Abstract
Fut2-LacZ-null mice, which are a model of the human ABO and Lewis nonsecretor group, display increased susceptibility to experimental yeast vaginitis, indicating a role for α(1,2)fucosylated cervical glycans in mucosal defense. However, the lack of significant effect of competitive inhibition by exogenous neoglycoproteins in this study emphasizes the complexity of Candida-epithelial cell adhesion events.
Recurrent vulvovaginal candidiasis (RVVC) is a mucosal infection caused by the opportunistic fungus Candida albicans which affects up to 5 to 10% of women of reproductive age (22, 23). The immune response elicited during an episode of RVVC differs from the classical host defense mechanism, as infection occurs despite normal Candida-specific Th1-type cell-mediated immunity (9, 16). Protection from RVVC is believed to be acquired locally, possibly involving incompletely defined vaginal epithelial carbohydrate adhesion molecules (2, 24).
A common null mutation within the coding region of the α(1,2)fucosyltransferase gene, FUT2 (secretor factor gene), leads to ABO and Lewis histo-blood group antigen nonsecretion from mucosal tissues in approximately 20% of humans, with ethnic variation (13, 14). Nonsecretor status has been associated with differences in susceptibility to several infections, including infections with Norwalk virus (15), human immunodeficiency virus (1), Escherichia coli (17, 21), Staphylococcus aureus (20), Campylobacter jejuni (19), calicivirus (18), and C. albicans (5). While the mechanism of how C. albicans interacts with nonsecretors is not known, a host-microbe adhesion mechanism is supported by in vitro studies that have demonstrated binding of various fucosylated oligosaccharides to germ tubes of C. albicans (3, 4, 6, 25). We report here the development of a mouse model for the nonsecretor phenotype to test the importance of α(1,2)fucosylated glycans in vivo during experimental vaginal candidiasis.
Absence of endocervical and vaginal α(1,2)fucosylated glycans in Fut2-LacZ-null mice.
The expression pattern of Fut2 and resulting α(1,2)fucosylated glycans in the female lower reproductive tract was examined in 8- to 10-week-old female mutant mice (Fut2-LacZ-null mice) that contained a targeted replacement of the Fut2 open reading frame with a bacterial lacZ reporter gene (8). The original mutant mice were backcrossed 10 generations to C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine), and the resulting congenic strain used for this study was designated B6.129X1-Fut2tm1Sdo (MGI accession ID no. 2183220). A second line of mutant mice lacking the coding region of another α(1,2)fucosyltransferase gene, Fut1, was similarly backcrossed for 10 generations to C57BL/6J mice and was designated B6.129X1-Fut1tm1Sdo (MGI accession ID no. 2183219) and used as genetic background controls.
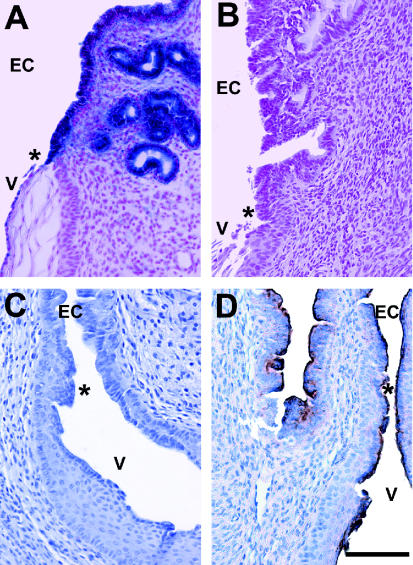
Using a 5-bromo-4-chloro-3-indolylβ-d-glucuronic acid (X-Gal) staining method (7), specific nuclear LacZ staining was detected in the glandular and lumenal epithelium of the endocervix of Fut2-LacZ-null mice in estrus (Fig. 1A), but not in wild-type mice (Fig. 1B). Lectin staining with Ulex europaeus agglutinin I (UEA-I) showed loss of α(1,2)fucosylated glycans from the endocervix and upper vagina of Fut2-LacZ-null mice (Fig. 1C), while lectin staining was preserved at the apical surface of the epithelium of the endocervix and upper vagina in wild-type mice (Fig. 1D), indicating that Fut2 is essential for the production of α(1,2)fucosylated glycans within the endocervix and vagina. Given epidemiological evidence that nonsecretors are more susceptible to RVVC (5) and the implication of fucose involvement in C. albicans adhesion from various in vitro studies (4, 25), we postulated that α(1,2)fucosylated glycans carried on secreted endocervical mucins protect the vaginal epithelium by sequestering and thereby preventing C. albicans from adhering to vaginal epithelial cells. This potential defense mechanism would be reduced in nonsecretor women. The estrogen-regulated expression of FUT2 may thus represent innate protection against monthly infection with C. albicans by increasing α(1,2)fucosylated glycan secretion at times most risky for colonization associated with elevated estrogen.
FIG. 1.
Cell-specific expression of Fut2-LacZ reporter gene activity in endocervical glandular cells and loss of α(1,2)fucosylated glycans from the lower reproductive tract of Fut2-LacZ-null mice. Fut2-LacZ-null and C57BL/6J wild-type mice were processed for X-Gal staining (top panels) and UEA-I lectin staining (bottom panels). Specific nuclear X-Gal staining was detected in endocervical glandular epithelium of Fut2-LacZ-null mice in estrus (A), while staining in wild-type mice was negative (B). UEA-I lectin staining was absent from Fut2-LacZ-null mice (C) but was associated with endocervical and upper vaginal epithelia of wild-type mice (D), indicating that Fut2 is essential for expression of lower reproductive tract α(1,2)fucosylated glycans. Endocervical (EC) and vaginal (V) epithelium are marked. The asterisk indicates the location of the squamocolumnar epithelial junction of the cervix. Bar, 100 μm.
Fut2-LacZ-null mice display increased susceptibility to C. albicans during experimental vaginal candidiasis compared to wild-type and Fut1-null control mice.
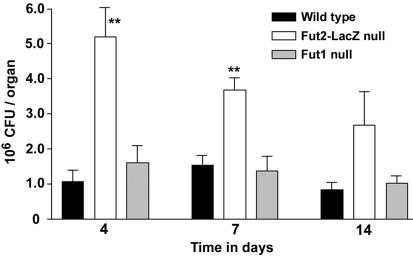
To test for and quantify potential differences between wild-type and mutant mice during experimental vaginal candidiasis, female C57BL/6J wild-type, Fut1-null, and Fut2-LacZ-null mice were maintained in pseudoestrus by using subcutaneous 0.5-mg, 21-day controlled release 17β-estradiol pellets (Innovative Research of America, Sarasota, Fla.). Following intravaginal inoculation with 10 μl containing 5 × 105 stationary-phase C. albicans (3153A) cells (10, 11) mice were euthanized at 4, 7, or 14 days, and the vagina and cervix were removed en bloc, homogenized in phosphate-buffered saline (12), and plated on Sabouraud dextrose agar plates to determine CFU counts (CFU per organ). This study was approved for humane animal use. Hematoxylin and eosin staining of paraffin-embedded vaginas of infected mice at 4 days displayed hyphal yeast and neutrophils throughout the vagina, confirming C. albicans infection (data not shown). Similar to human nonsecretors, Fut2-LacZ-null mice displayed an increased vaginal fungal burden compared to that in wild-type mice of 4.8- and 2.4-fold at 4 and 7 days postinoculation, respectively (Fig. 2). Analysis of variance (ANOVA) determined that the higher colonization rates in Fut2-LacZ-null mice compared to those in wild-type mice cannot be accounted for by expected variation. Upon post-hoc analysis using Tukey's honestly significant difference test and Dunnett's T3 test, Fut2-LacZ-null mice had statistically significant increased susceptibility (P < 0.01) versus wild-type mice at 4 and 7 days postinoculation. While displaying the same trend seen at earlier time points, there was an absence of statistical significance at 14 days postinoculation, perhaps as a result of the limited number of mice used in the study. There was no variation difference in fungal burden between the control Fut1-null and C57BL/6J wild-type mice.
FIG. 2.
Quantification of experimental C. albicans vaginitis in three mouse genotypes. Fut2-LacZ-null mice, Fut1-null mice, and wild-type mice were subjected to C. albicans vaginitis. The experiment was performed in triplicate with similar results; cumulative data are shown (n = 7 to 8 mice per group). One-way ANOVA and post-hoc analysis revealed that Fut2-LacZ-null mice display an increased susceptibility (**, P < 0.01) versus wild-type mice at 4 and 7 days postinoculation. Error bars show the standard errors of the means.
Neoglycoprotein competitive inhibitors to yeast colonization.
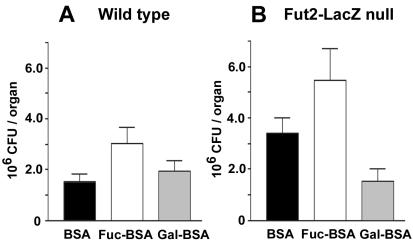
To address the mechanism of Fut2 involvement during C. albicans infection, we coinoculated mice with C. albicans and exogenous neoglycoproteins to directly saturate adhesin binding sites and potentially reduce colonization by sequestering, and thereby preventing, adhesion to host epithelium (25). Groups of pseudoestrous wild-type and Fut2-LacZ-null mice were intravaginally inoculated with a total of 13 μl composed of 10 μl of 5 × 105 stationary-phase C. albicans cells preincubated for 15 min with 3 μl of a 10-mg/ml solution of either bovine serum albumin (BSA), fucose-conjugated BSA (Fuc-BSA), or galactose-conjugated BSA (Gal-BSA) (EY Laboratories, Inc., San Mateo, Calif.). The vagina and cervix were removed en bloc from all mice 4 days postinoculation, and the CFU per organ was quantified as described above. Comparisons of the mean vaginal fungal burden between treatments in Fut2-LacZ-null and wild-type control mice did not show statistically significantly effects from Fuc-BSA or Gal-BSA pretreatments compared to BSA alone, based on a one-way ANOVA and Dunnett's T3 post-hoc test (Fig. 3). Possible explanations include an inability of simple neoglycoproteins to adequately bind C. albicans adhesins, insufficient dosing with the competitive sugars due to rapid turnover or elimination, or an inappropriate growth phase of C. albicans. Future studies will include more complex oligosaccharides and lectins that may show greater efficacies to sequester yeast and prevent adhesion to host epithelium. These data provide evidence that Fut2-LacZ-null mice may serve as an animal model to investigate host-microbe interactions in the gastrointestinal and genitourinary systems, where epidemiological data support an association between ABO and Lewis blood group secretion and genetic predisposition to infection.
FIG. 3.
Effects of neoglycoproteins on susceptibility of Fut2-LacZ-null and wild-type mice to experimental yeast vaginitis. C57BL/6J wild-type (A) and Fut2-LacZ-null (B) mice were inoculated with C. albicans cells preincubated with BSA, Fuc-BSA, or Gal-BSA (n = 9 to 11 mice per group). Error bars show the standard errors of the means. Under the conditions tested, exogenous neoglycoproteins were not effective in preventing C. albicans colonization in Fut2-LacZ-null or wild-type mice.
Acknowledgments
We thank personnel of the University of Michigan IPOX histology core and Histoserv Inc. for histological processing, Brady West of the University of Michigan Center for Statistical Consultation and Research, and the laboratory of Paul Fidel for the gift of C. albicans strain 3153A.
This work was supported in part by a University of Michigan Rackham Grant (S.E.D.). E.A.H. is a recipient of a University of Michigan Biomedical Research postdoctoral fellowship award.
Editor: T. R. Kozel
REFERENCES
- 1.Ali, S., M. A. Niang, I. N′Doye, C. W. Critchlow, S. E. Hawes, A. V. Hill, and N. B. Kiviat. 2000. Secretor polymorphism and human immunodeficiency virus infection in Senegalese women. J. Infect. Dis. 181:737-739. [DOI] [PubMed] [Google Scholar]
- 2.Barousse, M. M., C. Steele, K. Dunlap, T. Espinosa, D. Boikov, J. D. Sobel, and P. L. Fidel, Jr. 2001. Growth inhibition of Candida albicans by human vaginal epithelial cells. J. Infect. Dis. 184:1489-1493. [DOI] [PubMed] [Google Scholar]
- 3.Brassart, D., A. Woltz, M. Golliard, and J. R. Neeser. 1991. In vitro inhibition of adhesion of Candida albicans clinical isolates to human buccal epithelial cells by Fuc α1→2Galβ-bearing complex carbohydrates. Infect. Immun. 59:1605-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, B. J., and L. J. Douglas. 1996. Blood group glycolipids as epithelial cell receptors for Candida albicans. Infect. Immun. 64:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaim, W., B. Foxman, and J. D. Sobel. 1997. Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J. Infect. Dis. 176:828-830. [DOI] [PubMed] [Google Scholar]
- 6.Critchley, I. A., and L. J. Douglas. 1987. Role of glycosides as epithelial cell receptors for Candida albicans. J. Gen. Microbiol. 133:637-643. [DOI] [PubMed] [Google Scholar]
- 7.Domino, S. E., and E. A. Hurd. 2004. LacZ expression in Fut2-LacZ reporter mice reveals estrogen-regulated endocervical glandular expression during estrous cycle, hormone replacement, and pregnancy. Glycobiology 14:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domino, S. E., L. Zhang, P. J. Gillespie, T. L. Saunders, and J. B. Lowe. 2001. Deficiency of reproductive tract α(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 α(1,2)fucosyltransferase locus. Mol. Cell. Biol. 21:8336-8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidel, P. L., Jr. 2002. Immunity to Candida. Oral Dis. 8(Suppl. 2):69-75. [DOI] [PubMed] [Google Scholar]
- 10.Fidel, P. L., Jr., J. Cutright, and C. Steele. 2000. Effects of reproductive hormones on experimental vaginal candidiasis. Infect. Immun. 68:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1993. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect. Immun. 61:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly, R. J., S. Rouquier, D. Giorgi, G. G. Lennon, and J. B. Lowe. 1995. Sequence and expression of a candidate for the human secretor blood group α(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 270:4640-4649. [DOI] [PubMed] [Google Scholar]
- 14.Koda, Y., H. Tachida, H. Pang, Y. Liu, M. Soejima, A. A. Ghaderi, O. Takenaka, and H. Kimura. 2001. Contrasting patterns of polymorphisms at the ABO-secretor gene (FUT2) and plasma α(1,3)fucosyltransferase gene (FUT6) in human populations. Genetics 158:747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 16.Nawrot, U., K. Grzybek-Hryncewicz, U. Zielska, A. Czarny, and J. Podwinska. 2000. The study of cell-mediated immune response in recurrent vulvovaginal candidiasis. FEMS Immunol. Med. Microbiol. 29:89-94. [DOI] [PubMed] [Google Scholar]
- 17.Newburg, D. S., G. M. Ruiz-Palacios, M. Altaye, P. Chaturvedi, J. Meinzen-Derr, M. Guerrero, and A. L. Morrow. 2004. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 14:253-263. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson, M., K. O. Hedlund, M. Thorhagen, G. Larson, K. Johansen, A. Ekspong, and L. Svensson. 2003. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 77:13117-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fuc α1,2Gal β1,4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112-14120. [DOI] [PubMed] [Google Scholar]
- 20.Saadi, A. T., D. M. Weir, I. R. Poxton, J. Stewart, S. D. Essery, C. C. Blackwell, M. W. Raza, and A. Busuttil. 1994. Isolation of an adhesin from Staphylococcus aureus that binds Lewis a blood group antigen and its relevance to sudden infant death syndrome. FEMS Immunol. Med. Microbiol. 8:315-320. [DOI] [PubMed] [Google Scholar]
- 21.Schaeffer, A. J., N. Rajan, Q. Cao, B. E. Anderson, D. L. Pruden, J. Sensibar, and J. L. Duncan. 2001. Host pathogenesis in urinary tract infections. Int. J. Antimicrob. Agents 17:245-251. [DOI] [PubMed] [Google Scholar]
- 22.Sobel, J. D. 1988. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann. N. Y. Acad. Sci. 544:547-557. [DOI] [PubMed] [Google Scholar]
- 23.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14(Suppl. 1):S148-S153. [DOI] [PubMed] [Google Scholar]
- 24.Steele, C., J. Leigh, R. Swoboda, H. Ozenci, and P. L. Fidel, Jr. 2001. Potential role for a carbohydrate moiety in anti-Candida activity of human oral epithelial cells. Infect. Immun. 69:7091-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardar-Unlu, G., C. McSharry, and L. J. Douglas. 1998. Fucose-specific adhesins on germ tubes of Candida albicans. FEMS Immunol. Med. Microbiol. 20:55-67. [DOI] [PubMed] [Google Scholar]