Abstract
Immunity to Entamoeba species intestinal infection is associated with the presence of intestinal IgA antibodies against the parasite's galactose-inhibitable adherence lectin. We determined the epitope specificity of serum and intestinal antilectin IgA antibodies by enzyme-linked immunosorbent assay using overlapping fragments of a recombinant portion of the lectin heavy subunit, designated LC3. These findings were correlated with the effects of epitope-specific murine antilectin immunoglobulin A (IgA) monoclonal antibodies (MAbs) on amebic in vitro galactose-specific adherence. LC3 is a highly antigenic and immunogenic cysteine-rich protein (amino acids [aa] 758 to 1150) that includes the lectin's carbohydrate binding domain. The study subjects, from Durban, South Africa, were recently cured of amebic liver abscess (ALA) with or without concurrent Entamoeba histolytica intestinal infection or were infection free 1 year after cure. We also studied seropositive subjects that were infected with E. histolytica, disease free, and asymptomatic. Serum anti-LC3 IgA antibodies from all study groups exclusively recognized the third (aa 868 to 944) and the seventh (aa 1114 to 1134) LC3 epitopes regardless of clinical status; epitope 6 (aa 1070 to 1114) was also recognized by serum anti-LC3 IgG antibodies. However, IgG antibody recognition of epitope 6 but not 3 or 7 was lost 1 year following cure of ALA. We produced 14 murine anti-LC3 IgA MAbs which collectively recognized five of the seven LC3 epitopes. The majority of the murine MAbs recognized the first epitope (aa 758 to 826), which was not recognized by human IgA antibodies. Interestingly, adherence of E. histolytica trophozoites to CHO cells was inhibited by MAbs against epitopes 1, 3, 4 (aa 944 to 987), and 6 (P < 0.01). The LC3 epitopes recognized by human IgA antibodies (3 and 7) were further characterized by use of overlapping synthetic peptides. We identified four peptides (aa 891 to 903, 918 to 936, 1114 to 1134, and 1128 to 1150) that in linear or cyclized form were recognized by pooled intestinal IgA antibodies and serum IgG antibodies from subjects with ALA and asymptomatic, seropositive infected subjects. This study identifies the lectin epitopes to be studied in an amebiasis subunit vaccine designed to elicit mucosal immunity mimicking that of humans cured of ALA.
Colonization of the gut by the enteric protozoan Entamoeba histolytica is associated with adherence to the carbohydrate-rich mucin layer covering the colonic mucosa (8, 9), which forms a nonimmune barrier to parasitic invasion. In general, secretory IgA antibodies are thought to contribute to mucosal defense via immune exclusion. IgA antibodies prevent contact of enteric pathogens with the intestinal epithelial surface due to their agglutination, entrapment within immune complexes, and clearance within the mucous blanket (1, 21). Adherence of E. histolytica to colonic mucins and epithelial cells is mediated by the parasite's galactose-inhibitable surface lectin (8, 27). The carbohydrate binding domain of the lectin's 170-kDa heavy subunit (23, 24) is localized between amino acids (aa) 895 and 998 (13, 20, 26). Murine immunoglobulin G (IgG) monoclonal antibodies against the 170-kDa lectin subunit (23) completely eliminate the galactose-specific adherence of E. histolytica trophozoites to colonic mucins in vitro (8, 9), indicating that intestinal antilectin IgA antibodies could have an important role in mucosal immunity to E. histolytica. There is mounting evidence from epidemiologic studies that intestinal antilectin IgA antibodies mediate immunity to intestinal infection by E. histolytica (16, 17) and Entamoeba dispar trophozoites (29). The latter is a closely related but distinct species (11) that is morphologically identical to E. histolytica and that possesses a functional galactose-binding lectin with greater than 85% amino acid sequence homology to that of E. histolytica (25). The E. dispar lectin includes the complete carbohydrate binding domain (25); E. dispar induces an intestinal but not a humoral antilectin IgA antibody response (29).
A recombinant cysteine-rich fusion protein that includes aa 758 to 1134 of the lectin's 170-kDa subunit, designated LC3 (30), is recognized by adherence-inhibitory IgG monoclonal antibodies and includes the lectin's galactose-binding domain (13, 20, 26). The LC3 protein is highly antigenic and immunogenic; purified LC3 protein has a 70% vaccine efficacy in the gerbil model of amebic liver abscess (ALA) (30). Oral immunization of BALB/c mice with the LC3 protein, with cholera toxin as the adjuvant, induces an adherence-inhibitory intestinal anti-LC3 IgA antibody response (6). Anti-LC3 IgA and IgG antibodies are present in the sera of over 90% of patients with invasive amebiasis (colitis and ALA) and in the majority of subjects with asymptomatic E. histolytica intestinal infection (3, 28, 29). In several studies that encompassed large numbers of patients with amebic colitis or liver abscess, a mucosal IgA immune response to the recombinant LC3 antigen was detected (4, 29).
The purpose of this study was to identify the specific LC3 epitopes recognized by IgA antibodies associated with the putatively protective mucosal immune response that occurs following cure of ALA (29). We identified the IgA antibody epitopes by use of overlapping recombinant LC3 protein fragments, utilizing serum IgG antibodies for comparison, and confirmed our findings by studies with pooled intestinal IgA antibodies. We produced IgA monoclonal antibodies against the LC3 protein for use as specific probes to correlate epitope recognition with inhibition of amebic galactose-specific adherence. To further define the putative protective LC3 epitopes, overlapping peptides were prepared by using amino acid sequences of the reactive LC3 epitopes and screened for recognition with IgA antibodies from pooled human sera and feces.
MATERIALS AND METHODS
Subjects.
Sera and stool samples were obtained from control subjects without amebic infection, seropositive subjects with E. histolytica asymptomatic infection, patients recently (0 to 3 months) cured of ALA with or without a sustained E. histolytica intestinal infection, and ALA patients 1 year after cure who remained uninfected in an area of high endemicity in Durban, South Africa. Luminal amebicidal agents such as diloxanide furoate and paromomycin are unavailable in South Africa; therefore, ALA patients may remain infected for months to years after treatment (29). All human studies were reviewed and approved by the Institutional Review Board at the University of Minnesota and the University of Natal, Durban, South Africa. The presence of E. histolytica infection was determined by ribosomal DNA amplification using PCR and/or culture of stool with zymodeme analysis.
Detection of amebic infection by ribosomal DNA PCR.
Stool samples were stored at −70°C, and the extracted DNA was stored at −20°C in a fecal DNA bank. The QIAamp DNA stool minikit (QIAGEN, Hilden, Germany) was used to extract DNA human feces according to the manufacturer's protocol. Four separate laboratory areas were used for PCR analysis (7) to minimize the risk of contamination. DNA was extracted from stool in one area, and the PCR mixture was prepared and samples were added in another area. PCR was run in a third area, and the analysis and storage of the amplified PCR materials (materials, glassware, and equipment) occurred in the remaining area.
PCR was performed with deoxynucleoside triphosphates (Amersham Pharmacia Biotech; catalog no. 27-2035-01) by mixing 100 μl of each nucleotide (G, C, T, and A; each neocleotide was supplied as a 100 mM solution in H2O at pH 7.5) with 5 ml of 10× PCR buffer (7) and 4.6 ml of H2O (final concentration of each nucleotide was 10 μl/ml (5.8 μg/ml for A, 5.6 μg/ml for C, 5.9 μg/ml for G, and 5.7 μg/ml for T). The mixture was divided into 1-ml aliquots and stored at −20°C. The Taq polymerase (Amersham Pharmacia Biotech; catalog no. 270799) was diluted 1:20 immediately before use. The E. histolytica sense primer (5′-GTA CAA AAT GGC CAA TTC ATT CAA CG-3′), the E. dispar sense primer (5′-GTA CAA AGT GGC CAA TTT ATG TAA GCA-3′), and the E. histolytica and E. dispar antisense primer (5′-GAA TTG ATT TTA CTC AAC TCT AGA G-3′) (7) were prepared at 10 pmol/μl. Bovine serum albumin (BSA; Pierce; 200 mg/dl; catalog no. 23210) was diluted with an equal volume of H2O (500 μl of BSA plus 500 μl of H2O) and kept at 4°C. The DNA to be tested (5 μl) was added to 95 μl of PCR mixture to make 100 μl. Each DNA sample was tested twice, once with the E. histolytica sense primer and once with the E. dispar sense primer.
The conventional PCR machine thermocycling conditions were 1 cycle of 2 min at 95°C followed by 35 cycles of 1 min at 94°C, 1 min at 56°C, and 30 s at 72°C. The last single cycle was 3 min at 72°C.
Specific detection of amplified DNA was achieved by gel electrophoresis. Digested DNA was separated on a 2% agarose gel containing ethidium bromide.
Expression of recombinant LC3 fragments in Escherichia coli.
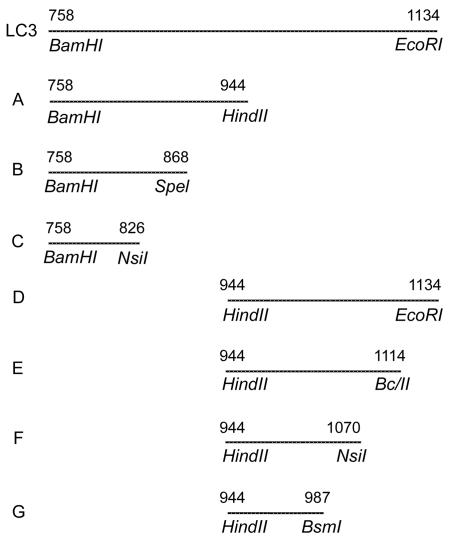
The DNA encoding LC3 (bp 2273 to 3397 of the lectin heavy subunit gene) (30, 33) was subjected to restriction enzyme digestion (Fig. 1), and the DNA fragments were ligated in frame into pREST expression vectors. Transformed bacteria were grown and the fusion proteins were expressed as detailed previously (30). Expression of each protein was verified by immunoblotting with a T7 tag IgG monoclonal antibody, which binds to the fusion leader sequence.
FIG. 1.
Recombinant protein fragments produced from restriction enzyme digestion of LC3 DNA. Restriction enzyme sites are indicated, and fragments are represented by their amino acid sequence numbers. Seven recombinant LC3 protein fragments (A through G) were produced.
Generation of IgA monoclonal antibodies against the LC3-encoded protein fragments.
BALB/c mice were obtained from the Jackson Laboratory (Bar Harbor, Maine), which maintains pathogen-free animal colonies. The mice were maintained in microisolation cages, free from Sendai virus and other pathogenic microorganisms.
IgA monoclonal antibodies were produced via a mucosal immunization protocol. BALB/c mice were immunized via the Peyer's patch twice with 200 μg of LC3 protein and boosted intravenously with 2 μg of epinephrine and 50 μg of LC3 protein the following day. Three days later, the mice were scarified and their spleen cells were hybridized to SP2/0 myeloma cells.
Enzyme-linked immunosorbent assay (ELISA) was utilized to identify hybridoma clones that secreted IgA monoclonal antibodies against the LC3-encoded protein. (22). Nunc-Immune plates were coated overnight with LC3 protein at 4°C and pH 9.6. The plates were blocked with 1% BSA in phosphate-buffered saline solution (PBS). Tissue culture supernatants from each fusion were incubated for 1 h at room temperature or overnight at 4°C. Following washing with PBS containing 1% BSA and 0.5% Tween 20, alkaline phosphatase-conjugated goat anti-mouse IgA antibodies were added as 100 μl/well at a concentration of 1 to 1,000 in PBS-Tween with 1% BSA. The enzymatic reaction was developed with 1 mg of p-nitrophenolphosphate substrate/ml, and the optical density (OD) was determined at a wavelength of 410 nm. An OD reading of 0.05 above that for the control well without LC3 present was considered positive. The isotype specificity of the anti-mouse IgA conjugate was confirmed with IgA, IgG, and IgM myeloma proteins. The isotype of the murine antibody was also confirmed with an Iso-strip mouse monoclonal antibody isotyping kit.
Epitope mapping of LC3 fragments recognized by human serum IgA and IgG antibodies.
ELISA was performed as described previously (2). LC3 protein fragments were purified as described previously (30). Briefly, 96-well microtiter flat-bottom polystyrene ELISA plates were coated with individual LC3 protein fragments (0.4 μg/well), and the nonreactive sites were blocked with 1% BSA. Serum samples were studied at a 1:100 dilution for IgA and 1:250 for IgG, all in PBS-Tween-1% BSA, and incubated for 2 h at room temperature. Alkaline phosphatase-conjugated goat anti-human IgA antibodies (ICN Biomedicals, Costa Mesa, Calif.) and anti-human IgG (Sigma, St. Louis, Mo.) were diluted (at 1:2,500 for IgA and 1:5,000 for IgG) in PBS-Tween-1% BSA for incubation in 100-μl wells for 2 h at room temperature. Developing, reading the plates, and correcting for nonspecific background binding were performed as described previously (28).
Epitope mapping of LC3 fragments recognized by murine IgA monoclonal antibodies.
Transformed bacteria (30) were pelleted, lysed in sodium dodecyl sulfate (SDS), and loaded into 10% Laemmli polyacrylamide gels. After electrophoresis, the proteins were transferred to nitrocellulose papers for immunoblotting with anti-LC3 IgA monoclonal antibodies. Horseradish peroxidase-conjugated anti-mouse IgA (1:1,000 dilution) was utilized as a secondary antibody, and 4-chloro-1 naphthol was used as a substrate for staining the bound secondary antibody.
Effect of monoclonal antibodies on amebic in vitro adherence to CHO cells.
E. histolytica trophozoites, strain HMI:IMSS, were maintained in axenic culture in TYI-S-33 culture medium, as described by Diamond et al. (12), and harvested as described previously (27). CHO cells obtained from the American Type Culture Collection were grown in F-12 medium (GIBCO) supplemented with 10% fetal bovine sera (FBS; GIBCO), penicillin (100 μg/ml), and streptomycin (100 μg/ml) as described previously.
Adherence studies were performed using a rosetting assay (27). Amebae (105/ml) were incubated in the hybridoma supernatants (dilution) at 4°C, and control amebae were incubated with RPMI medium-10% FBS or tissue culture supernatant containing an anti-Sendai virus IgA monoclonal antibody. After extensive washing, trophozoites (104) and CHO cells (105) were suspended in 1 ml of M199S, centrifuged at 250 × g, and incubated for 2 h at 4°C. After incubation, 0.8 ml of supernatant was removed and the pellet was suspended. The percentage of amebae that formed rosettes with CHO cells (three or more adherent cells) was determined in a hemocytometer chamber.
Synthesis and purification of peptides based on the amino acid sequences of the LC3 protein epitopes.
Ten overlapping peptides were prepared from the amino acid sequence of epitope number 3 (between aa 868 and 944). Two more overlapping peptides were synthesized from sequences between aa 1114 and 1150 (epitope 7).
Peptides were synthesized with a Perkin-Elmer Pioneer peptide synthesizer, by solid-phase fluorenylmethoxy carbonyl chemistry. Peptides were cleaved from the resin and deprotected with reagent R and then lyophilized. Lyophilized crude peptides were purified by preparative reverse-phase high-performance liquid chromatography (HPLC; Beckman 126) on a C-4 column by VYDAC. Solvent A is 0.1% trifluoroacetic acid (TFA) in water, and solvent B is 0.1% TFA in acetonitrile. Purification was performed on a gradient of 0 to 60% solvent B in 30 min. Assessment of purity and quality control of the peptides were performed by analytical HPLC with an HP1090 on a C-18 (VYDAC) column using the same gradient and by mass spectrometry on a Hewlett-Packard matrix-assisted laser desorption ionization-time of flight instrument (5, 10).
Peptide recognition by human IgA and IgG antibodies.
Pooled human sera and human feces (1.0 g) diluted in 1.0 μl of phenylmethylsulfonyl fluoride (2 mM) and 2.0 ml of PBS-Tween containing 1.0% BSA were obtained from ALA patients, subjects with asymptomatic E. histolytica infection, and controls and used in ELISA to determine recognition of the reactive peptides. ELISA was performed in an identical method as that used in assays for LC3 fragments except that equal volumes of sera or prepared feces from the study subjects were mixed and added in the previously mentioned concentration to wells coated with each peptide. The rest of the ELISA steps were identical to those performed with the protein fragments.
Statistics.
Results were expressed as means (plus 3 standard deviations of percent positive and percent negative). The Z test (converted to P value) and unpaired Student t test were used to determine the significance of difference (31). The Z test used the equation Z = (P1 − P2)/[PQ(1/N1 − 1/N2)]1/2, where P1 and P2 are the proportions of positives in groups 1 and 2, respectively, P is the pooled proportional estimate [(= X1 + X2)/(N1 + N2), where X1 and X2 are the numbers of positives in groups 1 and 2, respectively], N1 and N2 are the total numbers in groups 1 and 2, respectively, and Q = (1 − P).
In present study the Z test is used for percentages. The null hypothesis says that the percentages that test positive in the two groups are the same (difference in percentages = 0.0%). The alternative says that percentages that test positive in the two groups are different. With a type 1 error (alpha) equal to 0.05, it is found that significant changes are characterized by Z values >1.645 and that insignificant changes are characterized by Z values <1.645.
RESULTS
Production of LC3 recombinant protein fragments.
As illustrated in Fig. 1, the LC3 protein encompasses aa 758 to 1134 of the lectin heavy subunit. LC3 DNA was cleaved by restriction enzyme digestion into two fragments (A and B) encoding proteins consisting of aa 758 to 944 and 944 to 1134. Fragment A was further digested into fragments B (encoding aa 758 to 868) and C (encoding aa 758 to 826) (Fig. 1). Fragment D was further digested into fragments E, F, and G, encoding proteins consisting of aa 944 to 1114, 944 to 1070, and 944 to 987, respectively. Recombinant proteins were produced from each of these overlapping DNA fragments, and the pattern of their recognition by IgA antibodies allowed identification of seven distinct LC3 epitopes.
Epitope mapping of LC3 fragments by human anti-LC3 IgA and IgG antibodies.
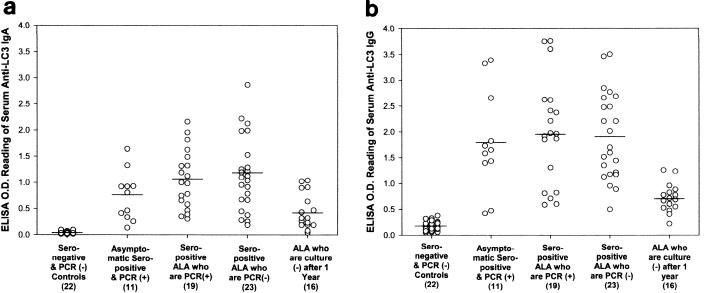
The serologic response to purified LC3 protein was determined by ELISA for each of the study groups. Five different groups of amebiasis subjects were studied to determine if there were differences in IgA epitope specificity: seropositive asymptomatic subjects with E. histolytica infection, subjects cured of ALA that had persistent asymptomatic intestinal infection or that had cleared the infection, and ALA subjects who were infection free 1 year after cure. As expected, subjects recently cured of ALA with or without a concurrent infection had higher OD readings for serum anti-LC3 IgA and IgG antibodies (P < 0.05 compared to controls and ALA subjects 1 year after cure; Fig. 2). Seropositive subjects with asymptomatic infection had levels of anti-LC3 IgA and IgG antibodies comparable to those of the recently cured ALA subjects (Fig. 2). Serum anti-LC3 IgG antibody ELISA OD values (at equal dilutions) were higher than those for IgA antibodies (P < 0.05). In the epitope mapping studies, nonpurified recombinant proteins present in cell supernatant were utilized as antigen in the ELISA. Therefore, nonpurified LC3 recombinant protein present in E. coli supernatant served as the positive control under experimental conditions identical to those for the recombinant LC3 fragments. Under these experimental conditions, ELISA for serum anti-LC3 IgA antibodies demonstrated reactivity in 56.3 (1 year after cure of ALA) to 90.1% (asymptomatic infected adults) of amebiasis subjects, all of whom were previously found to be IgA seropositive for the highly purified LC3 protein (Table 1).
FIG. 2.
ELISA OD results for serum anti-LC3 IgA (a) and IgG (b) antibodies using purified LC3 antigen (horizontal bars, means). Study groups include seronegative uninfected controls; asymptomatic, seropositive subjects with concurrent E. histolytica infection as determined by fecal PCR; subjects recently cured of ALA with and without infection (determined by PCR); and ALA subjects free of infection by culture criteria 1 year after cure. There were substantially higher IgA and IgG antibody levels in recently cured ALA subjects with or without infection and seropositive subjects with asymptomatic intestinal infection than in controls and ALA subjects studied 1 year after treatment (P < 0.05 for each).
TABLE 1.
Recognition of the E. histolytica LC3 recombinant protein fragments by serum IgA antibodies
| LC3 protein fragment (aa) | No.d (%) of subjects who werea:
|
||||
|---|---|---|---|---|---|
| Seronegative PCR− controls (n = 22) | Asymptomatic seropositive PCR+ (n = 11) | ALA seropositive PCR+ (n = 19) | ALA seropositive PCR− (n = 23) | ALA seropositive culture− after 1 yr (n = 16) | |
| Nonpurified LC3 | 0 | 10 (90.1) | 16 (84.2) | 16 (69.6) | 9 (56.3) |
| A (758-944) | 0 | 6 (54.5)b | 15 (78.9)b | 14 (60.8)b | 8 (50)b |
| B (758-868) | 0 | 0 | 1 (5.3) | 0 | 0 |
| C (758-826) | 0 | 0 | 0 | 0 | 0 |
| D (944-1134) | 0 | 6 (54.5)c | 10 (52.6)c | 10 (43.4)c | 5 (31.5)c |
| E (944-1114) | 0 | 1 (9.1) | 3 (15.8) | 1 (4.3) | 0 |
| F (944-1070) | 0 | 1 (9.1) | 0 | 0 | 1 (6.3) |
| G (944-987) | 0 | 0 | 0 | 0 | 0 |
Seronegative or seropositive is defined by ELISA of purified LC3 protein; nonpurified LC3 protein serves as a positive control for nonpurified LC3 protein fragments. Boldface indicates values significantly different from controls.
P < 0.05 for recognizing LC3 fragment A compared to LC3 fragments B, C, E, F, and G.
P < 0.05 for recognizing LC3 fragment D compared to LC3 fragments B, C, E, F, and G.
Serum IgA antibodies obtained from all four amebiasis study groups recognized only LC3 fragments A (aa 758 to 944) and D (aa 944 to 1134) (Table 1; P < 0.05 compared to all other fragments and sera from uninfected seronegative controls). ELISA for anti-LC3 serum IgG antibodies demonstrated a higher level of reactivity to the nonpurified LC3 in E. coli supernatant (81 to 100%; Table 2) than was found for IgA antibodies, concordant with the higher OD readings for serum IgG antibodies against the purified LC3 protein (Fig. 2B). Serum IgG antibodies from three of the four amebiasis subgroups (asymptomatically infected, ALA infected, and ALA uninfected) recognized fragments A (aa 758 to 944), D (aa 944 to 1134), and E (aa 944 to 1114) (P < 0.05; Table 2). However, IgG antibody recognition of fragment E was absent in sera from uninfected ALA subjects only 1 year after cure (Table 2).
TABLE 2.
Recognition of the E. histolytica LC3 protein fragments by serum IgG antibodies
| LC3 protein fragment (aa) | No.f (%) of subjects who werea:
|
||||
|---|---|---|---|---|---|
| Seronegative PCR− controls (n = 22) | Asymptomatic seropositive PCR+ (n = 11) | ALA seropositive PCR+ (n = 19) | ALA seropositive PCR− (n = 23) | ALA seropositive culture− after 1 yr (n = 16) | |
| Nonpurified LC3 | 0 | 11 (100) | 18 (94.7) | 22 (95.7) | 13 (81.3) |
| A (758-944) | 0 | 11 (100)b | 16 (84.2)b | 19 (82.6)b | 10 (62.5)b |
| B (758-868) | 0 | 2 (18.2) | 0 | 0 | 0 |
| C (758-826) | 0 | 1 (9.1) | 0 | 0 | 0 |
| D (944-1134) | 0 | 11 (100)c | 15 (78.9)c | 19 (82.6)c | 10 (62.5)c |
| E (944-1114) | 0 | 9 (81.2)d | 14 (73.7)d | 15 (65.2)d | 1 (6.3)e |
| F (944-1070) | 1 (4.5) | 0 | 0 | 0 | 0 |
| G (944-987) | 0 | 0 | 0 | 0 | 1 (6.3) |
Seronegative or seropositive is defined by ELISA of purified LC3 protein; nonpurified LC3 protein serves as a positive control for nonpurified LC3 protein fragments. Boldface indicates values significantly different from controls.
P < 0.05 for recognizing LC3 fragment A compared to LC3 fragments B, C, F, and G.
P < 0.05 for recognizing LC3 fragment D compared to LC3 fragments B, C, F, and G.
P < 0.05 for recognizing LC3 fragment E compared to LC3 fragments B, C, F, and G.
P < 0.05 for recognizing fragment E compared to other ALA subjects and asymptomatic seropositive controls.
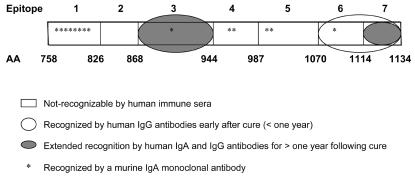
Based on the recognition pattern for the LC3 protein fragments, we determined that human serum anti-LC3 IgA antibodies exclusively recognized LC3 epitopes 3 (aa 868 to 944) and 7 (aa 1114 to 1134), regardless of study group (Fig. 3). Anti-LC3 IgG antibodies also recognized epitope 6 (aa 1070 to 1114), but as stated above this recognition was lost 12 months after cure of ALA (Fig. 3).
FIG. 3.
Recognition of LC3 epitopes by human anti-LC3 IgA and IgG antibodies and by anti-LC3 murine IgA monoclonal antibodies. By analysis of serum IgA and IgG antibody recognition of LC3 protein fragments, we found that IgA antibodies from ALA subjects (infected or uninfected) and from seropositive subjects with asymptomatic E. histolytica infection all recognized epitopes 3 and 7 (gray ovals). Only anti-LC3 IgG antibodies recognized epitope 6 (white oval), and recognition was lost only 1 year after cure of ALA. Anti-LC3 murine IgA monoclonal antibodies recognized epitopes 1, 2, 4, 5, and 6 (asterisks) and not epitopes 2 and 7.
Characterization of murine anti-LC3 IgA monoclonal antibodies.
We screened 1,300 hybridoma clones by ELISA for anti-LC3 IgA antibodies and found 85 positive secretors. Of the 85, by limiting dilution, we found 14 which were stable as single clones. We confirmed by immunoblotting whether the 14 hybridoma clones produced IgA monoclonal antibodies against the LC3 protein (Table 3). All but 2 of the 14 monoclonal antibodies, 244 and 728, recognized native E. histolytica lectin in ELISA. Monoclonal antibodies against Sendai virus IgA served as a negative control in all experiments.
TABLE 3.
Epitope specificity of 14 anti-LC3 IgA murine monoclonal antibodies with comparison to that of human serum IgA and IgG antilectin antibodies
| LC3 epitope (aa) | IgA monoclonal antibody(ies) (clone no.) | Specificity for human
|
|
|---|---|---|---|
| IgA | IgG | ||
| 1 (758-826) | 38, 41, 193, 244, 606, 728, 737, 854 | − | − |
| 2 (826-868) | —b | − | − |
| 3 (868-944) | 875 | + | + |
| 4 (944-987) | 580, 1152 | − | − |
| 5 (987-1070) | 676, 1059 | − | − |
| 6 (1070-1114) | 867 | − | +a |
| 7 (1114-1150) | — | + | + |
Epitope recognition lost 1 year after cure of ALA.
—, no antibodies.
Anti-LC3 monoclonal antibodies were further characterized by epitope mapping as shown in Table 3. The seven LC3 protein fragments (A to G) were immunoblotted with all 14 of the IgA monoclonal antibodies; the LC3 protein and an LC1 fusion protein (aa 1 to 346) were used as positive and negative controls, respectively. Eight monoclonal antibodies (no. 38, 41, 193, 244, 606, 728, 737, and 854) recognized fragments A, B, and C. Clone 875 reacted only with fragment A, indicating that it recognized epitope 3 (aa 868 to 944). We determined by similar analysis the epitope specificity of each anti-LC3 IgA monoclonal antibody (summarized in Table 3 and illustrated in Fig. 3). None of the IgA monoclonal antibodies were found to recognize the second or seventh LC3 epitopes (aa 826 to 868 and 1114 to 1134, respectively) (Fig. 2). Interestingly, the overwhelming majority (13 of 14) of the murine IgA monoclonal antibodies recognized LC3 epitopes not recognized by human anti-LC3 IgA antibodies (Table 3 and Fig. 3).
Effects of anti-LC3 IgA monoclonal antibodies on amebic adherence to CHO cells.
Except for clone 1152 (epitope 4), which demonstrated no inhibition, regardless of epitope specificity all of the anti-LC3 IgA monoclonal antibodies inhibited amebic galactose-specific adherence to CHO cells, by 25 to 87% (P < 0.01 compared to a control IgA monoclonal antibody; Table 4). Monoclonal antibody 580, which also recognized epitope 4, inhibited amebic adherence by 72% (P < 0.01; Table 4).
TABLE 4.
Inhibition of amebic adherence to CHO cells by anti-LC3 IgA murine monoclonal antibodies
| IgA-producing hypridomasupernatant (LC3 epitope) | Inhibition of adherencea (%) |
|---|---|
| 38 (1) | 73 |
| 41 (1) | 25 |
| 193 (1) | 70 |
| 244 (1) | 29 |
| 606 (1) | 73 |
| 728 (1) | 36 |
| 737 (1) | 87 |
| 854 (1) | 76 |
| 875 (3) | 61 |
| 580 (4) | 72 |
| 1152 (4) | 3 |
| 676 (5) | 79 |
| 1059 (5) | 68 |
| 867 (6) | 28 |
| Control anti-Sendai virus IgA | 1 |
Percent inhibition of adherence is defined as comparison to control test medium without antibodies present. Cell supernatants from all anti-LC3 IgA monoclonal antibodies inhibited amebic adherence except 1152 (P < 0.01 compared to control anti-Sendai virus IgA).
Fine mapping of LC3 epitopes by use of synthetic peptides.
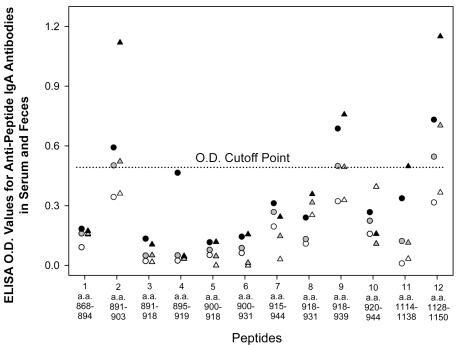
Pooled human sera and feces were studied for reactivity with 12 overlapping peptides to further define the LC3 epitopes recognized by human IgA antibodies. Ten peptides were prepared from the amino acid sequence of epitope 3, and two peptides were synthesized based on the sequence of epitope 7. Fecal anti-LC3 IgA antibodies from ALA patients with E. histolytica infection recognized the same four peptides (Fig. 4), three of which were also recognized by serum IgA antibodies. Fecal and serum IgA antibodies from asymptomatic seropositive infected subjects recognized three of the four peptides (no. 2, 9, and 12; Fig. 4). The amino acid sequences (associated nucleotide sequences) of the reactive peptide segments are as follows: peptide 2 (aa 891 to 903), CTYEITTRECKTC (TGT ACA TAC GAA ATA ACA ACA AGA GAA TGT AAA ACA TGT); peptide 9 (aa 918 to 939), CAEETKNGGVPFKCKNNNC (TGT GCA GAA GAG ACT AAG AAT GGA GGA GTT CCA TTC AAA TGT AAG); peptide 11 (aa 1114 to 1138), CDQTTGETIYTKKTCTVSEEF (TGT GAT CAA ACA ACT GGA GAA ACT ATT TAC ACA AAG AAA ACA TGT); peptide 12 (aa 1128 to 1150), CTVSEEFPTITPNQGRCFYCQCS (TGT ACT GTT TCA GAA GAA TTC CCA ACA ATC ACA CCA AAT CAA GGA). IgA antibodies from pooled control sera or in feces from controls did not react with any of the peptides, and the four reactive peptides were also recognized by serum anti-LC3 IgG antibodies pooled from the same study subjects (data not shown). Therefore, the LC3 epitopes recognized by human serum and intestinal IgA antibodies regardless of clinical status are aa 891 to 903 and aa 918 to 936 of epitope 3 and all of epitope 7 (aa 1114 to 1150).
FIG. 4.
Recognition by pooled human intestinal and serum anti-LC3 IgA antibodies of synthetic peptides based on the amino acid sequences of LC3 epitopes 3 and 7. Ten overlapping peptides were synthesized based on the sequence of epitope 3 and 2 peptides were synthesized based on epitope 7. Intestinal (triangles) and serum (circles) IgA antibodies were obtained from subjects cured of ALA with concurrent infection (black); gray symbols, seropositive, asymptomatic PCR-positive subjects; open symbols, seronegative PCR-negative control subjects. Peptides 2, 9, 11, and 12 were recognized by intestinal and serum IgA antibodies from all subjects, compared to the cutoff point established by seronegative controls. Peptides 2, 9, and 11 were recognized by serum IgA antibodies from ALA subjects and fecal and serum IgA antibodies from asymptomatic infected seropositive subjects.
DISCUSSION
Human infection with E. histolytica and E. dispar results in an intestinal IgA antibody response to the 170-kDa galactose-inhibitable lectin subunit (16, 17, 29). Antilectin IgA antibodies have been found in saliva and feces of patients with invasive amebiasis (colitis or ALA) and subjects asymptomatically infected with E. histolytica (4, 17, 18). After cure of ALA, secretory antilectin IgA antibodies can be recognized in stool for up to 36 months (29). The relationship between intestinal anti-amebic IgA antibodies and protection against parasitic infection has been recognized in children studied in Bangladesh; immunity was reported (16) to relate to individuals possessing anti-carbohydrate recognition domain (LC3 aa 895 to 998) IgA antibodies in feces; those with other antilectin IgA antibodies were reported not to be immune (17). However, these IgA antibodies were demonstrated to be present in children for a very short time (1 month) (16, 17). Following cure of ALA in Durban, South Africa, protective immunity to E. dispar infection persists for at least 3 years and ALA subjects demonstrated sustained secretion of high-titer intestinal antilectin IgA antibodies for up to 36 months (29). However, despite the presence of high-titer anti-amebic IgA antibodies in feces, in the absence of luminal amebicidal agents, a significant percentage of ALA subjects remain infected with E. histolytica, indicating that there is a difference between immune clearance of established infection and immunity to a new infection. Such differentiation requires genotyping to distinguish new infection from ongoing infection and to distinguish established infections from transient infections (30, 34). Genotyping has already succeeded in identifying different E. histolytica isolates in families residing in areas of hyperendemicity in South Africa. In most cases, members of individual family groups were infected with the same genotype of E. histolytica or E. dispar and the genotype remained constant over time (34).
By using seven overlapping fragments of the recombinant LC3 protein, we determined that serum IgA antibodies from asymptomatically infected subjects, seropositive ALA subjects (with or without concurrent E. histolytica infection), and uninfected ALA subjects 1 year after cure exclusively recognized LC3 epitopes 3 (aa 868 to 944) and 7 (aa 1114 to 1134). Serum anti-LC3 IgG antibodies from recently cured ALA subjects also recognized epitope 6 (aa 1070 to 1114), but this reactivity was lost by 1 year. There was no difference in epitope recognition between ALA subjects with or without sustained intestinal infection, suggesting that immunity to new asymptomatic Entamoeba species infection, as observed by Haque et al. (17) and Ravdin et al. (29), does not correlate with the ability to clear an established infection. Unlike Haque et al. (17), we found no clinical or immunologic subgroup that demonstrates a unique lectin epitope recognition pattern. Although we cannot rule out the presence of additional IgA lectin epitopes that were not identified due to the sensitivity of the assay, clearly, epitopes 3 and 7 were immunodominant. In fact, our previous studies indicate that the titer of antilectin IgA in stool may be the strongest predictor of effective mucosal immunity in adults (29). Infection by E. dispar does induce an intestinal antilectin IgA response, but it is of low titer and short-lived (29). Therefore, it is not surprising that, despite multiple shared lectin epitopes (24), E. dispar infection does not induce cross-species protection against E. histolytica (29).
We utilized murine anti-LC3 IgA monoclonal antibodies as epitope-specific probes to correlate in vitro adherence-inhibitory activity with human IgA epitope specificity. Of interest, immunization of BALB/c mice raised antibodies mainly against LC3 epitopes that are not recognized by humans (epitopes 1, 4, and 5, with epitope 1 predominate). Only 1 of 14 murine IgA antibodies recognized an epitope shared by human IgA antibodies (epitope 3). Clearly, vaccine studies using lectin-derived proteins in murine experimental models must be interpreted with caution due to clear differences in major histocompatibility complex-restricted immune recognition of the lectin protein structure. It seems unwise to jump directly from murine models to studies with humans without conducting vaccine studies with a more immunologically related model, such as primates.
Murine IgA monoclonal antibodies possessed adherence-inhibitory activity against amebic native surface lectin regardless of which LC3 epitope the antibody recognized. Interestingly, no adherence-enhancing activity (24) was observed with any of the IgA monoclonal antibodies studied. Analogous to results from studies of murine IgG or IgM antilectin monoclonal antibodies (23), adherence-inhibitory activity of these IgA antibodies did not correlate with direct recognition of the carbohydrate binding domain (aa 895 to 998) (13, 20, 26), which is contained within epitope 4 and which extends partially to epitopes 3 and 5. Therefore, the ability of antilectin IgA antibodies to mediate immunity in the gut may relate to multiple factors important in forming immune complexes and preventing the parasite from binding to colonic mucins or host cells.
The LC3 protein does not include the lectin's pseudo-repeat region (aa 436 to 624), against which Lotter and Tannich (19) raised adherence-inhibitory antibodies. However, as the LC3 protein includes the parasite's carbohydrate binding domain (13, 26), which is sufficient to induce immunity to ALA in gerbils (30) (as is a smaller 375-aa fragment of LC3 [14]), we chose this cysteine-rich recombinant protein for further study. We cannot exclude the possibility that IgA antibodies against the pseudo-repeat region may also be important in host mucosal immunity. However, compared to results from our study of anti-LC3 IgA antibodies (29), Lotter and Tannich found that immunity to the pseudo-repeat region waned more rapidly over time (19).
Peptide synthesis has been considered a productive tool for preparation of short protein segments with a limited number of amino acids. Overlapping subfragments from each epitope were engineered through peptide synthesis (5, 15) to better define the human IgA epitope specificity. Both serum IgA and IgG antibodies recognized 4 of 13 synthetic peptides when studied in either a linear or cyclized form. Therefore, the complete epitope specificity of human anti-LC3 IgA antibodies obtained from Durban, South Africa, is defined as aa 891 to 903, aa 918 to 936, and aa 1114 to 1150. Peptides such as these can be prepared in multiple forms (15) or attached to a polylysine backbone (32) to further enhance immunogenicity for use as a subunit vaccine.
In summary, based on previous epidemiologic studies (16, 29) and our present findings, we have defined potentially protective epitopes of the E. histolytica galactose-inhibitable lectin. Identification of the lectin epitopes by IgA antibody recognition of synthetic peptides provides a new opportunity for design of an experimental amebiasis subunit vaccine for prevention of amebic intestinal infection.
Acknowledgments
This work was supported by NIH grants PO1-AI36359-01 and UO1-AI35840 from NIAID and support from the MRC (South Africa).
We thank Egbert Tannich for providing the E. histolytica sense primer, the E. dispar sense primer, and the E. histolytica and E. dispar antisense primers. We thank Shana Brooks and Linda Andrean for expert secretarial assistance.
Editor: J. B. Bliska
REFERENCES
- 1.Abd-Alla, M. D., and J. I. Ravdin. 2004. Mucosal immune response to parasitic infections p. 2096-2099. In J. Mestecky (ed.), Mucosal immunology, 3rd ed. Elsevier, London, United Kingdom.
- 2.Abd-Alla, M. D., A. A. Wahib, and J. I. Ravdin. 2000. Comparison of antigen capture ELISA to stool culture in detection for asymptomatic Entamoeba species infection in Kafer Daoud, Egypt. Am. J. Trop. Med. Hyg. 65:579-582. [DOI] [PubMed] [Google Scholar]
- 3.Abd-Alla, M. D., and J. I. Ravdin. 2002. Diagnosis of amebic colitis by antigen capture ELISA in patients presenting with acute amebic diarrhea in Cairo, Egypt. Trop. Med. Intern. Health 7:365-370. [DOI] [PubMed] [Google Scholar]
- 4.Abou-E1-Magd, I., C. J. Soong, A. M. El-Hawey, and J. I. Ravdin. 1996. Humoral and mucosal IgA antibody response to a recombinant 52-kDa cysteine-rich portion of the Entamoeba histolytica galactose-inhibitable lectin correlates with detection of native 170-kDa lectin antigen in serum of patients with amebic colitis. J. Infect. Dis. 174:157-162. [DOI] [PubMed] [Google Scholar]
- 5.Atherton, E., H. Fox, C. Harkiss, R. C. Sheppard, and J. Williams. 1978. A mild procedure for solid phase peptide synthesis: use of fluorenylmerhoxy carbonyl amino acids. J. Chem. Soc. Chem. Commun. 13:537-543. [Google Scholar]
- 6.Beving, D. E., C. J. Soong, and J. I. Ravdin. 1996. Oral immunization with a recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin elicits an intestinal secretory immunoglobulin A response that has in vitro adherence inhibition activity. Infect. Immun. 64:1473-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blessman J., H. Buss, P. Antonnu, H. D. Thi, M. D. Abd Alla, T. F. H. G. Jackson, J. R. Ravdin, and E. Tannich. 2002. Real-time PCR detection and differentiation of Entamoeba histolytica and Entamoeba dispar in fecal samples. J. Clin. Microbiol. 40:4413-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chadee, K., W. A. Petri, J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit the adherence lectin of Entamoeba histolytica. J. Clin. Investig. 80:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadee, K., W. A. Petri, M. Johnson, M. E. Orozco, and J. I. Ravdin. 1988. Binding and internalization of rat colonic mucins by the Gal/GalNAc adherence lectin of Entamoeba histolytica. J. Infect. Dis. 158:398-406. [DOI] [PubMed] [Google Scholar]
- 10.Cleland, W. W. 1964. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 35:480-482. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn 1903 (emended Walker 1911), separating it from Entamoeba dispar Brumpt 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, L. S., D. R. Harlow, and C. C. Cunnnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 13.Dodson, J. M., C. G. Clark, L. A. Lockhart, et al. 1997. Comparison of adherence, cytotoxicity, and Gal/GalNAc lectin gene structure in Entamoeba histolytica and Entamoeba dispar. Parasitol. Int. 46:225-235. [Google Scholar]
- 14.Dodson, J. M., P. W. Lenkowski, A. C. Eubanks, T. F. G. Jackson, et al. 1999. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J. Infect. Dis. 179:460-466. [DOI] [PubMed] [Google Scholar]
- 15.Fields, C. G., G. B. Fields, R. L. Noble, and T. A. Cross. 1989. Solid phase peptide synthesis of 15N-gramicidins A, B, and C and high performance liquid chromatographic purification. Int. J. Pept. Protein Res. 33:1-41. [DOI] [PubMed] [Google Scholar]
- 16.Haque, R., I. M. Ali, R. B. Sack, B. M., Farr, G. Ramakrishnan, and W. A. Petri. 2001. Amebiasis and mucosal IgA antibody against Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 17.Haque, R., P. Duggal, I. M. Ali, M. B. Hossain, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed]
- 18.Kelsall, B. L., T. F. H. G. Jackson, V. Gathiram, S. B. Sails, M. Vaithilingum, R. D. Pearson, and J. I. Ravdin. 1994. Secretory immunoglobulin A antibodies to the galactose-inhibitable adherence protein in the saliva of patients with amebic liver disease. Am. J. Trop. Med. Hyg. 51:454-459. [PubMed] [Google Scholar]
- 19.Lotter, H., and E. Tannich. 1997. The ga1actose-inhibitable surface lectin of Entamoeba histolytica, a possible candidate for a subunit vaccine to prevent amoebiasis. Behring Inst. Mitt. 99:112-116. [PubMed] [Google Scholar]
- 20.Mann, B. J., C. Y. Chung, J. M. Dodson, L. S. Ashley, L. L. Braga, and T. L. Snodgrass. 1993. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesion map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect. Immun. 61:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGhee, J. R., J. Mestecky, T. Dertzbaugh, J. H. Eldridge, J. I. L. Nirasawa, and H. Kiyona. 1992. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10:75-88. [DOI] [PubMed] [Google Scholar]
- 22.Nedrud, J. G., X. P. Liang, N. Hague, and M. E. Lamm. 1987. Combined oral plus intranasal immunization protects mice from Sendai virus infection. J. Immunol. 139:3484-3492. [PubMed] [Google Scholar]
- 23.Petri, W. A., W. D. Smith, P. H. Schlesinger, and J. I. Ravdin. 1987. Isolation of the galactose-binding lectin which mediates the in vitro adherence to Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petri, W. A., T. F. H. F. Jackson, V. Gatheram, K. Kress, L. D. Saffer, T. L. Snodgrass, M. D. Chapman, Z. Keren, D. Mirelman, et al. 1990. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to galactose-specific adherence lectin. Infect. Immun. 58:1802-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillai, D. R., D. Britten, J. P. Ackers, J. I. Ravdin, and K. C. Kain. 1997. A gene homologous to hg12 of Entamoeba histolytica is present and expressed in Entamoeba dispar. Mol. Biochem. Parasitol. 87:101-105. [DOI] [PubMed] [Google Scholar]
- 26.Pillai, D. R., W. S. K. Wan, Y. C. W. Yau, J. I. Ravdin, and K. C. Kain. 1999. The cysteine-rich region of the Entamoeba histolytica adherence lectin (170-kilodalton subunit) is sufficient for high-affinity Gal/GalNAc-specific binding in vitro. Infect. Immun. 67:3836-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravdin, J. I., and R. I. Guerrant. 1981. The role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J. Clin. Investig. 68:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin, J. I., T. F. H. G. Jackson, W. A. Petri, et al. 1990. Association of serum anti-adherence lectin antibodies with invasive amebiasis and asymptomatic pathogenic Entamoeba histolytica infection. J. Infect. Dis. 162:768-772. [DOI] [PubMed] [Google Scholar]
- 29.Ravdin, J. I., M. D. Abd-Alla, S. L. Welles, S. Reddy, and T. F. H. G. Jackson. 2003. Intestinal antilectin immunoglobulin A antibody response and immunity to Entamoeba dispar infection following cure of amebic liver abscess. Infect. Immun. 71:6899-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soong, C. J., K. C. Kain, M. Abd-Alla, T. F. H. G. Jackson, and J. I. Ravdin. 1995. A recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 31.Sox, H. C., Jr. 1986. Probability theory in the use of diagnostic test. Ann. Intern. Med. 104:60-66. [DOI] [PubMed] [Google Scholar]
- 32.Tam, J. P. 1988. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 85:5400-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannich, E., F. Ebert, and D. Horstmann. 1991. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaki, M., S. Reddy, T. F. H. G. Jackson, J. I. Ravdin, and G. Clark. 2003. Genotyping of Entamoeba species in South Africa: diversity, stability, and transmission patterns within families. J. Infect. Dis. 187:1860-1869. [DOI] [PubMed] [Google Scholar]