Abstract
The modulation of risk-taking is critical for adaptive and optimal behavior. This study examined how oxytocin (OT) and arginine vasopressin (AVP) influence risk-taking in function of three parameters: sex, risk-valence, and social context. Twenty-nine healthy adults (14 males) completed a risk-taking task, the Stunt task, both in a social-stress (evaluation by unfamiliar peers) and non-social context, in three separate drug treatment sessions. During each session, one of three drugs, OT, AVP, or placebo (PLC), was administered intra-nasally. OT and AVP relative to PLC reduced betting-rate (risk-averse effect). This risk-averse effect was further qualified: AVP reduced risk-taking in the positive risk-valence (high win-probability), and regardless of social context or sex. In contrast, OT reduced risk-taking in the negative risk-valence (low win-probability), and only in the social-stress context and men. The reduction in risk-taking might serve a role in defensive behavior. These findings extend the role of these neuromodulators to behaviors beyond the social realm. How the behavioral modulation of risk-taking maps onto the function of the neural targets of OT and AVP may be the next step in this line of research.
Keywords: neuropeptides, motivated behavior, social stress, sex, risk-valence
Introduction
Risk-taking is a critical aspect of motivated behavior. It can be modulated by many factors, including the nature of risk (e.g., risk-valence: positive vs. negative probabilistic outcome), social context (e.g., presence or absence of peer groups), and individual differences (e.g., trait anxiety, sex). In addition to these factors, we propose that neuromodulators, such as the neuropeptides oxytocin (OT) and arginine vasopressin (AVP), can significantly influence risk-taking proclivity. This hypothesis stems from the fact that OT and AVP potently affect fundamental behaviors, specifically in the social realm. Social stimuli are probably the most powerful reinforcers of behavior, and thus a potential role of these neuromodulators on motivated behaviors, particularly risk-taking behaviors, is conceivable and deserves to be considered. Here, we foray a new untouched area of research.
Thus, OT and AVP may affect risk-taking indirectly through their documented influence on social and affective processes (Kosfeld, et al., 2005; Churchland and Winkielman, 2012), or perhaps directly. Studies have examined risk-taking in social exchange tasks that manipulate trust and cooperation (e.g., Declerck et al., 2010). However, no studies have yet examined the potential impact of these neuropeptides on risk-taking behavior outside social economic exchange tasks. The present study aimed to directly investigate the impact of OT and AVP on risk-taking behavior. To this aim, we assessed the influence of intra-nasal administration of OT, AVP, and placebo (PLC) on the performance of a risk-taking task.
The monetary risk-taking task used in the current study is a novel task that was developed to provide a unique parametrization of risk level over a wide range of nine levels. It also presents favorable (greater likelihood of winning than losing) and unfavorable (greater likelihood of losing than winning) contexts, in which individuals select a safe (pass) or risky (bet) option. Recent work suggests that the behavioral influence of OT, and perhaps AVP as well, varies as a function of environmental factors. This has been documented in the context of a social vs. non-social environment (e.g., Declerck et al., 2010), or in response to positive vs. negative social stimuli (e.g., Di Simplicio et al., 2009). In general, findings support stronger effects in a social vs. non-social situation, and towards positive vs. neutral/negative social stimuli. Here, we examine the effects of OT and AVP vs. PLC on risk-taking as a function of the risk-valence (positive vs. negative) and social context.
We address the social context by implementing the task in a social and a non-social situation. We selected a stress-related social situation (social stress by virtue of being judged by unfamiliar peers), because the administration of OT has been found to modulate neuroendocrine responses to external stressors (Heinrichs et al., 2003). Social stress, typically associated with anxiety, is expected to reduce risk-taking and promote risk-aversion as a protective response (Maner et al., 2007; Giorgetta et al., 2012). We expect OT to mitigate this effect. This prediction is based on work showing that OT can alleviate anxiety (Windle et al., 1997; Ring et al., 2006; see review, Neumann, 2008), and, perhaps in turn, stimulates trust, cooperation and other affiliative behaviors (Declerck et al., 2010; De Dreu et al., 2010; Churchland and Winkielman, 2012). As a result, OT would diminish the impact of social stress on risk-taking, and produce a relative decrease in risk-aversion. In contrast, AVP is thought to exacerbate anxiety (Bielsky et al., 2004; Thompson et al., 2006), associated with defensive responses. Accordingly, AVP would be expected to enhance the impact of social-stress on risk-taking, and amplify risk-aversion.
Finally, we expect individual differences to influence these pharmacological effects. For example, anxious individuals may be more sensitive to risk-valence and social stress, and may respond more strongly to the modulatory effects of OT and AVP on risk-taking, relative to non-anxious subjects. Sex may also be a powerful modulator of these effects, given the presence of sex differences in social responses and risk-taking. Very broadly, males tend to be more competitive and aggressive, as well as more risk-takers than females (Byrnes et al., 1999; Croson and Gneezy, 2009), suggesting that males may be more sensitive to the modulation of risk-taking by OT and AVP.
Based on this brief background, we hypothesize that, in general, OT will enhance risk-taking through favoring approach behavior, whereas AVP might reduce risk-taking through promoting defensive responses. We expect these effects to be modulated by risk-valence (positive vs. negative probability of winning) and social context (social-stress vs. non-social). Regarding risk-valence, the nature of its impact is difficult to predict without previous related work. Regarding social context, we could expect stronger effects in the social-stress vs. non-social context for both OT and AVP. Lastly, because of sex-differences in risk-taking and social behavior, the modulation of risk-taking is expected to be stronger in men than women.
Methods
Subjects
Thirty-two healthy adults were tested. Three subjects (2 men and 1 woman) were excluded because of unreliable performance (see below). The final sample included 14 men (age 21 to 35 years; mean = 26.67, SD = 4.68) and 15 women (age 20 to 38 years; mean = 27.43, SD = 4.59). Subjects were recruited through the intramural National Institutes of Health (NIH) volunteer system and general advertisements.
Participants were free of medical (determined by a clinical interview and physical exam) or psychiatric disorders (determined by the Structured Clinical Interview for DSM-IV; First et al., 1995) and were not taking any psychotropic medications, contraceptive hormones, or recreational drugs.
The protocol was approved by the National Institute of Mental Health Institutional Review Board. Written informed consent was obtained after the study was explained and all questions were answered. Participants were financially compensated.
Procedure
Study design
This study tested the effects of OT, AVP and PLC on risk-taking and its modulation by social context. A within-subjects, double-blind, placebo-controlled, randomized crossover design was adopted, in which a different drug treatment was administered on three separate days, at an average of five-week intervals. Women were tested during the same menstrual cycle phase (either in the follicular phase or during the luteal phase) across the three sessions to avoid intra-individual variability unrelated to the study manipulations.
Each session followed the same procedures. Subjects arrived in the morning. A state anxiety measure (State-Trait Anxiety Inventory, STAI; Spielberger, 1983) was collected twice, before and 50 minutes after drug administration. The task was initiated about 100 minutes after drug administration. For all sessions, during the drug treatment-to-task time interval, participants completed another independent study (Grillon et al., 2013). This independent study assessed the neuropeptides’ effects on anxiety. It involved a measure of physiological anxiety via eye-blink startle responses during conditions of threat (potential for mild electrical shocks) or safety (absence of electrical shocks). To minimize carry-over effects, a 20 minute break was implemented and our experiment was conducted in a different part of the clinic by a different research assistant.
We used a novel risk-taking task to manipulate the risk-level and risk-valence of the trials, a feature that has not been systematically included in existing risk-taking tasks (see review, Richards et al., 2013). It was designed to probe risk-taking decisions over a wide range of risk levels, nine different probabilities of negative/positive outcome. In addition, this novel task readily evoked the notion of risk by featuring a stunt-like behavior (motorcyclist jumping over buses). This design was thought to promote more “gut-feeling” rather than explicit calculation of risks (e.g., monetary type decision-making) and to tap more strongly emotional/motivational processing than cognitive evaluative functions. The Stunt task, was administered twice, at a 15-minute interval. During this time interval, participants were distracted from the study by performing an independent, irrelevant, simple attention task (passive eye movement attention task). The two Stunt task administrations were conducted in a social-stress and a non-social context, and the order of these contexts was counter-balanced across subjects. At the end of each session, a questionnaire was administered to obtain information on participants’ subjective experience regarding the risk-taking task and the social-stress/non-social contexts.
Drug administration
OT, AVP, and PLC were each administered intra-nasally in four doses over two minutes. Doses totaled 24 International Units (IU) (60 units/mL at .4 mL) for OT and 40 IU (100 units/mL at .4 mL) for AVP. Prior studies using similar drug administration (dosage and route) have found drug treatment effects on social information processing, such as facial emotion recognition (Di Simplicio et al., 2009; Fischer-Shofty et al., 2010; Zink et al., 2010). Additionally, intra-nasal administration of OT in rats has been found to increase OT levels in the brain (Neumann et al., 2013) and intra-nasal administration of AVP in humans has been found to increase AVP levels in cerebrospinal fluid (CSF) (Born et al., 2004). Subjects did not report side effects from drug administration. The order of drug administration was counter-balanced.
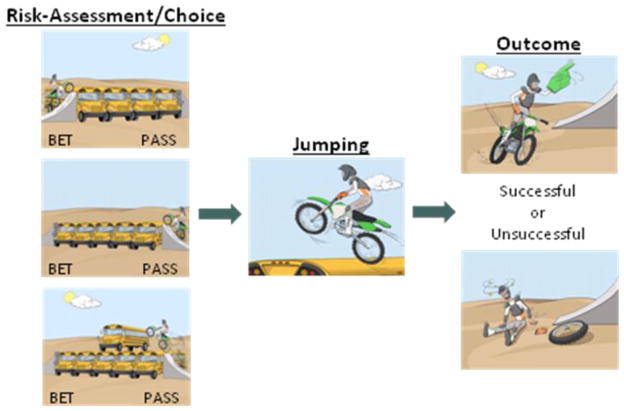
Stunt task
The Stunt task (see Figure 1) featured a motorcyclist who was challenged to jump over a variable number of buses. The number of buses ranged from 1 to 9 (i.e., 9 different risk levels), which corresponded to the probability of success. Specifically, the probability of a successful jump was 10% for the 9-bus trial, 20% for the 8-bus trial, 30% for the 7-bus trial, and so on, down to 90% for the 1-bus trial. These probabilities were not divulged to participants. Trials of each risk level were presented 10 times, and all trial types were fully randomized across the task.
Figure 1.
The Stunt Task
Each trial started with the presentation of the motorcyclist about to jump a certain number of buses (3000 ms) (see Figure 1). Participants were asked to bet or pass by button press, indicating whether they believed the stunt motorcyclist would succeed or not, respectively. Next, the motorcyclist was shown jumping (1000 ms), followed by the termination of the trial on a screen revealing the outcome, i.e., success or failure of the jump (1000 ms). A fixation-cross separated the trials (jitter applied across 750–1150 ms). Successful bets were rewarded with two points and unsuccessful bets were punished with the loss of two points. A “pass” response resulted in no exchange of points. Subjects were told that points represented money they would receive at the completion of the full study and that the more points they won, the more money they would receive. Subjects were compensated up to $25 per session. The task duration was 12 minutes.
Social-context
During the social-stress context, subjects were told that they were being observed by a group of peers through a video-camera feed. They were told that the observers would rate them on their performance during the task by answering a questionnaire, of which the subjects were given a preview. A tripod and video-camera were set-up, and the video camera was turned on for the social-stress context. Additionally, the research assistant made and received calls to establish that the live-feed was working and that the peers were ready. After these steps, the subject completed the Stunt task. During the non-social context, participants performed the Stunt task without the presence of the camera. Similar to the social-stress context, during the non-social context, the research assistant was present in the testing room but did not actively observe the participant’s performance or act in a manner that would influence the participant’s performance. The order of social-stress and non-social contexts was counter-balanced across participants, but it was maintained constant within participants for all three drug treatment sessions.
Questionnaires
At the end of each session, participants rated on a 1 (not at all) to 10 (very much) scale their experience during the social-stress and non-social context, e.g., how they thought the observers perceived their performance, and task difficulty in each context. Participants also rated on a scale of 1 (very negative) to 10 (very positive), how much they liked each risk level (1-bus through 9-bus trials), and they were asked to select the two trial-types that were the most difficult to respond to. Lastly, since the nature of the task might be more appealing to men than women, subjects were asked how much they enjoyed completing the task.
Data analysis
Missing trials and outliers of 2.5 standard deviations above and below the mean for individual reaction times (RTs; invalid) were removed from the analysis. Subjects who missed more than 10 trials during a visit (n = 1), or who did not vary their responses for more than 20 consecutive trials (n = 2) were considered to be disengaged from the task and were excluded from the data analysis.
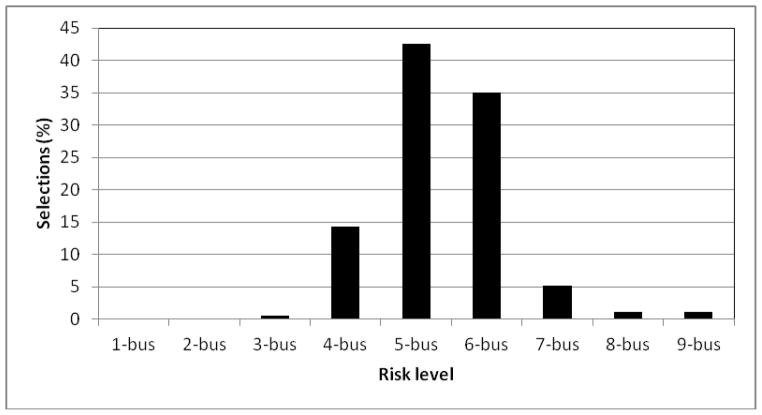
Task performance was measured using betting-rate (ratio of betting count over all valid trials) and RT. Because our hypotheses focused on risk-taking, which characterized performance on the most uncertain trials (Platt and Huettel, 2008), we focused our analyses on the trials featuring the most uncertain outcomes (60%, 50% and 40% likelihood to win). To verify that these trials were experienced as the most risky and difficult, we examined self-report ratings on the difficulty of making a decision. As expected, trials featuring 4, 5, and 6 buses (i.e., 60%, 50% and 40% win-probability) were experienced as the most uncertain and difficult trials on which to decide (see Figure 2). Therefore, the analyses were confined to these 3 trial types (4-bus, 5-bus, and 6-bus trials), which featured a positive, neutral and negative win-probability context, i.e., a positive, neutral and negative risk-valence.
Figure 2.
Self-report ratings of difficulty as a function of risk level. Subjects selected the two trial-types that were the most difficult for them. The 4-bus trial carries a positive win-probability (60% likelihood of win), the 5-bus trial is the riskiest with an equal chance of winning and losing (50% likelihood of win), and the 6-bus trial has a negative win-probability (40% likelihood of win).
Betting-rate was analyzed using a repeated-measures analysis of variance (rANOVA) with social-context (social-stress, non-social), drug treatment (OT, AVP, PLC) and risk-valence (4-bus, 5-bus, 6-bus trials) as the within-subjects factors, and sex as the between-subjects factor. RT was analyzed via a linear mixed model analysis with decision (bet, pass), social-context, drug treatment and risk-valence as the within-subjects factors and sex the between-subjects factor. The linear mixed model was used to account for missing values (Quene and van den Bergh, 2004) in betting and passing RTs (e.g., for the 4-bus trial, if only bets were placed, missing data would occur for passing RT). Additionally, because of the reported effects of OT and AVP on anxiety (Bielsky et al., 2004; see review, Neumann, 2008; Ring et al., 2006; Thompson et al., 2006; Windle et al., 1997), state anxiety (STAI-S component of the STAI) was examined pre- and 50-minutes post-drug administration using a rANOVA with time (pre- and post-drug administration), drug treatment, and sex as the factors of interest. Lastly, because the order of drug treatment was randomized in a crossover design, visit order was not included as a covariate in the data analysis. Alpha was set at 0.05. Huynh-Feldt corrections were made for violations of sphericity for rANOVA. Bonferroni post-hoc tests were conducted and Bonferroni corrections were used to adjust for multiple comparisons.
Results
Questionnaires
Participants rated how much they liked the different risk-valence situations after each visit. As expected, these responses showed a linear relationship with win-probability level, the 4-bus trial being the preferred (mean = 6.99) and the 6-bus trial the least liked (mean = 3.87) (Figure 6). A main effect of risk-valence (F(2,54) = 67.746, p < .01) indicated that the ratings differed between all the buses (ps < .01). Ratings did not differ among drug treatment sessions, or between men and women. Men and women also did not differ in ratings on task enjoyment (p > .1).
Figure 6.
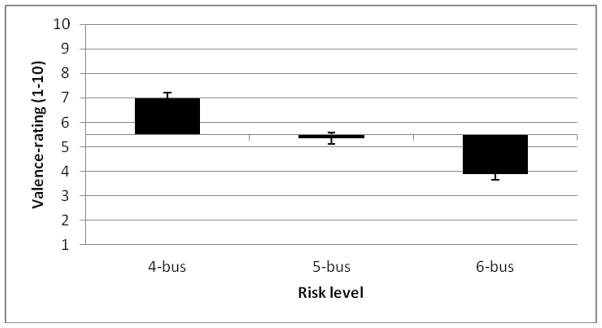
Self-report valence-ratings as a function of risk level. Ratings represent how much subjects liked the 4-bus, 5-bus, and 6-bus trials. Ratings of 5.5 or higher indicate positive subjective associations with the bus and ratings below 5.5 indicate negative subjective associations with the bus. Error bars represent ± SEM
The questionnaire about the social-stress vs. non-social context revealed that the social-stress context was experienced as more stressful. An rANOVA, with social-context and drug treatment as the within-subjects factors and sex the between-subjects factor, indicated that participants experienced the task as more difficult during the social-stress (mean = 2.73, SE = .30) than non-social context (mean = 2.48, SE = .31) (F(1,27) = 5.145, p < .05). Additionally, participants reported peers as being more than moderately critical (mean = 5.88, SE = .31; > 5= moderate, t(28) = 2.879, p < .01). Men and women did not differ on these ratings.
Betting-rate
The 4-way rANOVA of risk-valence by drug treatment by social-context by sex on betting-rate revealed two significant main effects, which confirmed that the task worked as expected. Most importantly, the 4-way interaction was statistically significant.
The two main effects involved the social-context and risk-valence factors. The main effect of social-context (F(1,27) = 4.514, p < .05, partial η2 = .143) indicated that bets were less frequent during the social-stress than the non-social context. The main effect of risk-valence (F(1.631,44.036) = 69.731, p < .01, partial η2 = .721) reflected decreased betting-rate with decreased win-probability.
The significant 4-way interaction of risk-valence by drug treatment by social-context by sex (F(4,108) = 2.704, p < .05, partial η2 = .091) indicated that betting-rate was indeed modulated by the experimental manipulations and by sex. This analysis was further decomposed by risk-valence (4-bus, 5-bus and 6-bus trials). For each of these three risk-valence conditions, a 3-way rANOVA was conducted with drug treatment and social-context as the within-subjects factors and sex as the between-subjects factor.
Positive win-probability trials (4-bus trial, 60% win-probability)
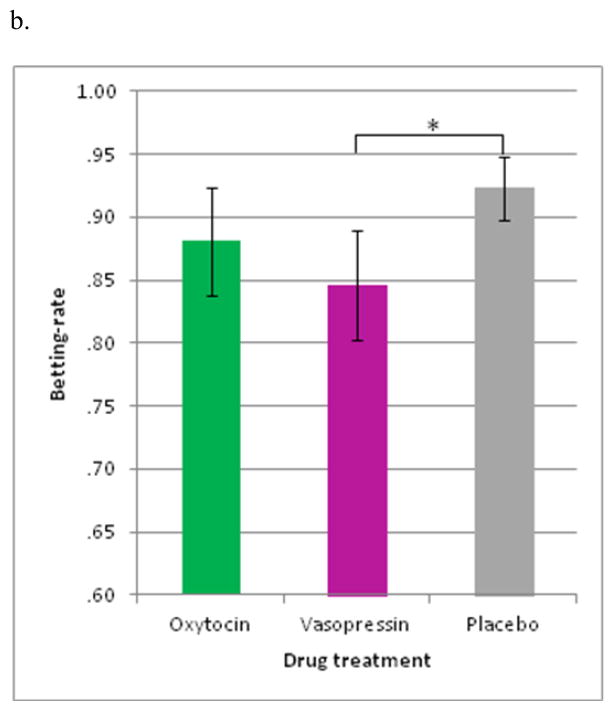
The 3-way rANOVA revealed a significant 2-way interaction of social-context by sex and a main effect of drug treatment. No other main effects or interactions emerged. The social-context by sex interaction (F(1,27) = 4.431, p < .05, partial η2 = .141) (Figure 3a) revealed that men placed fewer bets during the social-stress than non-social context (p < .05), whereas women showed no betting-rate differences between these contexts. Regarding the main effect, drug treatment was found to modulate betting-rate (F(2,54) = 4.710, p < .05, partial η2 = .149) (Figure 3b) such that AVP reduced betting-rate, relative to OT or PLC.
Figure 3.
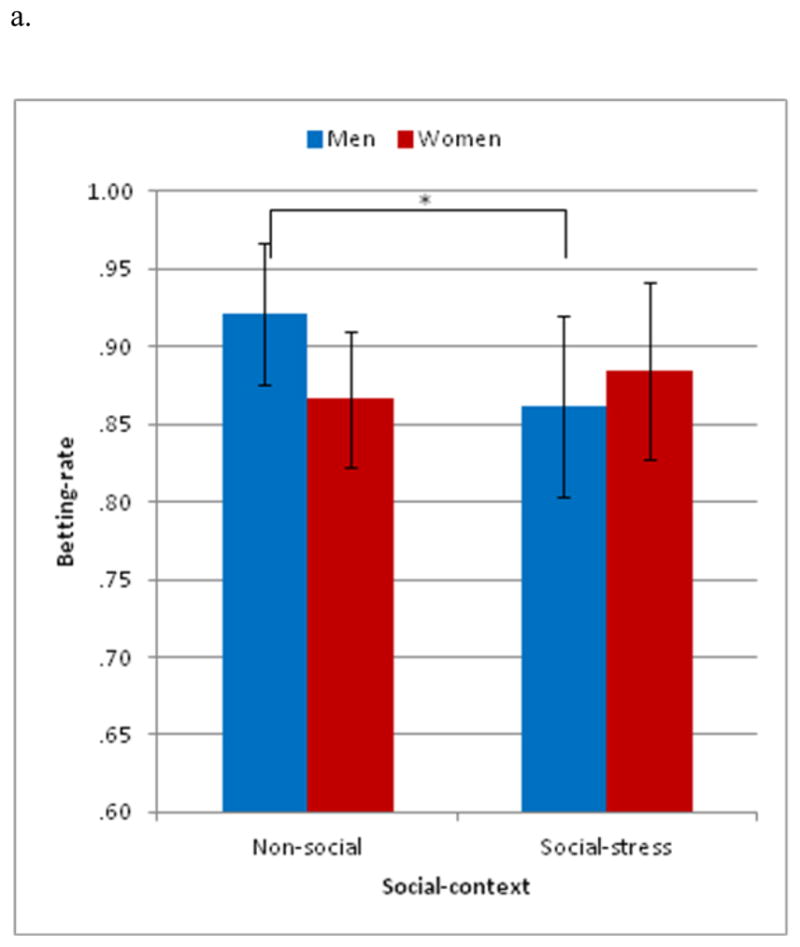
Positive win-probability trials (4-bus trial) on betting-rate. (a) The social-context by sex interaction indicated that men made fewer bets during the social-stress than non-social context. No differences were found in women. (b) The main effect of drug indicated that fewer bets were made after arginine-vasopressin (AVP) than placebo. Betting-rate during oxytocin was intermediate between that during AVP and placebo. *p < .05, error bars represent ± SEM
Altogether, in conditions of positive win-probability, social-stress modulated risk-taking in men but not women. Specifically, social-stress reduced risk-taking in males. In addition, AVP exerted a general risk-averse effect across both men and women and across the social-stress and non-social contexts.
Neutral win-probability trials (5-bus trial, 50% win-probability)
The 3-way drug treatment by sex by social-context rANOVA revealed only a main effect of social-context. No other main effects or interactions were found. In these neutral-valence trial-types, the main effect of social-context reflected a lower betting-rate in the social-stress than the non-social context (F(1,27) = 9.407, p < .01, partial η2 = .258).
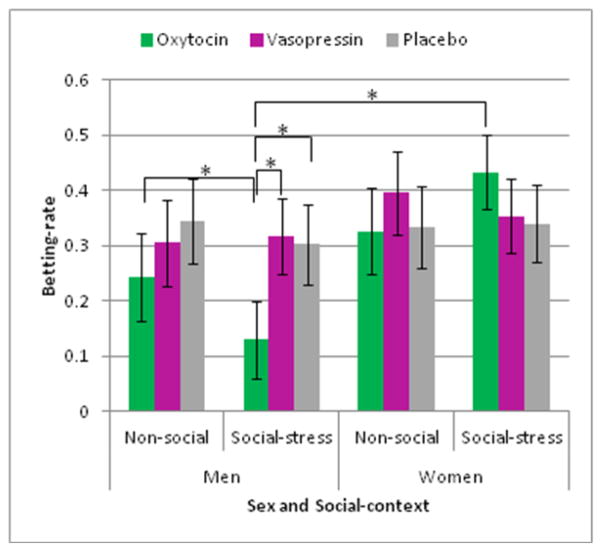
Negative win-probability trials (6-bus trial, 40% win-probability)
The 3-way rANOVA revealed a significant 3-way interaction of drug treatment by sex by social-context (F(2,54) = 4.030, p < .05, partial η2 = .13) (Figure 4). No other main effects or interactions were found. This 3-way interaction was decomposed by social-context. In the social-stress context, men made fewer bets after OT than AVP or PLC (ps >.05), and, after OT, they made fewer bets than women (p < .01). In the non-social context, betting-rate was not influenced by drug treatment or sex or drug treatment by sex.
Figure 4.
Negative win-probability trials (6-bus) on betting-rate. The drug by social-context by sex interaction showed that men made fewer bets after oxytocin (OT) than arginine vasopressin (AVP) or placebo (PLC) during the social-stress condition. On these trials, men also made fewer bets than women. No drug-related differences in betting-rates were found within women. * p < .05, error bars represent ± SEM
Taken together, in conditions of negative win-probability, betting-rate was decreased after OT in men, but not in women, and only in the social-stress context.
Reaction Time
The linear mixed model analysis with the factors of drug treatment, social-context, decision, risk-valence and sex failed to detect any effect of drug treatment or social-context on RT, as a main effect or in interaction. However, this analysis revealed a significant interaction of decision by risk-valence and a main effect of sex on RT. No other main effects or interactions were found.
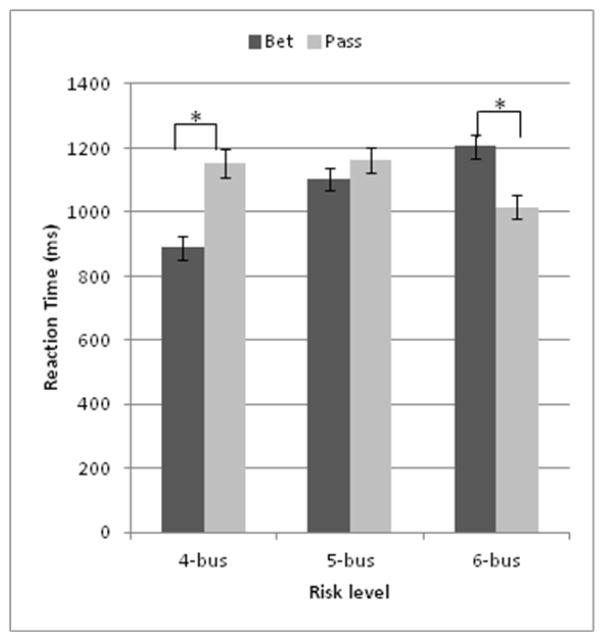
The significant decision by risk-valence interaction (F(2,761.557) = 42.006, p < .01) (Figure 5) indicated that RT was higher for the most conflicting decisions. Indeed, a post-hoc test showed that for the positive win-probability trials, RT was longer for passing than betting (p < .001). A similar trend was found for the 5-bus trial (p = .054). In contrast, the negative win-probability trials showed longer RT for betting than passing (p < .001). Lastly, the main effect of sex (F(1,27.522) = 7.848, p < .01) indicated that RT was longer in men (mean = 1175.8 ms, SE = 45.1) than women (mean = 1000.8 ms, SE = 43.2).
Figure 5.
Effects of choice by risk level on reaction time. Reaction time was faster for betting than passing for the 4-bus trial and slower for the 6-bus trial. * p < .001, error bars represent ± SEM
Anxiety
A rANOVA was used to examine the effects of drug treatment and sex on state anxiety measured pre- and 50 minutes post-drug administration. This analysis failed to reveal any effects of drug treatment or sex (male: mean = 28.13, SE = 1.73; female: mean = 28.94, SE = 1.67) on state anxiety.
Discussion
To our knowledge, this study is the first research foray into the potential role of OT and AVP on risk-taking behaviors. Healthy men and women were compared on the effects of intra-nasal OT and AVP on risk-taking behavior, under various conditions of uncertainty and social context. We expected that OT would enhance risk-taking through favoring approach behavior, whereas AVP would reduce risk-taking through promoting defensive responses. We anticipated these effects to be modulated by risk-valence (positive vs. negative probability of winning) and social context (social-stress vs. non-social). In general, the social-stress context was expected to stimulate protective behaviors, and, in turn, reduce risky behavior relative to the non-social context. This effect was indeed observed in the most risky (most uncertain) condition of 50% win-probability. This study yields two main findings on betting-rate, the central measure of risk-taking.
In line with prediction, the first finding revealed a unique risk-averse effect of AVP on favorable risky trials (positive win-probability). In other words, the stimulation of AVP function, in a safer context, seemed to produce a protective response, akin to defensive responses associated with enhanced anxiety. This finding is consistent with reports of an anxiogenic role attributed to AVP (Bielsky et al., 2004; Thompson et al., 2006). The absence of AVP effect in a negative win-probability is interesting as it may complement the effects of OT in a positive win-probability as discussed below. Furthermore, in contrast to our predictions, this risk-averse effect was not modulated by social-stress or sex. We expected an exacerbation of the AVP-related risk-averse effect during social-stress (vs. a non-social) based on the role of AVP in social processes (e.g., in aggression and social bonding) (Winslow et al., 1993; Thompson et al., 2006). This lack of influence of social-stress and sex was in contrast to the effect of OT, our second main finding.
The second finding differed from prediction. Accordingly, OT was associated with reduced risk-taking. This effect was specific to unfavorable risky trials (negative win-probability), and only seen in males and social-stress. In contrast to the generally accepted anxiolytic effects of OT (Windle et al., 1997; Ring et al., 2006; see review, Neumann, 2008), the negative risk-valence context, in which this OT risk-averse effect emerged, might have promoted anxiogenic-like effects. Indeed, OT has also been linked to defensive responses in potentially dangerous situations when facing “out-group” protagonists (De Dreu et al., 2010). Here, risk-aversion might have been compounded by the social-stress context. In line with this notion, OT has been found to reduce cooperativeness with opponents in conditions of social unfamiliarity (Declerck et al., 2010). Reduced cooperativeness could be interpreted as reticent behavior, manifest as risk-averse in a social-stress situation involving unfamiliar peers. Finally, Grillon and colleagues (2013) recently reported that during aversive and unpredictable situations, OT administration increased anxiety (as measured by startle response). These findings fit with the exacerbation of defensive behaviors by OT in social-stress.
Importantly, the effect of OT was detected only in males. Generally, men have been found to be greater risk-takers (for meta-analysis see Byrnes et al., 1999), perhaps making them more sensitive to manipulations that affect risk-taking behavior. In addition, studies report sex differences in OT social effects. However, the nature of these sex differences vary by studies, probably reflecting the unique social manipulations used in these studies. For example, OT reduced distress in men but enhanced anger in women in response to social stress (Kubzansky et al., 2012). In contrast, in a study using an Interpersonal Perception Task, OT facilitated competition in men relative to women, whereas women showed improvement on kinship recognition relative to men (Fischer-Shofty et al., 2013). These sex-specific effects of OT need to be better understood via studies that systematically manipulate environmental and social parameters. Nevertheless, the present study provides another instance of such sex-specific effects of OT, this time in relation to risk-taking in a social-stress context.
Taken together, the differences between OT and AVP on their modulation of risk-taking may be important. They suggest that context (i.e., social-context, sex, and risk-valence) may regulate distinctly the function of these two molecules, which can affect the coordination of their complementary effects. In the future, it will be important to examine the influence of dose strength and delay-to-testing on these differential effects.
Some limitations need to be mentioned. First, the Stunt task was administered about 100 minutes after drug administration. This delay could moderate the direct drug action, although evidence suggests that a direct drug action might still be present beyond 100 min post administration. Indeed, OT levels in saliva have been detected at four hours post-administration of intra-nasal OT at 24 IU (Weisman et al., 2012). In addition, behavioral effects have been shown to last at least two hours after administration (e.g., see Heinrichs et al., 2004). However, the observed effects after a 100 min delay might also result from the cascade of neurochemical events initiated by the drug action, in addition to the direct drug action on the OT/AVP receptors (Churchland and Winkielman, 2012). Second, subjects completed an independent study (Grillon et al., 2013) prior to our experiment. The most likely carry-over effect would be a higher level of state anxiety at initiation of our study. However, the delay between both studies, and the change in context (examination room and research assistant) are expected to have minimized the impact of the previous study on our experiment. Third, the Stunt task is a novel task, limiting comparisons of the results to other risk-taking/decision-making tasks. However, performance on the task was as expected, showing differential sensitivity to the range of risk levels provided by the task. Fourth, biological measures of stress, such as cortisol levels, were not collected. Although self-report ratings and behavioral responses were consistent with the aversive quality of the social-stress context, a biological measure would have been an additional validator. We also lacked measures of individual anxiety levels other than subjective state ratings. It would be important to add other measures, both behavioral (e.g., temperament) and physiological (e.g., fear-potentiated startle reflex) in the next set of studies.
In conclusion, this study extends the growing literature on OT and AVP by investigating risk-taking behavior. Specifically, risk-averse effects emerged with both OT and AVP, but OT’s effects were specific to men and to decisions on unfavorable gambles, while AVP’s effects only pertained to decisions on favorable gambles and affected both men and women similarly. These findings are preliminary, but promising. The effects of OT and AVP on risk-taking should be further investigated using different drug treatment dosages and various time intervals between drug administration and testing, as well as systematically manipulating risk-valence and social parameters of the context where risk-taking is tested. Finally, given that these molecules’ sites of action involve the amygdala, prefrontal cortex and striatum (Barberis and Tribollet, 1996; Kirsch et al., 2005; Smeltzer et al., 2006; Skuse and Gallagher, 2009), the neural systems that together modulate motivated behavior (Ernst and Fudge, 2009), it would be of great interest to follow the present work with a functional neuroimaging study investigating how the behavioral modulation of risk-taking by OT and AVP maps onto the function of their neural targets.
Highlights.
Oxytocin (OT) and arginine vasopressin (AVP) influence risk-taking
OT and AVP induce risk-aversiveness, but in different outcome probabilities
OT’s influence on risk-taking is modulated by sex and social stress
Findings extend the role of OT and AVP beyond social-context to risk-taking
Acknowledgments
This study was funded by the Intramural Research Program of the National Institutes of Mental Health.
Footnotes
Dr. Pine has received compensation for activities related to teaching, editing, and clinical care that pose no conflicts of interest. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: a meta-analysis. Psychol Bull. 1999;125:367–383. [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? what does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croson R, Gneezy U. Gender differences in preferences. J Econ Lit. 2009;47:448–474. [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm Behav. 2010;57:368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SWW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen P, Harmer C. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG. Oxytocin facilitates accurate perception of competition in men and kinship in women. Soc Cogn Affect Neurosci. 2013;8:313–317. doi: 10.1093/scan/nsr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–184. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Giorgetta C, Grecucci A, Zuanon S, Perini L, Balestrieri M, Bonini N, Sanfey AG, Brambilla P. Reduced risk-taking behavior as a trait feature of anxiety. Emotion. 2012;12:1373–1383. doi: 10.1037/a0029119. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. Selective amnesic effects of oxytocin on human memory. Physiol Behav. 2004;83:31–38. doi: 10.1016/j.physbeh.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE, Schmidt NB. Dispositional anxiety and risk-avoidant decision-making. Personality and Individual Differences. 2007;42:665–675. [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 2008;11:398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quene H, van den Bergh H. On multi-level modeling of data from repeated measures designs: a tutorial. Speech Commun. 2004;43:103–121. [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms in adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Adults (Forms Y1 and Y2) Mind Garden; Menlo Park, CA: 1983. [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012;37:1582–1586. doi: 10.1016/j.psyneuen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]