Abstract
Eukaryotic cytosolic ACBPs (acyl-CoA-binding proteins) bind acyl-CoA esters and maintain a cytosolic acyl-CoA pool, but the thermodynamics of their protein–lipid interactions and physiological relevance in plants are not well understood. Arabidopsis has three cytosolic ACBPs which have been identified as AtACBP4, AtACBP5 and AtACBP6, and microarray data indicated that all of them are expressed in seeds; AtACBP4 is expressed in early embryogenesis, whereas AtACBP5 is expressed later. ITC (isothermal titration calorimetry) in combination with transgenic Arabidopsis lines were used to investigate the roles of these three ACBPs from Arabidopsis thaliana. The dissociation constants, stoichiometry and enthalpy change of AtACBP interactions with various acyl-CoA esters were determined using ITC. Strong binding of recombinant (r) AtACBP6 with long-chain acyl-CoA (C16- to C18-CoA) esters was observed with dissociation constants in the nanomolar range. However, the affinity of rAtACBP4 and rAtACBP5 to these acyl-CoA esters was much weaker (dissociation constants in the micromolar range), suggesting that they interact with acyl-CoA esters differently from rAtACBP6. When transgenic Arabidopsis expressing AtACBP6pro::GUS was generated, strong GUS (β-glucuronidase) expression in cotyledonary-staged embryos and seedlings prompted us to measure the acyl-CoA contents of the acbp6 mutant. This mutant accumulated higher levels of C18:1-CoA and C18:1- and C18:2-CoAs in cotyledonary-staged embryos and seedlings, respectively, in comparison with the wild type. The acbp4acbp5acbp6 mutant showed the lightest seed weight and highest sensitivity to abscisic acid during germination, suggesting their physiological functions in seeds.
Keywords: acyl-CoA-binding protein, isothermal titration calorimetry, lipid metabolism, seed
Abbreviations: ABA, abscisic acid; ABRC, Arabidopsis Biological Resource Center; ACB, acyl-CoA-binding; ACBP, acyl-CoA-binding protein; CBF, C-repeat binding factor; CRT, C-repeat; EMSA, electrophoretic mobility shift assay; ER, endoplasmic reticulum; GUS, β-glucuronidase; ITC, isothermal titration calorimetry; MBS, MYB-binding site; PC, phosphatidylcholine
Short abstract
The binding affinities of the three Arabidopsis cytosolic ACBPs (AtACBP4, AtACBP5 and AtACBP6) with acyl-CoA esters were investigated by ITC. When the biological significance of these AtACBPs was analysed using mutants, results indicated their overlapping functions in seed acyl-lipid metabolism.
INTRODUCTION
ACBPs (acyl-CoA-binding proteins) have a highly conserved 10-kDa domain that binds long-chain acyl-CoA esters with high affinity [1–5]. ACBPs were first identified in mammals as a DBI (diazepam-binding inhibitor) or EP (endozepine) inhibiting diazepam binding to the GABA (γ-aminobutyric acid) receptor [6]. ACBPs were later demonstrated to be involved in multiple physiological processes such as fatty acid biosynthesis, enzyme regulation, intracellular acyl-CoA transport, and maintenance of intracellular acyl-CoA pools [7,8]. Homologues of ACBPs have been identified in eukaryotes (Animalia, Plantae, Fungi and Protista) as well as in Eubacteria, but not in Archaebacteria [7]. Multiple isoforms of ACBPs are present in fungi as well as in animals [9]. In mammals, three isoforms have been identified in different tissues: L-ACBP in liver, B-ACBP in brain and T-ACBP in testis [9]. The 10-kDa ACBP is a cytosolic protein that has been reported to be associated with the smooth ER (endoplasmic reticulum), Golgi bodies and the outer membrane of the mitochondria [10,11]. Bovine L-ACBP localizes to the ER and Golgi complex in a ligand-dependent manner [11].
Recombinant rat, bovine, human and Plasmodium falciparum ACBPs have been reported to bind to acyl-CoA esters in vitro [1,12–14]. Similar observations have been reported for recombinant AtACBPs using Lipidex assays in the model plant Arabidopsis [15–20]. In yeast, ACBPs are required for membrane assembly and fatty acid chain elongation [21]. ACBP-null mice lacking the 10-kDa ACBP were embryo-lethal, suggesting that the protein is essential during early development [22]. Besides lipid biosynthesis, membrane biogenesis, and the regulation of gene expression and enzyme activities, ACBPs also function in development and stress responses in Caenorhabditis elegans [23], mammals [24] and plants [25,26]. The appearance of ACBPs very early in evolution points to their fundamental role in acyl-CoA metabolism [7].
In Arabidopsis, six isoforms have been identified, designated as AtACBP1 to AtACBP6. Despite being conserved within the ACB (acyl-CoA-binding) domain, their molecular weights range from 10.4 to 73.1 kDa because of the additional domains in AtACBP1 to AtACBP5 [25]. The 37.5 kDa AtACBP1 and 38.5 kDa AtACBP2 share 72% amino acid identity throughout their sequences, and each includes a C-terminal domain of ankyrin repeats which mediates protein–protein interactions [15,18,27,28], and an N-terminal hydrophobic transmembrane domain that targets it to the ER and the plasma membrane [27,29–31]. Both proteins have been shown to participate in embryogenesis [30,32] and heavy metal stress tolerance [18,28,33], whereas AtACBP1 also plays a role in stem cuticle formation [34] and AtACBP2 in drought tolerance [35]. Their recombinant proteins have been reported to bind acyl-CoA esters in vitro as well as to PC (phosphatidylcholine) [15,18,29,32], while recombinant (r) AtACBP1 also binds to phosphatidic acid, and rAtACBP2 to lysophophatidylcholine (lysoPC) [28,32,36]. The 39.3-kDa AtACBP3 contains a cleavable N-terminal signal peptide and is the only member with an ACB domain localized at the C-terminus [17]. The extracellularly-targeted AtACBP3 [17,20,37] has been shown to bind acyl-CoA esters, PC and PE (phosphatidylethanolamine) [17,20].
The two largest AtACBPs, the 73.1-kDa AtACBP4 and 71.0-kDa AtACBP5, have an N-terminal ACB domain followed by two kelch motifs [16]. Both are localized in the cytosol [38]. Likewise, the 10-kDa AtACBP6 is cytosolic; this protein comprises only the ACB domain, and has well-characterized homologues in other species [39]. The recombinant forms of these three cytosolic proteins (AtACBP4, AtACBP5 and AtACBP6) [38,39] have been previously reported to bind acyl-CoA esters and PC in Lipidex assays [16,19,39]. Microarray data from the Arabidopsis eFP browser indicated that all three cytosolic AtACBPs are expressed in Arabidopsis seeds (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi).
Herein, the binding affinities of the three recombinant cytosolic AtACBPs to acyl-CoA esters were investigated by ITC (isothermal titration calorimetry), which represents a more precise method to measure protein–ligand binding compared with Lipidex assays [40]. To correlate the ITC data with the physiological roles of AtACBP6, its promoter was cloned and transgenic Arabidopsis AtACBP6pro::GUS lines were generated, allowing us to study the spatial pattern of expression. The acyl-CoA contents of the acbp6 mutant were also measured in comparison with the wild-type. To investigate the biological significance of recombinant AtACBP4, AtACBP5 and AtACBP6 in the binding of various acyl-CoA esters, double and triple mutants of AtACBP4, AtACBP5 and AtACBP6 [41] were further analysed. Given indications of the overlapping functions of AtACBP4, AtACBP5 and AtACBP6 in flower development [41], the seed morphology and seedling development of these mutants were also studied.
EXPERIMENTAL
Preparation of recombinant (His)6-AtACBP4, (His)6-AtACBP5 and (His)6-AtACBP6 proteins
The protein expression plasmids containing full-length AtACBP4 and AtACBP5 cDNA were previously constructed in the vector pRSETB (Invitrogen) as pAT184 and pAT185, respectively [16]. Recombinant (His)6-AtACBP4 and (His)6-AtACBP5 proteins (rAtACBP4 and rAtACBP5) were expressed in Escherichia coli, purified and refolded according to Leung et al. [16]. The coding sequence of AtACBP6 was amplified by PCR with primer pairs ACBP-F1 and ACBP-R1 and cloned into expression vector pEXP5-NT/TOPO (Invitrogen) to generate plasmid pEXP-ACBP6. Sequences of primers are listed in Supplementary Table S1. Recombinant (His)6-AtACBP6 (rAtACBP6) was expressed in E. coli and purified following Chye [29]. The three recombinant proteins were dialysed in 10 mM sodium phosphate buffer (pH 7.0). Protein concentrations were determined by the standard Bradford assay (Bio-Rad).
ITC
Calorimetric titration was performed with a MicroCal iTC200 system (GE Healthcare) at 30°C. Long-chain acyl-CoA esters (Avanti Polar Lipids, Inc.) were dissolved in 10 mM sodium phosphate buffer (pH 7.0) to a final concentration of 0.25, 0.5 and 1 mM. The solution was degassed and loaded into a syringe, while 15 μM rAtACBP6, 6 μM rAtACBP4 or 60 μM rAtACBP5, was placed into the sample cell. For all titrations, 1.8 μl ligand was injected into the sample cell at 150 s intervals with a stirring speed of 1000 rpm. The titrations were completed after 20 injections. All data were collected automatically, and non-specific heat effects were estimated and corrected after saturation following the instructions of the manufacturer. Raw data were integrated and analysed using the Origin v.7.0 software accompanying the calorimeter.
Plant material and growth conditions
Seeds of wild-type Arabidopsis Col-0 were purchased from the ABRC (Arabidopsis Biological Resource Center). The double and triple mutants of AtACBP4, AtACBP5 and AtACBP6 were generated by crossing the respective single mutants [41]. To sterilize Arabidopsis seeds, a solution containing 20% bleach and 0.1% (v/v) Tween 20 was used. Seeds were subjected to 20 min vigorous shaking in this solution, washed six times in distilled water, and then spread on MS (Murashige and Skoog) medium (Sigma-Aldrich) containing 2% (w/v) sucrose, and 0, 0.2 or 0.4 μM ABA (abscisic acid). After 4°C stratification for 2 days, the plates were placed under 16 h-light/8 h-dark cycles (22°C) in a tissue culture room. The rates of seed germination were scored when radicle protrusion occurred.
Promoter analysis
A 1.5-kb 5′-flanking region of AtACBP6 was amplified by PCR with primer pair ML795 and ML814. Deletion derivatives of the 1.5-kb 5′-flanking region of AtACBP6, 1.0, 0.6 and 0.3 kb in size were further amplified by PCR with corresponding forward primers ML1166, ML1167 and ML1168, and the reverse primer ML814. All forward primers contained a BamHI site, and the reverse primer, a SmaI site. Sequences of primers are listed in Supplementary Table S1. Fragments were purified and cloned into the pGEM-T EASY Vector system (Promega). Each BamHI–SmaI fragment was subcloned into corresponding restriction sites on the binary vector pBI101.3 (Clontech) to generate the AtACBP6pro::GUS fusion plasmids (pAT452, pAT590, pAT591 and pAT592) that were used in Agrobacterium tumefaciens transformation of wild-type Arabidopsis Col-0 by the floral dip method.
For GUS (β-glucuronidase) histochemical staining, samples of seedlings and embryos were immersed in a GUS substrate solution (50 mM sodium phosphate buffer, pH 7.0, 0.2% Triton X-100, 10 mM EDTA, 2 mM potassium ferricyanide, 2 mM potassium ferrocyanide and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-glucuronide), for 30 min with vacuum infiltration, after which samples were incubated at 37°C for 4 h (for seedlings) or 12 h (for embryos). Following a series of ethanol washes, tissues were observed and images were captured with a Nikon 80i Microscope.
Plant nuclear protein extraction was performed at 4°C using 10 g of 7-day-old Arabidopsis seedlings following the instructions of the Plant Nuclei Isolation/Extraction Kit (Sigma). Biotin-labelled DNA probes were prepared as specified in the Pierce Biotin 3′ End DNA Labeling Kit (Thermo Scientific). Probe 1 (ML2044/ML2045), Probe 2 (ML2046/ML2047) and Probe 3 (ML2048/ML2049) corresponded to each of the three putative Dof-boxes (−490/−486, −421/−417 and −353/−349, respectively). Sequences of the probes used are listed in Table S1. EMSAs (electrophoretic mobility shift assays) were performed on 6% native PAGE, and protein–DNA interactions subsequently revealed using the Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific).
Acyl-CoA profiling
Samples for acyl-CoA profiling were collected from 5-day-old seedlings and cotyledonary-staged embryos of wild-type Col-0 and the acbp6 mutant. Samples were extracted following Larson and Graham [42]. Seedling samples were analysed by reversed-phase high-performance liquid chromatography [32], and embryo samples were analysed by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring, operated in positive ion mode, according to Haynes et al. [43].
RESULTS
Recombinant AtACBPs bind to long-chain acyl-CoA esters
Long-chain acyl-CoA esters (C16- to C18-CoA) are major intermediates in lipid metabolism in plants [44], and cytosolic AtACBPs are potential participants in acyl-CoA trafficking, besides maintaining an acyl-CoA pool in the cytosol [8,25]. Therefore the binding of the recombinant cytosolic AtACBP4, AtACBP5 and AtACBP6 to these acyl-CoA esters was studied using ITC, to understand how their biochemical properties may be correlated with their physiological functions.
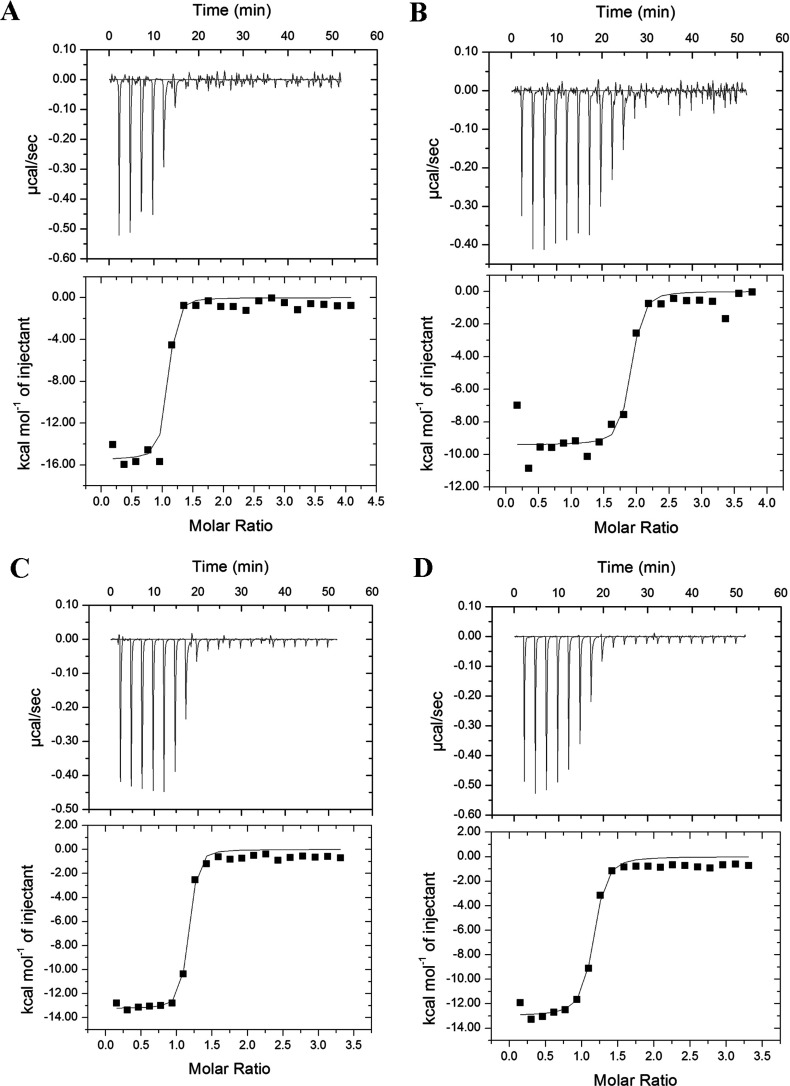
ITC thermograms indicated that rAtACBP6 binds to palmitoyl-CoA (C16:0-CoA), oleoyl-CoA (C18:1-CoA), linoleoyl-CoA (C18:2-CoA) and linolenoyl-CoA (C18:3-CoA) (Figure 1). Theoretical fits to the experimental data were obtained using a single binding site model. Table 1 lists the thermodynamic parameters obtained, including dissociation constant (Kd), stoichiometry of the ligand-to-protein binding (n), enthalpy change (ΔH), free energy change (ΔG) and the entropy change (ΔS). Our results indicated that rAtACBP6 has high binding affinity to long-chain acyl-CoA esters, with Kd values ranging from 35.9 to 84.1 nM (Table 1). Interestingly, among all the tested acyl-CoA esters, the interaction between rAtACBP6 and C18:1-CoA was unique, with n=1.9, suggesting that one molecule of rAtACBP6 binds to two acyl-CoA ligands (Table 1).
Figure 1. Binding isotherms of rAtACBP6 titrated with (A) palmitoyl-CoA (C16:0-CoA), (B) oleoyl-CoA (C18:1-CoA), (C) linoleoyl-CoA (C18:2-CoA) and (D) linolenoyl-CoA (C18:3-CoA) at 30°C.
Top panels show the raw data from 20 injections of 1.8-μl aliquots of a 0.25 mM (A, C and D) or 0.5 mM (B) solution of acyl-CoA into a cell containing 0.015 mM rAtACBP6 at 30°C; bottom panels show the integrated area of each injection after background correction.
Table 1. Thermodynamic parameters for acyl-CoA binding to rAtACBP6.
ITC experiments were carried out at 30°C in 10 mM sodium phosphate buffer (pH 7.0). The values are the means of three experiments±S.D.
| Acyl-CoAs | n (kcal mol−1) | ΔH (cal mol−1 K−1) | ΔS (kcal mol−1) | ΔG | Kd |
|---|---|---|---|---|---|
| 14:0 | 1.0±0.0 | −11.6±1.4 | −4.6 | −10.2±0.2 | 38.7±4.5 |
| 16:0 | 1.0±0.1 | −13.7±2.4 | −11.5 | −10.2±0.0 | 35.9±8.4 |
| 18:0 | 1.0±0.0 | −12.0±2.5 | −6.6 | −10.0±0.3 | 60.6±2.4 |
| 18:1 | 1.9±0.1 | −6.7±3.8 | 11.4 | −10.2±0.1 | 45.3±4.3 |
| 18:2 | 1.1±0.0 | −12.3±1.4 | −6.7 | −10.2±0.2 | 36.4±4.9 |
| 18:3 | 1.1±0.0 | −12.1±1.3 | −7.7 | −9.7±0.1 | 84.1±2.9 |
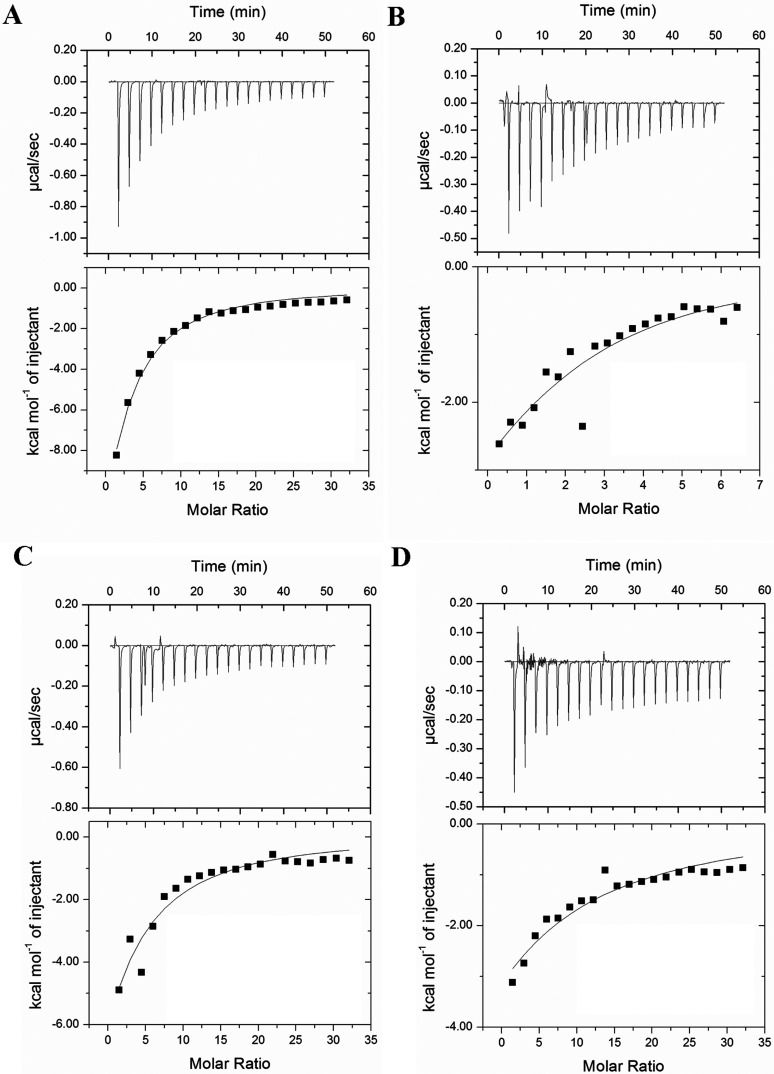
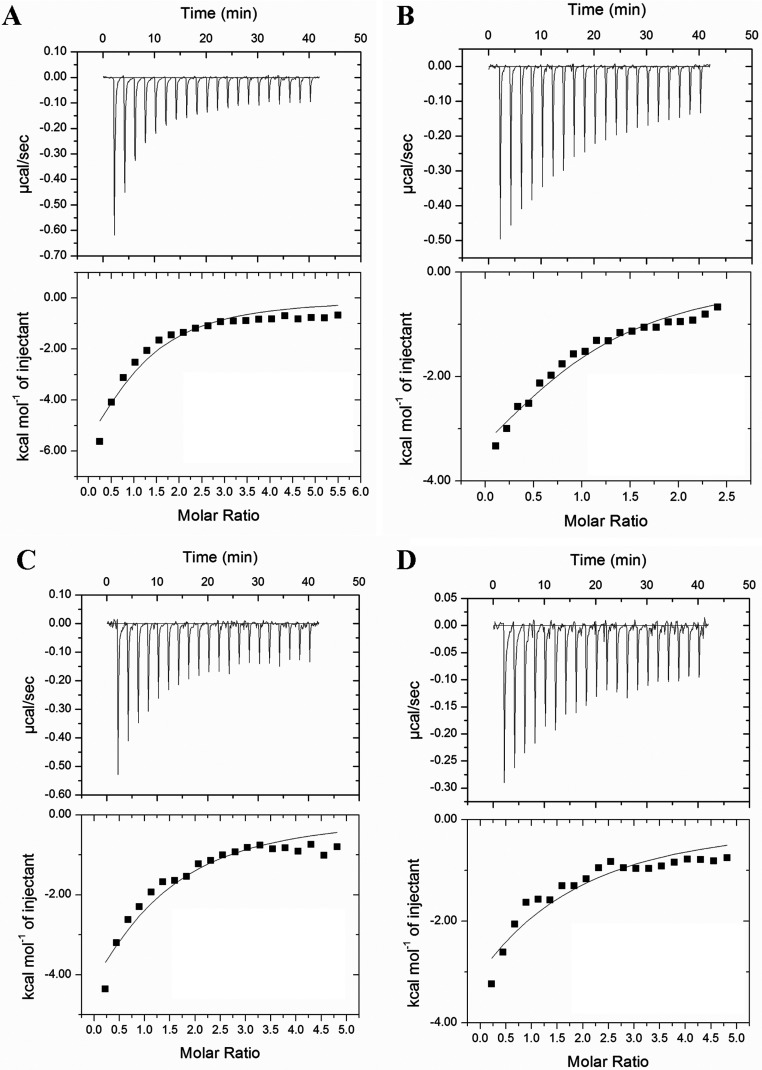
When the binding of rAtACBP4 and rAtACBP5 to acyl-CoA esters was investigated, ITC thermograms revealed that both bind to the same acyl-CoA esters tested for rAtACBP6 (Figures 2 and 3). Theoretical fits to the experimental data were again obtained using a single binding site model. Thermodynamic parameters for interaction between acyl-CoA esters and rAtACBP4 and rAtACBP5 are listed in Tables 2 and 3, respectively. The binding with both proteins was weaker than that observed for rAtACBP6. Recombinant AtACBP4 has affinities to long-chain acyl-CoA esters ranging from 2.7 to 189.0 μM (Table 2), while values for rAtACBP5 ranged from 35.4 to 92.4 μM (Table 3). An aberrant stoichiometry (n~2) for C18:1-CoA was observed for rAtACBP4, but not rAtACBP5.
Figure 2. Binding isotherms of rAtACBP4 titrated with (A) palmitoyl-CoA (C16:0-CoA), (B) oleoyl-CoA (C18:1-CoA), (C) linoleoyl-CoA (C18:2-CoA) and (D) linolenoyl-CoA (C18:3-CoA) at 30°C.
Top panels show the raw data from 20 injections of 1.8-μl aliquots of a 1 mM solution of acyl-CoA into a cell containing 0.006 mM rAtACBP4 at 30°C; bottom panels show the integrated area of each injection after background correction.
Figure 3. Binding isotherms of rAtACBP5 titrated with (A) palmitoyl-CoA (C16:0-CoA), (B) oleoyl-CoA (C18:1-CoA), (C) linoleoyl-CoA (C18:2-CoA) and (D) linolenoyl-CoA (C18:3-CoA) at 30°C.
Top panels show the raw heat signal from 20 injections of 1.8-μl aliquots of a 1 mM solution of acyl-CoA into a cell containing 0.06 mM rAtACBP5 at 30°C; bottom panels show the integrated area of each injection after background correction.
Table 2. Thermodynamic parameters for acyl-CoA binding to rAtACBP4.
ITC experiments were carried out at 30°C in 10 mM sodium phosphate buffer (pH 7.0). The values are the means of three experiments±S.D.
| Acyl-CoAs | n (kcal mol−1) | ΔH (cal mol−1 K−1) | ΔS (kcal mol−1) | ΔG | Kd |
|---|---|---|---|---|---|
| 14:0 | 1.0±0.0 | −53.5±6.9 | −157.5 | −5.8±0.1 | 65.3±22.6 |
| 16:0 | 1.0±0.0 | −77.4±2.5 | −235.5 | −6.1±0.2 | 23.5±27.1 |
| 18:0 | 1.1±0.1 | −8.9±1.0 | −0.6 | −8.7±1.0 | 2.7±1.6 |
| 18:1 | 2.1±0.0 | −6.9±0.1 | −4.3 | −5.6±0.0 | 95.2±0.0 |
| 18:2 | 1.0±0.0 | −73.7±2.6 | −224.5 | −5.8±0.2 | 73.8±0.3 |
| 18:3 | 1.0±0.0 | −96.2±5.3 | −300.5 | −5.1±0.0 | 189.0±0.5 |
Table 3. Thermodynamic parameters for acyl-CoA binding to rAtACBP5.
ITC experiments were carried out at 30°C in 10 mM sodium phosphate buffer (pH 7.0). The values are the means of three experiments±S.D
| Acyl-CoAs | n (kcal mol−1) | ΔH (cal mol−1 K−1) | ΔS (kcal mol−1) | ΔG | Kd |
|---|---|---|---|---|---|
| 14:0 | 0.9±0.0 | −3.7±1.2 | 7.7 | −6.1±0.0 | 41.9±2.2 |
| 16:0 | 0.9±0.0 | −11.2±0.5 | −16.7 | −6.2±0.0 | 35.7±1.0 |
| 18:0 | 1.0±0.0 | −17.4±0.1 | −37.2 | −6.2±0.0 | 35.4±1.4 |
| 18:1 | 0.9±0.0 | −6.2±0.2 | −1.8 | −5.7±0.0 | 74.4±3.5 |
| 18:2 | 1.0±0.0 | −10.2±0.2 | −14.7 | −5.8±0.0 | 64.1±0.0 |
| 18:3 | 1.2±0.1 | −7.8±1.3 | −7.4 | −5.6±0.1 | 92.4±10.7 |
AtACBP6 was expressed in cotyledonary-staged embryos and seedlings
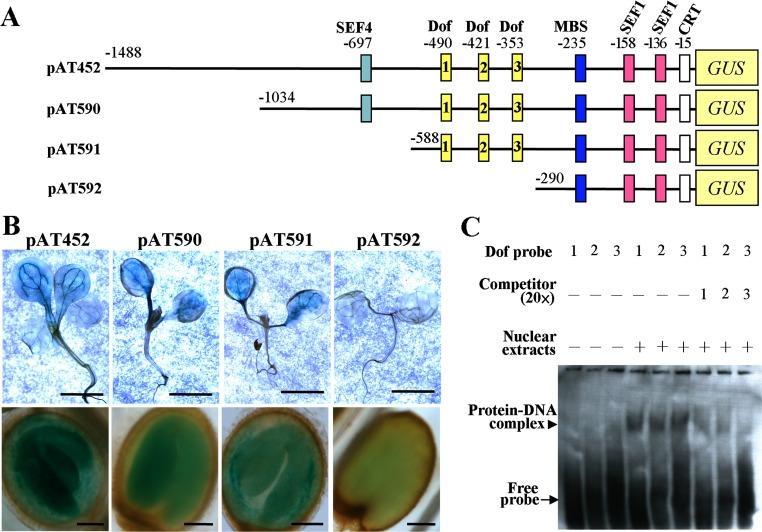
To correlate the ITC results on rAtACBP6 binding with its biological role, the spatial pattern of AtACBP6 expression was elucidated using AtACBP6pro::GUS fusions in transgenic Arabidopsis. To this end, the 1.5-kb 5′-flanking region of AtACBP6 and its three deletion derivatives were fused to GUS to generate plasmids pAT452, pAT590, pAT591 and pAT592 (Figure 4A). Analysis using the SoftBerry PlantProm database [45] suggested that the putative cis-elements that may be involved in AtACBP6 regulation include the light-responsive DNA-binding with one finger boxes (Dof-boxes [46] at nucleotide positions −490/−486, −421/−417 and −353/−349 in Figure 4A), soya bean embryo factors SEF1 (−158/−150 and −136/−128) and SEF4 (−697/−686) which are motifs that bind plant embryo factors [47], and an MBS (MYB-binding site) (−235/−230), which binds the MYB transcription factor [48]. A putative CRT (C-repeat) element (−15/−11) associated with dehydration and freezing responses [49] was also identified (Figure 4A).
Figure 4. Analysis of the AtACBP6 5′-flanking region.
(A) Schematic diagram of AtACBP6pro::GUS constructs in which the AtACBP6 5′-flanking sequence was transcriptionally fused to the GUS reporter gene. Putative cis-elements on the AtACBP6 5′-flanking region (pAT452) and its deletion derivatives (pAT590, pAT591 and pAT592) are represented by boxes. SEF1, SOYBEAN EMBRYO FACTOR1; SEF4, SOYBEAN EMBRYO FACTOR4; Dof, DNA-binding with one finger; MBS, MYB-binding site; CRT, C-repeat; GUS, β-glucuronidase. The putative Dof-boxes are numbered 1, 2, and 3, corresponding to −490/−486, −421/−417 and −353/−349, respectively. (B) GUS staining of 7-day-old seedlings and cotyledonary-staged embryos from T3 generation of Arabidopsis transformants containing AtACBP6pro::GUS construct pAT452 and its deletion derivatives (pAT590, pAT591 and pAT592). Bar in the upper panel represents 5 mm. Bar in the bottom panel represents 100 μm. (C) EMSA analysis showing the binding of nuclear extracts to the Dof-boxes in the AtACBP6 5′-flanking region. Probe 1 (ML2044/2045), Probe 2 (ML2046/2047) and Probe 3 (ML2048/2049), containing the three Dof-boxes at −490/−486, −421/−417 and −353/−349, respectively, are numbered 1, 2 and 3. Competitor contains 20×non-biotin-labelled probe; −, no competitor in the reaction. Nuclear proteins were extracted from 7-day-old Arabidopsis seedlings; −, no nuclear proteins in the reaction; +, nuclear proteins added in the reaction. Arrowhead indicates the DNA–protein binding complex formed in the presence of nuclear proteins extracted from 7-day-old Arabidopsis seedlings. Arrow indicates free unbound biotin-labelled probe.
Histochemical staining of seedlings and the cotyledon-staged embryos from transgenic Arabidopsis expressing AtACBP6pro::GUS constructs derived from pAT452 and its deletion derivatives (pAT590 to pAT592) revealed that GUS was expressed in pAT452, pAT590 and pAT591 transformants, but not pAT592 transformants (Figure 4B), suggesting that loss of promoter activity occurred in the pAT592-transformed seedling and embryo. Also, pAT592 does not contain any putative Dof boxes, whereas pAT452, pAT590 and pAT591 contain all three Dof boxes; these Dof boxes may inherently affect the expression of AtACBP6 during seed and seedling development.
Dof-boxes are related to AtACBP6 expression
To investigate the function of the putative Dof-boxes in the AtACBP6 5′-flanking region, EMSAs were performed using double-stranded biotin-labelled DNA Probes 1, 2 and 3 representing each of the three Dof-boxes (−490/−486, −421/−417 and −353/−349, respectively). When crude nuclear extracts from 7-day-old seedlings were tested, band shifts caused by DNA–protein complexes were observed with all three probes (Figure 4C). Non-biotin-labelled probes outcompeted the binding of nuclear protein (Figure 4C), suggesting that the three Dof-boxes are involved in the expression of AtACBP6.
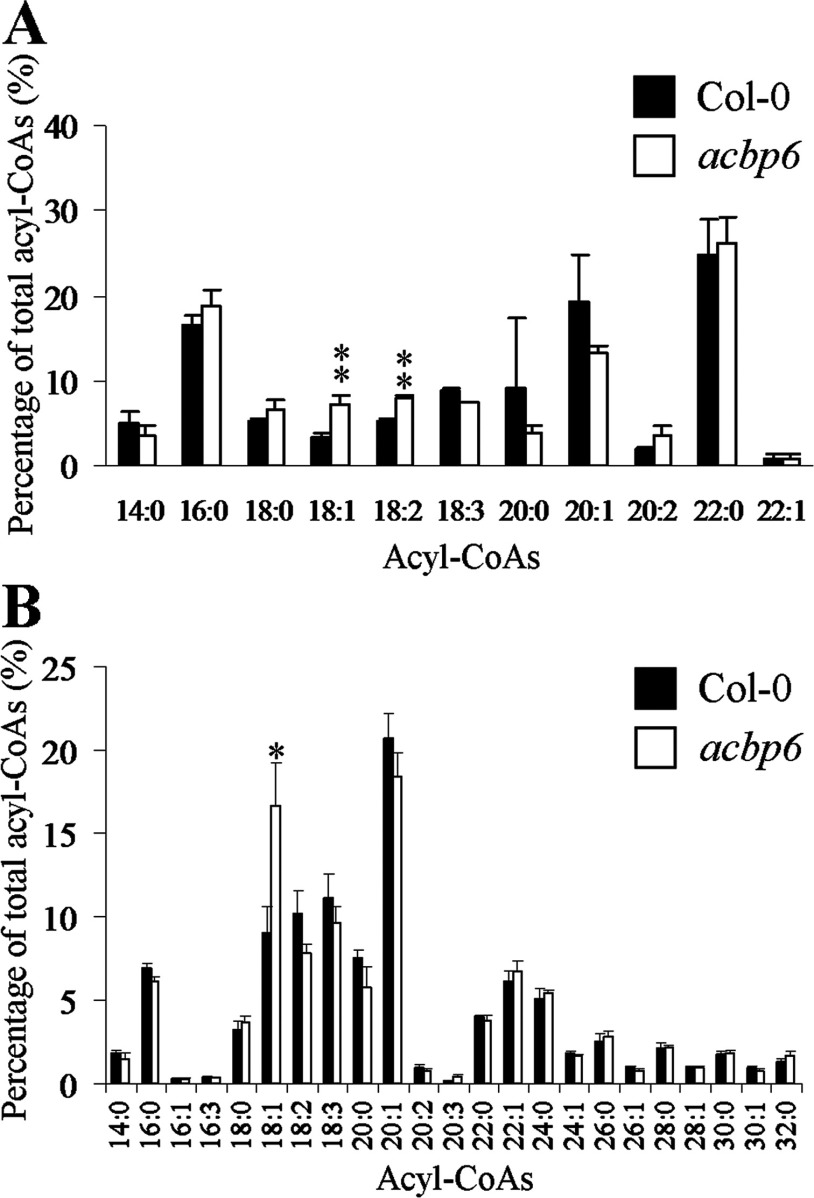
C18:1-CoA ester accumulated in the acbp6 seedlings and embryos
The pattern of GUS expression of AtACBP6 in seedlings and the cotyledonary-staged embryos prompted us to test whether the acyl-CoA content was altered in 5-day-old seedlings and the cotyledonary-staged embryos of the acbp6 mutant in comparison with the wild-type, using HPLC and liquid chromatography-tandem mass spectrometry. The results revealed that the acbp6 mutant, but not the wild-type, accumulated C18:1-CoA in both 5-day-old seedlings and the cotyledonary-staged embryos (Figure 5). The acbp6 mutant also showed C18:2-CoA accumulation in 5-day-old seedlings in comparison to the wild-type (Figure 5A). No other obvious differences were noted for other acyl-CoA esters in the cotyledonary-staged embryos (Figure 5B).
Figure 5. Acyl-CoA profiles of the acbp6 mutant in comparison with wild-type Arabidopsis Col-0.
(A) Acyl-CoA profiling in 5-day-old seedlings of the acbp6 mutant in comparison to Col-0. Values are mean±S.D. of measurements made on three separate batches of samples. Student's t test for **, P<0.01. (B) Acyl-CoA profiling in cotyledonary-staged embryos of the acbp6 mutant in comparison with Col-0. Values are mean±S.E.M. of measurements made on four separate batches of samples. Student's t test for *, P<0.05.
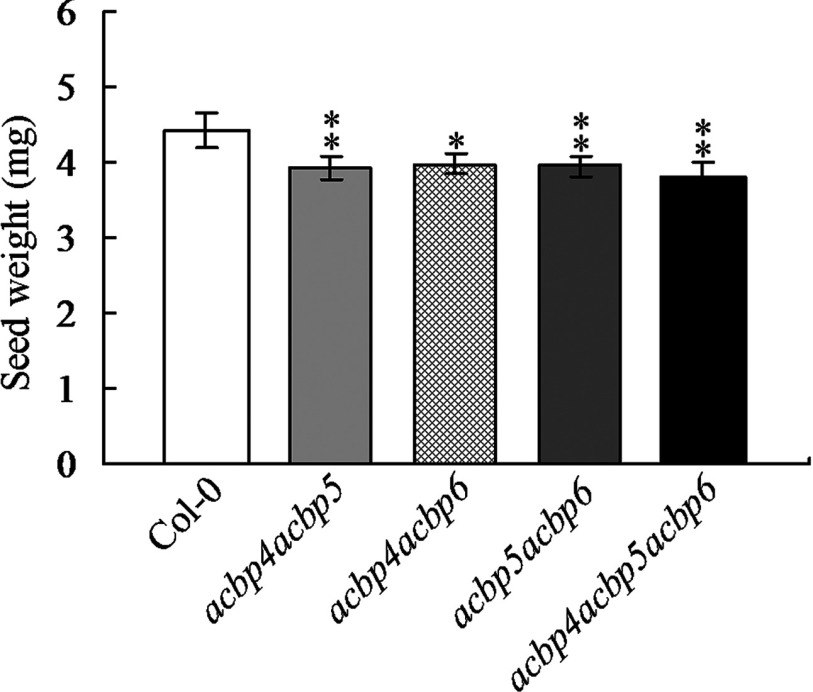
The acbp4acbp5acbp6 mutant showed the lightest seed weight
Results from microarray data on a comparison in the expression of AtACBP4, AtACBP5 and AtACBP6 in seeds (Supplementary Figure S1), together with observations that rAtACBP6, rAtACBP4 and rAtACBP5 bind to acyl-CoA esters, AtACBP6pro::GUS is highly expressed in cotyledonary-staged embryos, and acyl-lipid metabolism is important during seed development, prompted examination of the acbp4acbp5, acbp4acbp6, acbp5acbp6 and acbp4acbp5acbp6 mutants [41] to better understand the roles of these cytosolic AtACBPs in seed biology. There were no obvious variations in seed morphology and size among the double/triple mutants in comparison to the wild-type (Supplementary Figures S2 and S3). However, when seeds were weighed, the acbp4acbp5acbp6 mutant seeds weighed less than wild-type (Figure 6).
Figure 6. Seed weight of acbp4acbp5, acbp4acbp6, acbp5acbp6 and acbp4acbp5acbp6 mutants in comparison with wild-type Arabidopsis Col-0.
Weight of 250 seeds from the acbp4acbp5, acbp4acbp6, acbp5acbp6 and acbp4acbp5acbp6 mutants versus Col-0. Data represent a mean value of four trials±S.D. Student's t test for *, P<0.05; **, P<0.01.
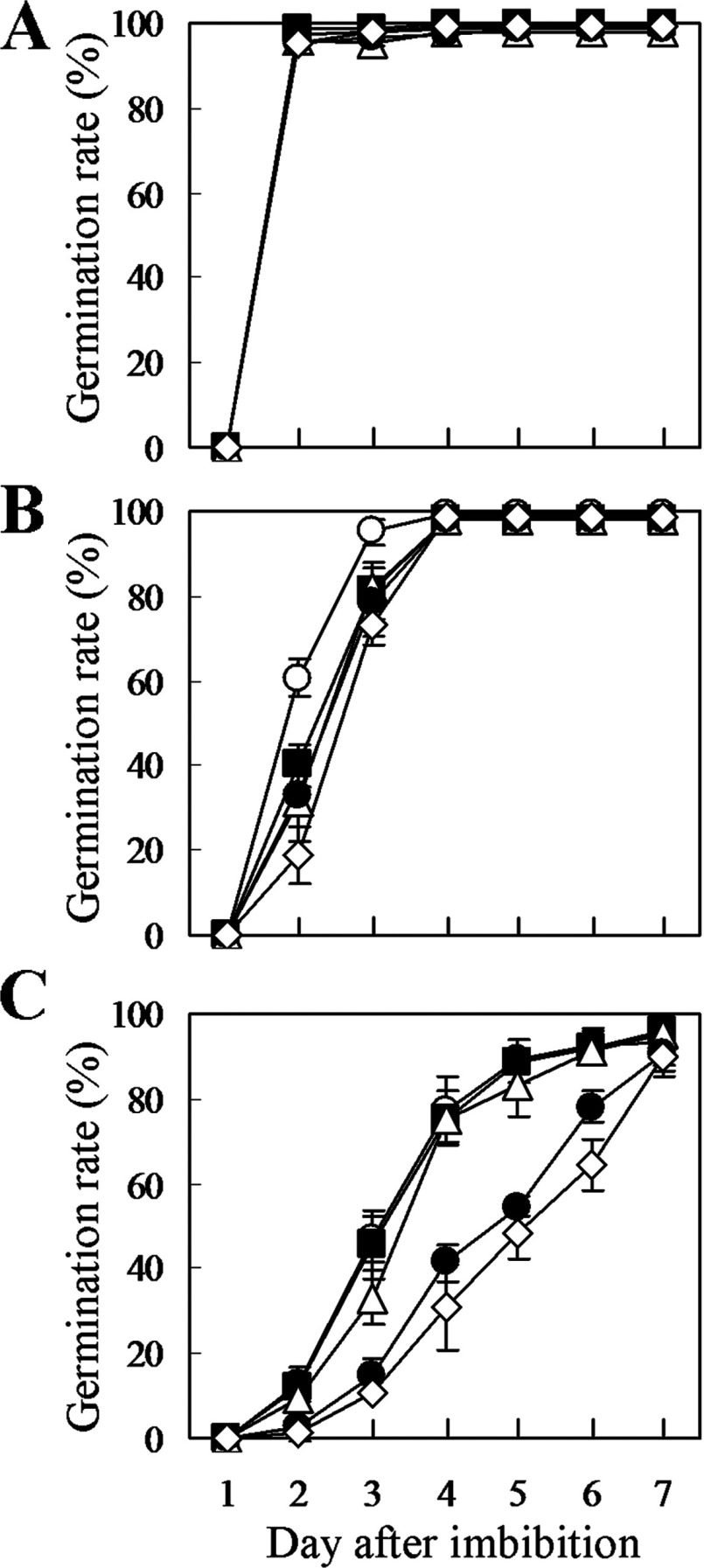
Hypersensitivity to ABA in seed germination in the double/triple mutants of AtACBP4, AtACBP5 and AtACBP6
ABA treatment was used to evaluate the physiological roles of AtACBP4, AtACBP5 and AtACBP6 during seed germination. Under normal conditions, all double/triple mutants showed a similar rate in seed germination to Col-0 (Figure 7A). However, the germination of the mutants was delayed by ABA treatment. As shown in Figure 7(B), only 19% of acbp4acbp5acbp6 seeds germinated within 2 days on the medium containing 0.2 μM ABA, compared with 60% of Col-0 seeds. Double mutants of acbp4acbp5, acbp4acbp6 and acbp5acbp6 showed intermediate germination rates of 40, 32 and 33%, respectively (Figure 7B). At a 0.4 μM ABA concentration, all the double and triple mutants showed lower germination rates in comparison with Col-0, whereas the triple mutant showed highest sensitivity to ABA (Figure 7C), suggesting that these three cytosolic AtACBPs play overlapping roles in seed germination.
Figure 7. Germination rate of the double/triple mutants of cytosolic AtACBPs under ABA treatment in comparison with wild-type Arabidopsis Col-0.
Seeds of Col-0 (open circle), the acbp4acbp5 (closed square), acbp4acbp6 (open triangle), acbp5acbp6 (closed circle) and acbp4acbp5acbp6 (open rhombus) mutants were cultured on MS medium supplemented with 0 (A), 0.2 (B) or 0.4 (C) μM ABA. Values are mean±S.D. of measurements of four separate batches of 33–44 seeds.
DISCUSSION
Cytosolic AtACBPs bind to acyl-CoA esters in vitro
The small (~10 kDa), highly conserved cytosolic ACBPs from ox, rat, armadillo and yeast have been reported to bind long-chain acyl-CoA esters with high affinity using Lipidex assays [1,2], fluorescence and CD spectroscopy studies [3], as well as titration microcalorimetry [4,5]. In the present study, the Arabidopsis homologue, AtACBP6, was shown in ITC-binding assays to bind tightly to several long-chain acyl-CoA esters with n=1 (Figure 1; Table 1). However, for C18:1-CoA, the observation of n=1.9 (Table 1) suggests a binding stoichiometry of 2 moles of C18:1-CoA to 1 mole of rAtACBP6. That multiple molecules of ligand and protein can be involved in some cases is supported by earlier structural work with myristoyl-CoA and human cytosolic liver ACBP [50]. In the acyl-CoA profiling of the cotyledonary-staged embryos, C18:1-CoA was identified as the only acyl-CoA ester that accumulated in the acbp6 mutant in comparison with the wild type (Figure 5B), suggesting that the physiological function of AtACBP6 in the cotyledonary-staged embryos is indeed related to this compound. In the acyl-CoA profiling of seedlings, both C18:1-CoA and C18:2-CoA accumulated in the acbp6 mutant when compared with the wild type, suggesting the physiological function of AtACBP6 in seedlings is related to the binding of C18:2-CoA, as well as C18:1-CoA (Figure 5A). In plants, the cytosolic acyl-CoA pool includes C18:1-CoA derived from de novo fatty acid biosynthesis and acyl-editing, and C18:2-CoA, the product of acyl-editing [51]. The results of the acyl-CoA profiling and binding assay suggest that AtACBP6 plays a major role in maintaining the cytosolic acyl-CoA pool during seed and seedling development; this is supported by its strong expression in the embryo and seedling as demonstrated using AtACBP6pro::GUS transformants.
The recombinant 10-kDa ACBP from Brassica napus has been reported to bind acyl-CoA esters in vitro and enhance acyl exchange between acyl-CoA and PC through incorporation of C18:1-CoA [52]. Previous studies have indicated that AtACBP6 responds to freezing stress; furthermore AtACBP6 overexpressors exhibit a decline of PC in rosettes [39] and an elevation of PC in flowers [53] after freezing. Recombinant AtACBP6 has been reported to bind PC in filter-binding assays [39]. We demonstrate here by ITC that rAtACBP6 binds to acyl-CoA esters, suggesting that this protein may also function in maintaining a balance between acyl-CoA esters and PC.
Recombinant ACBPs, rAtACBP6 (Figure 1; Table 1) as well as rAtACBP4 (Figure 2; Table 2) and rAtACBP5 (Figure 3; Table 3) showed binding affinity to C16:0-CoA, C18:1-CoA, C18:2-CoA and C18:3-CoA. However, the binding trend of rAtACBP6 (C16:0~C18:2>C18:1>C18:3), rAtACBP4 (C16:0>C18:2>C18:1>C18:3) and rAtACBP5 (C16:0>C18:2>C18:1>C18:3) revealed in ITC analysis differed from our previous results of rAtACBP6 (C18:2>C18:1~C16:0>C18:3), rAtACBP4 (C18:1>C16:0>C18:2>C18:3) and rAtACBP5 (C18:1>C16:0>C18:2>C18:3) in Lipidex assays [16,19]. This variation may have arisen from the difference in the methods used. ITC can measure heat change directly during the binding interaction and therefore provides direct access to thermodynamic parameters and Kd [54], while the Lipidex assay is more likely to have imprecise Kd values since it is based on competition and not directly measuring the binding event [25,54].
The binding affinity of the 10-kDa bovine ACBP for C16:0-CoA was estimated to be 4.5 pM [5], while armadillo ACBP showed a range between 34 and 75 nM [3], and yeast ACBP gave 5.5 nM [4]. In Arabidopsis, the binding affinity of the 10-kDa rAtACBP6 was estimated by ITC to be 35.9 nM (Table 1), similar to armadillo ACBP [3]. In contrast, the binding affinity of rAtACBP4 to C16:0-CoA was 23.5 μM (Table 2) and that of rAtACBP5, 35.7 μM (Table 3), which represents 1000-fold weaker binding than armadillo ACBP. The substantially larger molecular weights of AtACBP4 (73 kDa) and AtACBP5 (71 kDa) reflect the presence of multiple structural/functional domains, and both of these recombinant proteins had to be purified from refolded inclusion bodies [16], in contrast to the soluble fraction for rAtACBP6. It is possible that this fact explains the relatively weaker binding for rAtACBP4 and rAtACBP5. However, the stronger binding affinity of rAtACBP6 to long-chain acyl-CoA esters compared to rAtACBP4 and rAtACBP5 may suggest that AtACBP6 interacts with acyl-CoA esters differently from AtACBP4 and AtACBP5. As only AtACBP6 responds to freezing stress [39,53], the stronger binding affinity of rAtACBP6 to long-chain acyl-CoA esters may imply that tight acyl-lipid binding becomes more critical under stress conditions than AtACBP4 and AtACBP5.
The Dof-boxes in the AtACBP6 5′-flanking region bind nuclear proteins
The expression of AtACBP6 has been reported based on Northern blot analysis to be cold inducible [39] and AtACBP6-overexpressing rosettes [39] and flowers [53] have been shown to be conferred freezing tolerance. When AtACBP6-overexpressing flowers and rosettes were subject to freezing treatment, the expression of one or more cold-related CBFs (C-repeat binding factors) [49,55] appeared to be affected [53]. In AtACBP6-overexpressing flowers, the expression of CBF1, CBF2 and CBF3 was higher than the wild-type after cold acclimation and freezing, while in AtACBP6-overexpressing rosettes, only CBF3 showed higher expression than the wild-type after freezing and recovery [53]. CBF3 is known to bind the CRT element and CBF-induced genes contain one or more putative CRT elements in their 5′-flanking regions [55]. Using the PlantProm database, a putative CRT element (−15/−11) was predicted in the 5′-flanking region of AtACBP6, suggesting that this CRT may be involved in the AtACBP6-mediated freezing response.
Furthermore, analysis of the 5′-flanking region of AtACBP6 revealed that the Dof-boxes function in regulating AtACBP6 expression (Figure 4). A previous report has demonstrated that the Dof-box activates dark-responsive regulation of AtACBP3 expression [56], suggesting the importance of Dof-boxes in AtACBP regulation. Dof proteins are unique to plants [57], and members form a major family of transcription factors [46]. Their diverse roles in plants include light responses, phytohormone and defence responses, and seed development and germination [46,58]. Although only one Dof-box (−341/−338) was shown to regulate AtACBP3 expression in response to darkness [56], all three Dof-boxes (−490/−486, −21/−417 and −353/−349) in the AtACBP6 5′-flanking region were shown here using EMSAs to bind nuclear proteins from seedlings (Figure 4). In addition, the loss of the Dof-boxes at −490/−486, −421/−417 and −353/−349 adversely affected AtACBP6pro::GUS expression in 7-day-old seedlings and embryos (Figure 4).
Acyl-CoA transport during seed development to seed germination
It has been reported that recycled acyl groups (C16:0, C18:1, C18:2 and C18:3) in the acyl-CoA pool in the soybean embryo provide the majority of acyl chains for de novo glycerol-3-phosphate acylation, representing the major flux in TAG formation in oil bodies [59]. In Arabidopsis, AtACBP1 and AtACBP2 are expressed in the embryo [30,32], whereas AtACBP4, AtACBP5 and AtACBP6 are predicted (Supplementary Figure S1) to be expressed during seed development. To address the role of the three cytosolic AtACBPs in seeds, recombinant cytosolic AtACBPs (rAtACBP6, rAtACBP4 and rAtACBP5) were verified to bind long-chain acyl-CoA esters, i.e. C16:0-CoA, C18:1-CoA, C18:2-CoA, C18:3-CoA (Figures 1–3; Tables 1–3), suggesting that these AtACBPs can assist in the maintenance of an acyl-CoA pool and represent potential candidates for acyl-CoA trafficking during lipid metabolism from seed development to seed germination [25]. It was observed that rAtACBP6 exhibited binding to long-chain acyl-CoA (C16- to C18-CoA) esters with dissociation constants in the nanomolar range, the affinity of rAtACBP4 and rAtACBP5 to these acyl-CoA esters was much weaker (dissociation constants in the micromolar range), suggesting that they interact with acyl-CoA esters differently from rAtACBP6. Furthermore, microarray data indicated a difference in the expression pattern between AtACBP4 and AtACBP5 during embryogenesis; AtACBP4 was expressed in early embryogenesis while AtACBP5 was expressed in late embryogenesis (Supplementary Figure S1).
AtACBP6 may play an important role in acyl-CoA transport in seed and seedling development, given that C18:1-CoA was observed to accumulate in the acbp6 embryos, while both C18:1-CoA and C18:2-CoA accumulated in the acbp6 seedlings (Figure 5). Consistently, a decline in C18:1-CoA was observed in transgenic Arabidopsis developing seeds (20 days after flowering) that were ectopically expressing the Brassica 10-kDa ACBP [60]. Taken together, these results support a role for the 10-kDa ACBP in acyl-CoA transport in seeds. In seed germination assays under ABA treatment, all the double and triple mutants of cytosolic AtACBPs showed a more sensitive germination phenotype in comparison with the wild type (Figure 7), suggesting that the change in the acyl-CoA pool size may have contributed to their sensitivity to ABA during seed germination. The degree of sensitivity in the double/triple mutants was acbp4acbp5acbp6> acbp5acbp6> acbp4acbp6> acbp4acbp5 (Figure 7), indicating that AtACBP6 plays the most important role among the three cytosolic AtACBPs, and that AtACBP5 may be more important than AtACBP4. Hence, it was not surprising that the acbp4acbp5acbp6 mutant yielded the lowest seed weight (Figure 6). Taken together this study suggests that the three cytosolic AtACBPs have overlapping roles in seed acyl-lipid metabolism, which is intimately linked to their ability to bind acyl-CoA esters.
ACKNOWLEDGEMENTS
We thank the ABRC for providing the Arabidopsis seeds.
AUTHOR CONTRIBUTION
An-Shan Hsiao performed all of the bench work with the following exceptions: the acyl-CoA profiling was jointly performed by Richard P. Haslam, Louise V. Michaelson and Pan Liao; Qin-Fang Chen generated the pAT452 construct and transgenic Arabidopsis expressing pAT452; and Sanjeewani Sooriyaarachchi and Sherry L. Mowbray made the pEXP-ACBP6 construct. Julian A. Tanner helped analyse ITC data in Figures 1–3 and Tables 1–3. An-Shan Hsiao and Mee-Len Chye conceived the study and wrote the paper, with the help of Richard P. Haslam, Pan Liao, Sanjeewani Sooriyaarachchi, Sherry L. Mowbray and Julian A. Tanner. Johnathan A. Napier, Sherry L. Mowbray and Mee-Len Chye coordinated the project.
FUNDING
This work was supported by HKU Postgraduate Studentships (A.S.H. and Q.F.C.), a University Postgraduate Fellowship (P.L.), the Research Grants Council of Hong Kong [HKU765813M], the Wilson and Amelia Wong Endowment Fund, and a grant from the Swedish Natural Science Research Foundation (Vetenskapsrådet) to S.L.M. Work done by J.A.N., L.V.M. and R.P.H. investigating the dynamics of seed oil synthesis was funded by an Institute Strategic Program Grant (Designing Seeds) from the BBSRC (U.K.).
References
- 1.Rasmussen J.T., Börchers T., Knudsen J. Comparison of the binding affinities of acyl-CoA-binding protein and fatty-acid-binding protein for long-chain acyl-CoA esters. Biochem. J. 1990;265:849–855. doi: 10.1042/bj2650849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosendal J., Ertbjerg P., Knudsen J. Characterization of ligand binding to acyl-CoA-binding protein. Biochem. J. 1993;290:321–326. doi: 10.1042/bj2900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavagnari B.M., Milikowski D., Haller J.F., Zanek M.C., Santomé J.A., Ermácora M.R. Optical characterization of armadillo acyl-CoA binding protein. Int. J. Biol. Macromol. 2002;31:19–27. doi: 10.1016/S0141-8130(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen J., Færgeman N.J., Skøtt H., Hummel R., Børsting C., Rose T.M., Andersen J.S., Højrup P., Roepstorff P., Kristiansen K. Yeast acyl-CoA-binding protein: acyl-CoA-binding affinity and effect on intracellular acyl-CoA pool size. Biochem. J. 1994;302:479–485. doi: 10.1042/bj3020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen J.T., Færgeman N.J., Kristiansen K., Knudsen J. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for β-oxidation and glycerolipid synthesis. Biochem. J. 1994;299:165–170. doi: 10.1042/bj2990165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidotti A., Forchetti C.M., Corda M.G., Konkel D., Bennett C.D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc. Natl. Acad. Sci. U. S. A. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton M., Rose T.M., Færgeman N.J., Knudsen J. Evolution of the acyl-CoA binding protein (ACBP) Biochem. J. 2005;392:299–307. doi: 10.1042/BJ20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham I.A., Li Y., Larson T.R. Acyl-CoA measurements in plants suggest a role in regulating various cellular processes. Biochem. Soc. Trans. 2002;30:1095–1099. doi: 10.1042/BST0301095. [DOI] [PubMed] [Google Scholar]
- 9.Færgeman N.J., Wadum M., Feddersen S., Burton M., Kragelund B.B., Knudsen J. Acyl-CoA binding proteins; structural and functional conservation over 2000 MYA. Mol. Cell Biochem. 2007;299:55–65. doi: 10.1007/s11010-005-9040-3. [DOI] [PubMed] [Google Scholar]
- 10.Schultz R., Pelto-Huikko M., Alho H. Expression of diazepam binding inhibitor-like immunoreactivity in rat testis is dependent on pituitary hormones. Endocrinology. 1992;130:3200–3206. doi: 10.1210/endo.130.6.1597138. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J., Faergeman N., Kragelund B., Knudsen J. Acyl-CoA-binding protein (ACBP) localizes to the endoplasmic reticulum and Golgi in a ligand-dependent manner in mammalian cells. Biochem. J. 2008;410:463–472. doi: 10.1042/BJ20070559. [DOI] [PubMed] [Google Scholar]
- 12.Abo-Hashema K.A., Cake M.H., Lukas M.A., Knudsen J. The interaction of acyl-CoA with acyl-CoA binding protein and carnitine palmitoyltransferase I. Int. J. Biochem. Cell Biol. 2001;33:807–815. doi: 10.1016/S1357-2725(01)00049-8. [DOI] [PubMed] [Google Scholar]
- 13.Augoff K., Kolondra A., Chorzalska A., Lach A., Grabowski K., Sikorski A.F. Expression, purification and functional characterization of recombinant human acyl-CoA-binding protein (ACBP) from erythroid cells. Acta Biochim. Pol. 2010;57:533–540. [PubMed] [Google Scholar]
- 14.van Aalten D.M., Milne K.G., Zou J.Y., Kleywegt G.J., Bergfors T., Ferguson M.A., Knudsen J., Jones T.A. Binding site differences revealed by crystal structures of Plasmodium falciparum and bovine acyl-CoA binding protein. J. Mol. Biol. 2001;309:181–192. doi: 10.1006/jmbi.2001.4749. [DOI] [PubMed] [Google Scholar]
- 15.Chye M.L., Li H.Y., Yung M.H. Single amino acids substitutions at the acyl-CoA-binding domain interrupt 14[C]palmitoyl-CoA binding of ACBP2, an Arabidopsis acyl-CoA-binding protein with ankyrin repeats. Plant Mol. Biol. 2000;44:711–721. doi: 10.1023/A:1026524108095. [DOI] [PubMed] [Google Scholar]
- 16.Leung K.C., Li H.Y., Mishra G., Chye M.L. ACBP4 and ACBP5, novel Arabidopsis acyl-CoA-binding proteins with kelch motifs that bind oleoyl-CoA. Plant Mol. Biol. 2004;55:297–309. doi: 10.1007/s11103-004-0642-z. [DOI] [PubMed] [Google Scholar]
- 17.Leung K.C., Li H.Y., Xiao S., Tse M.H., Chye M.L. Arabidopsis ACBP3 is an extracellularly targeted acyl-CoA-binding protein. Planta. 2006;223:871–881. doi: 10.1007/s00425-005-0139-2. [DOI] [PubMed] [Google Scholar]
- 18.Gao W., Xiao S., Li H.Y., Tsao S.W., Chye M.L. Arabidopsis thaliana acyl-CoA-binding protein ACBP2 interacts with a heavy-metal-binding protein AtFP6. New Phytol. 2009;181:89–102. doi: 10.1111/j.1469-8137.2008.02631.x. [DOI] [PubMed] [Google Scholar]
- 19.Xiao S., Chen Q.F., Chye M.L. Light-regulated Arabidopsis ACBP4 and ACBP5 encode cytosolic acyl-CoA-binding proteins that bind phosphatidylcholine and oleoyl-CoA ester. Plant Physiol. Biochem. 2009;47:926–933. doi: 10.1016/j.plaphy.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Xiao S., Gao W., Chen Q.F., Chan S.W., Zheng S.X., Ma J., Wang M., Welti R., Chye M.L. Overexpression of Arabidopsis acyl-CoA-binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell. 2010;22:1463–1482. doi: 10.1105/tpc.110.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaigg B., Neergaard T.B., Schneiter R., Hansen J.K., Færgeman N.J., Jensen N.A., Andersen J.R., Friis J., Sandhoff R., Schrøder H.D., Knudsen J. Depletion of acyl-coenzyme A-binding protein affects sphingolipid synthesis and causes vesicle accumulation and membrane defects in Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:1147–1160. doi: 10.1091/mbc.12.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landrock D., Atshaves B.P., McIntosh A.L., Landrock K.K., Schroeder F., Kier A.B. Acyl-CoA binding protein gene ablation induces pre-implantation embryonic lethality in mice. Lipids. 2010;45:567–580. doi: 10.1007/s11745-010-3437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elle I.C., Simonsen K.T., Olsen L.C., Birck P.K., Ehmsen S., Tuck S., Le T.T., Færgeman N.J. Tissue- and paralogue-specific functions of acyl-CoA-binding proteins in lipid metabolism in Caenorhabditis elegans. Biochem. J. 2011;437:231–241. doi: 10.1042/BJ20102099. [DOI] [PubMed] [Google Scholar]
- 24.Fan J., Liu J., Culty M., Papadopoulos V. Acyl-coenzyme A binding domain containing 3 (ACBD3; PAP7; GCP60): an emerging signaling molecule. Prog. Lipid Res. 2010;49:218–234. doi: 10.1016/j.plipres.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao S., Chye M.L. New roles for acyl-CoA-binding proteins (ACBPs) in plant development, stress responses and lipid metabolism. Prog. Lipid Res. 2011;50:141–151. doi: 10.1016/j.plipres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Yurchenko O.P., Weselake R.J. Involvement of low molecular mass soluble acyl-CoA-binding protein in seed oil biosynthesis. New Biotechnol. 2011;28:97–109. doi: 10.1016/j.nbt.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Li H.Y., Chye M.L. Arabidopsis acyl-CoA-binding protein ACBP2 interacts with an ethylene-responsive element-binding protein, AtEBP, via its ankyrin repeats. Plant Mol. Biol. 2004;54:233–243. doi: 10.1023/B:PLAN.0000028790.75090.ab. [DOI] [PubMed] [Google Scholar]
- 28.Gao W., Li H.Y., Xiao S., Chye M.L. Acyl-CoA-binding protein 2 binds lysophospholipase 2 and lysoPC to promote tolerance to cadmium-induced oxidative stress in transgenic Arabidopsis. Plant J. 2010;62:989–1003. doi: 10.1111/j.1365-313X.2010.04209.x. [DOI] [PubMed] [Google Scholar]
- 29.Chye M.L. Arabidopsis cDNA encoding a membrane-associated protein with an acyl-CoA binding domain. Plant Mol. Biol. 1998;38:827–838. doi: 10.1023/A:1006052108468. [DOI] [PubMed] [Google Scholar]
- 30.Chye M.L., Huang B.Q., Zee S.Y. Isolation of a gene encoding Arabidopsis membrane-associated acyl-CoA binding protein and immunolocalization of its gene product. Plant J. 1999;18:205–214. doi: 10.1046/j.1365-313X.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 31.Li H.Y., Chye M.L. Membrane localization of Arabidopsis acyl-CoA binding protein ACBP2. Plant Mol. Biol. 2003;51:483–492. doi: 10.1023/A:1022330304402. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q.F., Xiao S., Qi W., Mishra G., Ma J., Wang M., Chye M.L. The Arabidopsis acbp1acbp2 double mutant lacking the acyl-CoA-binding proteins ACBP1 and ACBP2 is embryo lethal. New Phytol. 2010;186:843–855. doi: 10.1111/j.1469-8137.2010.03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Z.Y., Chen M.X., Chen Q.F., Gu J.D., Chye M.L. Expression of Arabidopsis acyl-CoA-binding proteins AtACBP1 and AtACBP4 confers Pb(II) accumulation in Brassica juncea roots. Plant Cell Environ. 2015 doi: 10.1111/pce.12382. doi: 10.1111/pce.12382. [DOI] [PubMed] [Google Scholar]
- 34.Xue Y., Xiao S., Kim J., Lung S.C., Chen L., Tanner J.A., Suh M.C., Chye M.L. Arabidopsis membrane-associated acyl-CoA-binding protein ACBP1 is involved in stem cuticle formation. J. Exp. Bot. 2014;65:5473–5583. doi: 10.1093/jxb/eru304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Z.Y., Chen M.X., Chen Q.F., Xiao S., Chye M.L. Overexpression of Arabidopsis acyl-CoA-binding protein ACBP2 enhances drought tolerance. Plant Cell Environ. 2013;36:300–314. doi: 10.1111/j.1365-3040.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- 36.Du Z.Y., Xiao S., Chen Q.F., Chye M.L. Depletion of the membrane-associated acyl-CoA-binding protein ACBP1 enhances the ability of cold acclimation in Arabidopsis. Plant Physiol. 2010;152:1585–1597. doi: 10.1104/pp.109.147066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao S., Chye M.L. The Arabidopsis thaliana ACBP3 regulates leaf senescence by modulating phospholipid metabolism and ATG8 stability. Autophagy. 2010;6:802–804. doi: 10.4161/auto.6.6.12576. [DOI] [PubMed] [Google Scholar]
- 38.Xiao S., Li H.Y., Zhang J.P., Chan S.W., Chye M.L. Arabidopsis acyl-CoA-binding proteins ACBP4 and ACBP5 are subcellularly localized to the cytosol and ACBP4 depletion affects membrane lipid composition. Plant Mol. Biol. 2008;68:571–583. doi: 10.1007/s11103-008-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q.F., Xiao S., Chye M.L. Overexpression of the Arabidopsis 10-kilodalton acyl-coenzyme A-binding protein ACBP6 enhances freezing tolerance. Plant Physiol. 2008;148:304–315. doi: 10.1104/pp.108.123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen J.T., Færgeman N.J., Kristiansen K., Knudsen J. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for β-oxidation and glycerolipid synthesis. Biochem. J. 1994;299:165–170. doi: 10.1042/bj2990165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsiao A.S., Yeung E.C., Ye Z.W., Chye M.L. The Arabidopsis cytosolic acyl-coA-binding proteins play combinatory roles in pollen development. Plant Cell Physiol. 2015 doi: 10.1093/pcp/pcu163. doi: 10.1093/pcp/pcp163. [DOI] [PubMed] [Google Scholar]
- 42.Larson T.R., Graham I.A. Technical advance: a novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 2001;25:115–125. doi: 10.1046/j.1365-313x.2001.00929.x. [DOI] [PubMed] [Google Scholar]
- 43.Haynes C.A., Allegood J.C., Sims K., Wang E.W., Sullards M.C., Merrill A.H., Jr Quantitation of fatty acyl-coenzyme As in mammalian cells by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Lipid Res. 2008;49:1113–1125. doi: 10.1194/jlr.D800001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohlrogge J.B., Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahmuradov I.A., Gammerman A.J., Hancock J.M., Bramley P.M., Solovyev V.V. PlantProm: a database of plant promoter sequences. Nucleic Acids Res. 2003;31:114–117. doi: 10.1093/nar/gkg041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanagisawa S. The Dof family of plant transcription factors. Trends Plant Sci. 2002;7:555–560. doi: 10.1016/S1360-1385(02)02362-2. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara T., Beachy R.N. Tissue-specific and temporal regulation of a β-conglycinin gene: roles of the RY repeat and other cis-acting elements. Plant Mol. Biol. 1994;24:261–272. doi: 10.1007/BF00020166. [DOI] [PubMed] [Google Scholar]
- 48.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Knight H., Zarka D.G., Okamoto H., Thomashow M.F., Knight M.R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol. 2004;135:1710–1717. doi: 10.1104/pp.104.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taskinen J.P., van Aalten D.M., Knudsen J., Wierenga R.K. High resolution crystal structures of unliganded and liganded human liver ACBP reveal a new mode of binding for the acyl-CoA ligand. Proteins. 2007;66:229–238. doi: 10.1002/prot.21124. [DOI] [PubMed] [Google Scholar]
- 51.Li-Beisson Y.H., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D., Baud S., Bird D., DeBono A., Durrett T.P., et al. Acyl-lipid metabolism. Arabidopsis Book. 2010;8:e0133. doi: 10.1199/tab.0133. doi:10.1199/tab.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurchenko O.P., Nykiforuk C.L., Moloney M.M., Ståhl U., Banaś A., Stymne S., Weselake R.J. A 10-kDa acyl-CoA-binding protein (ACBP) from Brassica napus enhances acyl exchange between acyl-CoA and phosphatidylcholine. Plant Biotechnol. J. 2009;7:602–610. doi: 10.1111/j.1467-7652.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- 53.Liao P., Chen Q.F., Chye M.L. Transgenic Arabidopsis flowers overexpressing acyl-CoA-binding protein ACBP6 are freezing tolerant. Plant Cell Physiol. 2014;55:1055–1071. doi: 10.1093/pcp/pcu037. [DOI] [PubMed] [Google Scholar]
- 54.Hanhoff T., Lücke C., Spener F. Insights into binding of fatty acids by fatty acid binding proteins. Mol. Cell Biochem. 2002;239:45–54. doi: 10.1023/A:1020502624234. [DOI] [PubMed] [Google Scholar]
- 55.Maruyama K., Sakuma Y., Kasuga M., Ito Y., Seki M., Goda H., Shimada Y., Yoshida S., Shinozaki K., Yamaguchi-Shinozaki K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- 56.Zheng S.X., Xiao S., Chye M.L. The gene encoding Arabidopsis acyl-CoA-binding protein 3 is pathogen inducible and subject to circadian regulation. J. Exp. Bot. 2012;63:2985–3000. doi: 10.1093/jxb/ers009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riechmann J.L., Heard J., Martin G., Reuber L., Jiang C., Keddie J., Adam L., Pineda O., Ratcliffe O.J., Samaha R.R., et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–2110. doi: 10.1126/science.290.5499.2105. [DOI] [PubMed] [Google Scholar]
- 58.Gualberti G., Papi M., Bellucci L., Ricci I., Bouchez D., Camilleri C., Costantino P., Vittorioso P. Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell. 2002;14:1253–1263. doi: 10.1105/tpc.010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates P.D., Durrett T.P., Ohlrogge J.B., Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yurchenko O., Singer S.D., Nykiforuk C.L., Gidda S., Mullen R.T., Moloney M.M., Weselake R.J. Production of a Brassica napus low-molecular mass acyl-coenzyme A-binding protein in Arabidopsis alters the acyl-coenzyme A pool and acyl composition of oil in seeds. Plant Physiol. 2014;165:550–560. doi: 10.1104/pp.114.238071. [DOI] [PMC free article] [PubMed] [Google Scholar]