of Viral IFNα Sensitivity
Negatively selected CD4+ T cells were either pretreated with 500 U/ml IFNα2, or not, for 24 hr and subsequently infected with viral stocks containing 2 ng reverse transcriptase activity. Virus production was monitored by p24 ELISA.
Statistical Analysis
All statistical calculations were performed with a two-tailed unpaired Student’s t test using GraphPad Prism 5.0. p values < 0.05 were considered significant. Correlations were calculated with the linear regression module.
Supplementary Material
Acknowledgments
We thank Jan Münch for critical reading of the paper, Valerie Oberhardt and Vibor Laketa for help with the subcellular localization analyses, and Susanne Engelhart, Daniela Krnavek, Kerstin Regensburger, and Martha Mayer for excellent technical assistance. This work was supported by grants from the NIH (R01 AI058715, R37 AI050529, R01 AI 111789), grants from the Deutsche Forschungsgemeinschaft (to O.T.F., FA 378/11-1; and to S.M.U., US116/1-1) and European FP7 “HIT HIDDEN HIV” (305762), a Leibniz award of the Deutsche Forschungsgemeinschaft, and an Advanced ERC investigator grant (to F.K.). M.G. is supported by the DFG Excellence Cluster ImmunoSensation.
Footnotes
ACCESSION NUMBERS
The GenBank accession numbers for the group M, O, and N nef MRCA sequences reported in this paper are KP059118–KP059121.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2014.10.002.
AUTHOR CONTRIBUTIONS
S.F.K., K.M., and S.S.I. performed most experiments. A.H., D.H., S.J., F.B.-R., and M.P. provided viral constructs and protocols. G.H.L., L.J.P., and P.M.S. generated the HIV-1 MRCA nef sequences. F.M.P. and O.T.F. performed microinjection studies, and M.G. performed structural analyses. S.F.K., K.M., S.S.I., D.S., B.H.H., and F.K. planned experiments and evaluated results. B.H.H. and F.K. wrote the manuscript.
References
- Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaugerre C, De Oliveira F, Lascoux-Combe C, Plantier JC, Simon F. HIV-1 group N: travelling beyond Cameroon. Lancet. 2011;378:1894. doi: 10.1016/S0140-6736(11)61457-8. [DOI] [PubMed] [Google Scholar]
- Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JF, Barat C, Beauséjour Y, Barbeau B, Tremblay MJ. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-kappaB, and AP-1 induction. J Biol Chem. 2004;279:39520–39531. doi: 10.1074/jbc.M407477200. [DOI] [PubMed] [Google Scholar]
- Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFkB-dependent proinflammatory responses. Cell Host Microbe. 2012;12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz N, Sauter D, Usmani SM, Fritz JV, Goffinet C, Heigele A, Geyer M, Bibollet-Ruche F, Learn GH, Fackler OT, et al. Reacquisition of Nef-mediated tetherin antagonism in a single in vivo passage of HIV-1 through its original chimpanzee host. Cell Host Microbe. 2012;12:373–380. doi: 10.1016/j.chom.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Towers GJ. A tail of Tetherin: how pandemic HIV-1 conquered the world. Cell Host Microbe. 2009;6:393–395. doi: 10.1016/j.chom.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc Natl Acad Sci USA. 2009;106:20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Weber E, Tokarev A, Lewinski M, Rizk M, Suarez M, Guatelli J, Xiong Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife. 2014;3:e02362. doi: 10.7554/eLife.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemey P, Pybus OG, Rambaut A, Drummond AJ, Robertson DL, Roques P, Worobey M, Vandamme AM. The molecular population genetics of HIV-1 group O. Genetics. 2004;167:1059–1068. doi: 10.1534/genetics.104.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108:18097–18101. doi: 10.1073/pnas.1113694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourez T, Simon F, Plantier JC. Non-M variants of human immunodeficiency virus type 1. Clin Microbiol Rev. 2013;26:448–461. doi: 10.1128/CMR.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit SJ, Blondeau C, Towers GJ. Analysis of the human immunodeficiency virus type 1 M group Vpu domains involved in antagonizing tetherin. J Gen Virol. 2011;92:2937–2948. doi: 10.1099/vir.0.035931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemée V, Damond F, Robertson DL, Simon F. A new human immunodeficiency virus derived from gorillas. Nat Med. 2009;15:871–872. doi: 10.1038/nm.2016. [DOI] [PubMed] [Google Scholar]
- Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Specht A, Kirchhoff F. Tetherin: holding on and letting go. Cell. 2010;141:392–398. doi: 10.1016/j.cell.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Sauter D, Hué S, Petit SJ, Plantier JC, Towers GJ, Kirchhoff F, Gupta RK. HIV-1 group P is unable to antagonize human tetherin by Vpu. Env or Nef Retrovirology. 2011;8:103. doi: 10.1186/1742-4690-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Unterweger D, Vogl M, Usmani SM, Heigele A, Kluge SF, Hermkes E, Moll M, Barker E, Peeters M, et al. Human tetherin exerts strong selection pressure on the HIV-1 group NVpu protein. PLoS Pathog. 2012;8:e1003093. doi: 10.1371/journal.ppat.1003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M, Münch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Müller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Fritz JV, Bitzegeio J, Fackler OT, Keppler OT. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. MBio. 2011;2:e00036–e11. doi: 10.1128/mBio.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Moreno R, Zimmermann K, Stern LJ, Evans DT. Tetherin/BST-2 antagonism by Nef depends on a direct physical interaction between Nef and tetherin, and on clathrin-mediated endocytosis. PLoS Pathog. 2013;9:e1003487. doi: 10.1371/journal.ppat.1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebit DM, Zekeng L, Kaptué L, Gürtler L, Fackler OT, Keppler OT, Herchenröder O, Kräusslich HG. Construction and characterization of an HIV-1 group O infectious molecular clone and analysis of vpr- and nef-negative derivatives. Virology. 2004;326:329–339. doi: 10.1016/j.virol.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Vallari A, Holzmayer V, Harris B, Yamaguchi J, Ngansop C, Makamche F, Mbanya D, Kaptué L, Ndembi N, Gürtler L, et al. Confirmation of putative HIV-1 group P in Cameroon. J Virol. 2011;85:1403–1407. doi: 10.1128/JVI.02005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessié re A, Rousset D, Kfutwah A, Leoz M, Depatureaux A, Simon F, Plantier JC. Diagnosis and monitoring of HIV-1 group O-infected patients in Cameroun. J Acquir Immune Defic Syndr. 2010;53:107–110. doi: 10.1097/QAI.0b013e3181b97ec1. [DOI] [PubMed] [Google Scholar]
- Vigan R, Neil SJ. Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin. J Virol. 2011;85:9737–9748. doi: 10.1128/JVI.00479-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Lopez LA, Hauser H, Exline CM, Haworth KG, Cannon PM. Anti-tetherin activities in Vpu-expressing primate lentiviruses. Retrovirology. 2010;7:13. doi: 10.1186/1742-4690-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Lopez LA, Exline CM, Haworth KG, Cannon PM. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology. 2011;8:78. doi: 10.1186/1742-4690-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Landford WN, Ng M, McNatt MW, Bieniasz PD, Hatziioannou T. SIV Nef proteins recruit the AP-2 complex to antagonize Tetherin and facilitate virion release. PLoS Pathog. 2011;7:e1002039. doi: 10.1371/journal.ppat.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


