Abstract
Cigarette smoking is associated with a higher prevalence of depressive symptoms and individuals with elevated symptoms of depression have more difficulty quitting smoking. Depression is accompanied by cognitive deficits similar to those observed during nicotine withdrawal. Depressed smokers may smoke to alleviate these cognitive symptoms, which are exacerbated upon smoking abstinence. We hypothesized that following overnight abstinence, depression-prone smokers (DP+; past history and current depression symptoms; n = 34) would exhibit deficits in short-term and working memory, and experience greater attentional bias for affective stimuli, compared with smokers with no history or current symptoms of depression (DP−; n = 34). All participants underwent two laboratory sessions, once while smoking abstinent and once while smoking ad libitum (order counterbalanced, abstinence biochemically verified). Smokers completed measures of short-term memory (STM; word recognition task), working memory (N-back task), and attentional bias (Emotional Stroop task). The DP+ group showed declines in STM during abstinence compared with smoking, whereas the DP− group did not (interaction p = .02). There were small decrements in working memory accuracy during abstinence (p = .05), but this did not interact with depression status. During the Emotional Stroop task, the DP+ group showed an attentional bias toward positive versus neutral stimuli during abstinence compared with smoking (interaction p = .01). This study provides initial evidence that depressive symptoms may moderate abstinence-induced deficits in STM and shift attentional bias toward emotionally salient stimuli during abstinence. These cognitive changes may prompt relapse and may help identify novel targets for nicotine dependence treatment aimed at attenuating these deficits to improve cessation rates.
Keywords: smoking, depression, memory, attentional bias, nicotine withdrawal
Cigarette smoking is associated with a higher prevalence of life-time major depressive disorder (MDD) as well as greater symptoms of depression (Lasser et al., 2000; Wilhelm, Wedg-wood, Niven, & Kay-Lambkin, 2006; Ziedonis et al., 2008). Smokers with elevated depression symptoms, current MDD, and a history of MDD are less likely to quit smoking and have higher relapse rates (Japuntich et al., 2007; Sonne et al., 2010; Weinberger, Pilver, Desai, Mazure, & McKee, 2012). This is important as half of smokers enrolled in smoking cessation programs report elevated symptoms of depression (Ginsberg, Hall, Reus, & Muñoz, 1995; Lerman et al., 1996; Levine, Marcus, & Perkins, 2003). Although greater difficulties achieving and maintaining abstinence among smokers prone to depression has been attributed to negative affect (Brown et al., 2007; Cinciripini et al., 2003; Evins et al., 2008; Strong et al., 2009), converging research suggests that cognitive aspects of depression may also contribute to these difficulties (Murrough, Iacoviello, Neumeister, Charney, & Iosifescu, 2011).
Cognitive theories of depression have typically focused on two domains: cognitive deficits and cognitive biases. A systematic review of cognitive deficits concluded that depressed patients exhibit poorer performance on executive function and verbal memory tasks (Bora, Harrison, Yucel, & Pantelis, 2013). Deficits in memory are observed primarily in free recall, recognition, and working memory tasks, though deficits may be greater for free recall and recognition (Gotlib & Joormann, 2010). Furthermore, some suggest that depression-related memory impairment may be exacerbated in specific contexts, such as when increased cognitive effort is required or when attention is allocated to task-irrelevant thoughts (Ellis & Ashbrook, 1988; Gotlib & Joormann, 2010). For instance, smoking abstinence may require increased cognitive effort to implement smoking cessation strategies and attention may be directed to withdrawal symptoms and cigarette cravings. Depression-prone smokers may be more vulnerable to the increased cognitive demands associated with strategies to maintain smoking abstinence because of depleted cognitive resources and impaired memory performance.
Cognitive models of depression also propose that depressed individuals possess a cognitive bias, or a tendency to direct attention and memory toward negative or emotionally salient stimuli (Murrough et al., 2011). This attentional bias may reflect an inability to suppress task-irrelevant negative thoughts, or a predisposition to allocate resources to the processing of negative stimuli (Bradley, Mogg, & Lee, 1997; Gotlib & Joormann, 2010). Attentional bias is typically operationalized as slower reaction times (RTs) to negative compared with neutral stimuli (Phaf & Kan, 2007; Williams, Mathews, & MacLeod, 1996). However, consistent with evidence that depression is associated with reduced positive affect in addition to increased negative affect (Forbes, 2009), depressed individuals also experience an attentional bias for positive stimuli, suggesting a general emotional bias (Epp, Dobson, Dozois, & Frewen, 2012). Whether this emotional bias hinders successful quitting or interacts with cognitive deficits is unclear. Cognitive deficits and cognitive biases have typically been studied separately and little is known about the relationship between these two domains.
The cognitive deficits associated with depression are similar to those observed during nicotine withdrawal emphasizing that cognition plays an important role in both depression and smoking behavior. Several studies have demonstrated deficits in working memory performance during smoking abstinence, particularly as task difficulty increases (Jacobsen et al., 2005; Jacobsen, Mencl, Constable, Westerveld, & Pugh, 2007; Mendrek et al., 2006; Myers, Taylor, Moolchan, & Heishman, 2008). There is also emerging evidence that withdrawal-related deficits in working memory predict relapse to smoking (Patterson et al., 2010). Indeed, cognitive deficits in attention and working memory (Ashare, Falcone, & Lerman, 2014) may represent a core dependence phenotype and a target for smoking cessation treatment efforts (Lerman et al., 2007; Sofuoglu, 2010). Furthermore, smokers exhibit an attentional bias for smoking-related stimuli, which is predictive of smoking relapse (Waters et al., 2009; Waters & Feyerabend, 2000; Waters et al., 2003) and smoking abstinence attenuates attentional bias toward positive stimuli (Dawkins, Powell, West, Powell, & Pickering, 2006; Powell, Pickering, Dawkins, West, & Powell, 2004).
Based on evidence that nicotine enhances cognition (Levin, McClernon, & Rezvani, 2006), it is conceivable that depressed individuals smoke to mitigate cognitive decrements observed in depression. Although depression is often associated with cognitive deficits observed during nicotine withdrawal (Ashare et al., 2014; Bora et al., 2013; Gotlib & Joormann, 2010; Murrough et al., 2011), it is not clear whether cognitive deficits associated with depression are worsened upon smoking cessation. Smokers with a history of depression may experience greater deficits in short-term recall memory during smoking abstinence, which may, in part, maintain smoking behavior. Although depression is also associated with working memory deficits, several studies have shown abstinence-induced deficits in working memory among nonde-pressed smokers (Myers et al., 2008; Patterson et al., 2009) suggesting that these deficits may be common across all smokers. Despite these connections, we are unaware of research that has specifically evaluated whether depression exacerbates the effects of abstinence on deficits in memory and attentional bias for emotional stimuli. We predicted that after overnight abstinence, depression-prone smokers would show diminished short-term memory (STM) during a word recognition task and experience greater attentional bias for affective stimuli during an Emotional Stroop task, compared with smokers with no history of depression. In contrast, we predict that all smokers will exhibit poorer working memory performance during an N-back task. We included these specific domains of cognitive function to examine whether nicotine withdrawal produces changes in cognitive function that are specific to depression-prone smokers. Identifying cognitive deficits specific to depression-prone smokers may guide treatment strategies to target these symptoms during a quit attempt.
Method
Participants
Cigarette smokers were recruited from the community through print advertisements. Eligible smokers were between the ages of 18–65, who smoked at least 10 cigarettes a day for 6 months and met criteria for one of two depression status groups: (DP−) those without a past history of major depression or current depression symptoms, or (DP+) those with a past history of major depression and current depression symptoms. Past history of major depression was determined by the Inventory to Diagnose Depression-Lifetime (IDD; Zimmerman & Coryell, 1987; Zimmerman, Coryell, Corenthal, & Wilson, 1986). The IDD is a 22-item self-report scale that assesses symptom severity on a scale from 0 to 4 as well as the persistence of the symptom for ≥2 weeks. Criteria for past depression is met on the IDD if the individual scores two or more on the low mood, irritability, or hopelessness items, or three or more on the decreased pleasure or decreased interest items, indicating either a positive or negative history of depression. In the current sample, the IDD had high internal consistency (α= .93). The presence of current depressive symptoms was defined as a score greater than 16 as measured by the 20-item CESD, Center for Epidemiological Studies on Depression Scale (CESD) as in previous studies (Brown et al., 2007). The CESD had high internal consistency (α= .94) and a CESD score of >16 correlates with clinical ratings of depression (Radloff, 1977).
Exclusion criteria included: pregnancy, lactation, chronic medical condition (e.g., heart disease), current diagnosis or history of bipolar disorder, schizophrenia or substance abuse (other than nicotine dependence), and current or recent use of smoking cessation medications, antidepressant or antipsychotic medications. During an initial eligibility visit, participants provided a carbon monoxide (CO) breath sample to verify smoking status and a urine sample for a drug screen. Participants who had a CO <10 ppm or a positive urine drug screen for illicit drugs and/or psychotropic medication were excluded. The final sample consisted of 34 DP+ smokers and 53 DP− smokers. Table 1 contains demographic and smoking characteristics for each group.
Table 1.
Demographic and Smoking Characteristics of DP– and DP+ for the Full Sample (n = 87) and for the Subset Matched for Age and Sex (n = 68)
| DP+ (n = 34) |
DP– (n = 53) |
DP– matched for age and sex (n = 34) |
||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Mean | SD | Mean | SD | p valuea | Mean | SD | p valueb |
| Age | 34.3 | 13.7 | 41.03 | 14.4 | 0.03 | 36.8 | 14.4 | 0.46 |
| Smoking rate | 17.7 | 6.7 | 17.4 | 10.1 | 0.89 | 17.4 | 10.1 | 0.73 |
| Nicotine dependence | 5.81 | 1.53 | 5.54 | 1.79 | 0.45 | 5.54 | 1.79 | 0.09 |
| Baseline CO (ppm) | 21.7 | 10.5 | 20.2 | 11.6 | 0.73 | 20.2 | 11.6 | 0.99 |
| CESD | 31.3 | 10.9 | 7.3 | 5.3 | <0.001 | 7.3 | 5.3 | <0.001 |
| IDD | 41.1 | 7.3 | 6.1 | 8.1 | <0.001 | 7.5 | 8.5 | <0.001 |
| Withdrawal symptoms | ||||||||
| Abstinent | 19.9 | 7.0 | 9.2 | 6.4 | <0.001 | 10.2 | 6.9 | <0.001 |
| Ad-lib smoking | 13.4 | 7.4 | 6.4 | 4.5 | <0.001 | 6.6 | 4.6 | <0.001 |
| % | Count | % | Count | % | Count | |||
|---|---|---|---|---|---|---|---|---|
| Sex (female) | 44% | (15) | 26% | (14) | 0.09 | 41% | (14) | 0.81 |
| Race | 0.57 | 0.74 | ||||||
| American Indian | 0 | (0) | 2% | (1) | 3% | (1) | ||
| Asian | 3% | (1) | 0 | (0) | 0 | (0) | ||
| Black | 47% | (16) | 57% | (30) | 47% | (16) | ||
| White | 47% | (16) | 40% | (21) | 47% | (16) | ||
| More than one race | 3% | (1) | 2% | (1) | 3% | (1) |
Note. CESD = Center for Epidemiological Studies on Depression Scale.
p value represents main effect of group for full sample (n = 87).
p value represents main effect of group for sample matched for age and sex (n = 68).
Procedures
All procedures were approved by the University of Pennsylvania's Institutional Review Board and all participants provided written informed consent. The current data are part of a larger study examining reward and affect regulation in depression-prone smokers (Audrain-McGovern, Wileyto, Ashare, Cuevas, & Strasser, in press). After eligibility was confirmed, participants completed a baseline assessment of demographics, nicotine dependence (Fagerström Test for Nicotine Dependence [FTND]; Heath-erton, Kozlowski, Frecker, & Fagerstrom, 1991), smoking history, and mood. They were then scheduled for two morning laboratory sessions: once after overnight abstinence from smoking (~9 hr) and once while smoking as usual (order counterbalanced). Abstinence was biochemically confirmed with CO <8 ppm (SRNT, 2002). Self- reported withdrawal symptoms were measured with the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986), which had high internal consistency (α= .92).
Participants were compensated $30 cash for each laboratory session. In addition to the cognitive tasks described below, participants completed computer tasks designed to measure the relative reinforcing value of smoking and reward sensitivity, which are described elsewhere (Audrain-McGovern et al., in press).
Cognitive Tasks
Word Recognition Memory (WRM)
This task is a computerized version of WRM tasks used in prior work (Dawkins, Turner, Hasna, & Soar, 2012; Peterson & Peterson, 1959). Ten target words were presented (1,000 ms) in succession on a computer monitor via E-Prime 2.0 software. Participants then completed the N-back task (described below). After this 16-min delay period, participants viewed another series of 10 words on the monitor: 6 targets and 4 distractor words. Participants were asked whether the word on the screen appeared in the original list presented earlier in the session and were given 10 s to respond to each item followed by feedback about the accuracy of each response. A failure to respond within the 10 s window was coded as an incorrect response. Two sets of target words and distractor words were equated for frequency, length, concreteness, and low imageability so that participants did not view the same set of words at each session. The outcome measure was the total number of correct responses, including correct responses to target words and correctly identifying a distractor word. Two subjects were excluded for poor performance (i.e., number correct was less than two SD below the mean). Test–retest reliability across sessions for number correct was r = .36, p < .01.
Visuospatial Working Memory N-Back Task
This N-back is a commonly used paradigm in studies investigating the maintenance and retrieval of information in working memory (Owen, McMillan, Laird, & Bullmore, 2005). The current study uses a visuospatial working memory task that is based on paradigms used in prior research (Ehlis, Bahne, Jacob, Herrmann, & Fallgatter, 2008; Green et al., 2005). During the N-back, participants are instructed to remember the location of a stimulus, a gray circle ~5 cm in diameter, as it appears randomly in eight possible locations around the perimeter of a computer screen. The stimulus appears for 200 ms, followed by an interstimulus interval (ISI) of 2,800 ms. A cross hair remains visible during the stimulus presentation to cue participants to look at the center of the screen. The N-back includes four conditions of varying difficulty levels presented in pseudorandomized counterbalanced order: the 0-back, 1-back, 2-back, and 3-back. Each block consists of 50 trials with 15 targets per block. Participants respond only to targets (30% of stimuli) by pressing the spacebar. During the 0-back, participants are instructed to press the spacebar if the stimulus appears in a predetermined location (upper left corner of the computer screen). During the 1-back, participants are instructed to press the spacebar whenever the stimulus appears in the same location as the stimulus that immediately preceded it. During the 2- and 3-back conditions, participants are instructed to press the spacebar whenever the stimulus appears in same location as the stimulus that preceded it by two or three trials, respectively. The primary dependent variables for this task are total number of correct responses and median RT during correct hits. Secondarily, signal detection analyses were conducted to examine the discrimination index (i.e., the difference between the hit rate and the false alarm rate) and the response bias (i.e., the tendency to respond irrespective of accuracy). Larger values on the discrimination index indicate better discrimination between stimuli. For the response bias, values greater than 0.5 indicate a liberal pattern of responding, values less than 0.5 indicate a conservative pattern, and values equal to 0.5 are considered neutral (Snodgrass, 1988). Three subjects were excluded from the N-back analysis for poor performance (i.e., number correct less than two SD below the mean). Test–retest reliability across sessions for accuracy and RT were rs > 0.60, ps < 0.01.
Emotional Stroop Task (E-Stroop)
The emotional Stroop task was used to assess attentional bias by positive (16 words) and negative (16 words) emotion-related stimuli compared with neutral stimuli (16 words). This task assesses the degree to which salient stimuli automatically capture and hold participants’ attention, thereby interfering with ability to execute other actions. Adapted from previous variants of this task (Drobes, Elibero, & Evans, 2006; Leventhal et al., 2007), words were written in one of three colors (red, green, or blue) and presented on the screen with a 500 ms ISI. The participant was instructed to ignore the meaning of the word and indicate as quickly and as accurately as possible which color the word was written in, by pressing one of three colored buttons on a keyboard. Stimuli were matched for length and usage frequency. Each stimulus was presented once in each of the three colors and presented in blocked order. Participants completed a practice sequence (24 trials) of letter strings (e.g., HHHH), then an experimental sequence (144 trials), lasting about 10 min. The dependent variables are “Stroop Effects,” which are the median correct RT on an emotionally valenced category's 48 trials (i.e., positive or negative) minus the median correct RT on 48 neutral trials. Subjects were excluded if the mean correct RT was greater than 2 SD above the mean (n = 3). Test–retest reliability across sessions for the positive versus neutral and negative versus neutral contrasts were rs < 0.2, ps > 0.2.
Data Analysis
Univariate statistics were generated to describe the study population in terms of demographics and smoking characteristics. Behavioral task performance was examined using regression with subject-level random effects and estimated using maximum likelihood techniques (Stata xtreg; Stata Corporation, College Station, TX). Models included terms for the main effects of group (DP− vs. DP+), session (abstinent vs. smoking as usual), and relevant covariates (age and sex). For the N-back, condition (0-, 1-, 2-, and 3-back) was a within-subject factor. Given the sex differences in rates of depression and smoking behavior (Peiper & Rodu, 2013; Pratt & Brody, 2010; Weinberger et al., 2012), interactions among sex, session, and depression status were tested in an exploratory analysis.
Results
Participant Characteristics
Table 1 depicts the characteristics for the full sample (n = 87). The DP+ group was significantly younger than the DP− group and tended to be more female. Because of the uneven sample sizes between groups and the association of sex and age with depression status and smoking behavior, we examined a subset of the sample matched for age and sex. The matched groups were used for all subsequent analyses and their demographic and smoking characteristics are included in Table 1.1 Except for a marginally higher level of nicotine dependence in the DP+ group (p = .09), there were no other significant differences between groups. CO values during the abstinent and ad libitum smoking sessions were 2.2 ppm (SD = 1.6) and 11.5 ppm (SD = 3.4), respectively, and did not differ between groups (ps > 0.4). For self-reported withdrawal symptoms, there were main effects of abstinence and group, but the interaction was marginal, p = .08. As expected, withdrawal symptoms were higher during abstinence and the DP+ reported significantly more withdrawal, compared with the DP− group, irrespective of smoking status, ps < 0.001 (see Table 1).
Word Recognition Memory
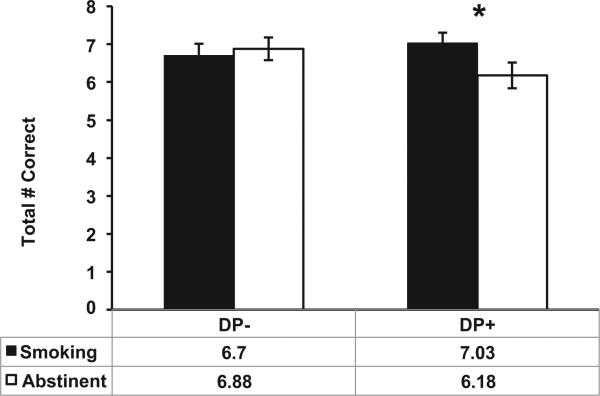
As shown in Figure 1, there was a significant group × session interaction on number correct, β=−0.91, CI: −1.67 to −0.14, p = .02, Cohen's d = 0.48. Follow-up tests indicated that the DP+ group recalled fewer words during abstinence compared with smoking, mean difference = 0.85, p = .02, whereas there was no session effect for the DP− group, mean difference = 0.18, p = .60. There were no main or interacting effects with sex, ps > 0.11.
Figure 1.
Mean number of correct responses during the word recognition task for each group (DP+ vs. DP−) by session (abstinent vs. smoking-as-usual) condition. Error bars represent the SEM. * p < .05 for the effect of abstinence in the DP+ group.
N-Back Working Memory
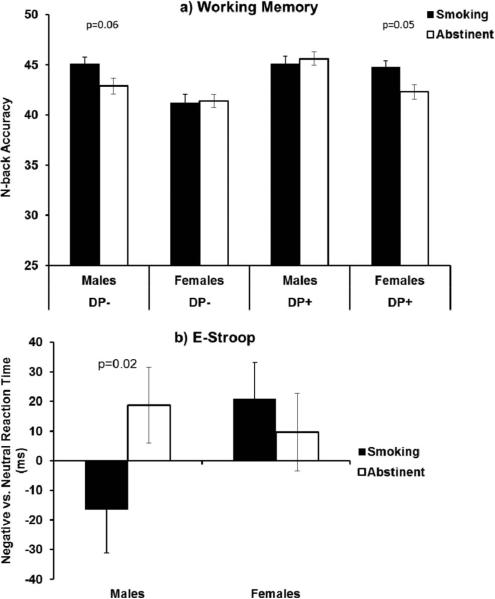
For number correct on the N-back, there were no main or interacting effects of group or session (ps > 0.13). However, there was a marginal sex × group × session interaction, p = .06. As shown in Figure 2, the pattern of means suggested that females in the DP+ group and males in the DP− group performed worse during abstinence compared with smoking, ps < 0.06, whereas there were no abstinence effects for the other two groups, ps > 0.71. There was also a main effect of abstinence on the discrimination index (p = .05, Cohen's d = 0.14), indicating that the ability to distinguish between stimuli was modestly better during ad libitum smoking (M = 0.70, SD = 0.01) compared with abstinence (M = 0.67, SD = 0.01). There were no significant effects of abstinence, group, or their interaction for RT or response bias, ps > 0.2. There was a main effect of N-back condition such that participants responded more slowly as difficulty increased, Wald's χ2(3) = 168.8, p < .001.
Figure 2.
(a) Mean number of true positives during the N-back task for males and females by group (DP+ vs. DP−) and session (abstinent vs. smoking-as-usual). (b) Difference in median RT (milliseconds) to negative versus neutral stimuli during the Emotional Stroop task for males and females by session (abstinent vs. smoking-as-usual) condition. Error bars represent the SEM.
E-Stroop
For the positive versus neutral Stroop effect, there was a significant group × session interaction, β= 64.3, CI: 14.0 to 114.5, p = .01, Cohen's d = 0.25. Follow-up tests indicated that the DP+ group responded significantly slower to positive, compared with neutral words, during abstinence, compared with smoking as usual, p = .02 (see Figure 3). In contrast, the DP− group showed no effect of abstinence for the positive versus neutral contrast, p = .26. For the negative versus neutral Stroop effect, there were no main or interacting effects with depression status. However, there was a sex × session interaction, β=−46.4, CI: −92.9 to 0.18, p = .05. The pattern of means suggested that males responded more slowly to negative versus neutral words during abstinence, compared with smoking, p = 0.02, whereas there was no abstinence effect among females, p = 0.53 (see Figure 2).
Figure 3.
Median RT (milliseconds) to positive, neutral, and negative stimuli during the Emotional Stroop task for each group (DP+ vs. DP−) by session (abstinent vs. smoking-as-usual) condition. Error bars represent the SEM.
Discussion
This is the first study to evaluate whether depression-prone smokers are more sensitive to cognitive deficits during smoking abstinence compared with smokers who are not prone to depression. We provided initial evidence that depression-prone smokers exhibited abstinence-induced deficits in recognition memory and a shift in attentional bias for positive emotional stimuli that were not apparent in smokers not prone to depression. Both groups exhibited abstinence-induced deficits in working memory, compared with smoking as usual and males demonstrated a stronger abstinence-induced attentional bias toward negative stimuli, compared with females. These findings suggest that depression-prone smokers may experience a unique pattern of cognitive deficits during abstinence characterized by difficulty encoding and recalling new information and increased attentional capture of emotionally salient stimuli.
More importantly, these changes may add to the difficulties quitting and maintaining smoking abstinence in this population and suggest that improving cognition may be a plausible target for enhancing quit rates among depressed smokers.
The finding that the DP+ group performed worse on the recognition memory task during abstinence may partially be explained by the “resource-allocation hypothesis,” which postulates that the cognitive deficits associated with depression may be because of a reduced cognitive capacity, which limits the ability to remember and engage in effortful cognitive processing (Ellis & Ashbrook, 1988). In the current task, there was a 16-min delay between the initial presentation of the words and the recognition phase, during which they completed the N-back working memory task. Working memory tasks, such as the N-back task, are thought to require both storage and processing of information, whereas short-term recognition memory tasks may only require storage (Cowan, 2008). All smokers in our study tended to exhibit abstinence- induced deficits in working memory. However, only the DP+ group performed worse on the recognition memory task during abstinence compared with smoking-as-usual. The demanding N-back task may have depleted cognitive resources in the DP+ group and produced the observed abstinence-induced decrement in recognition memory suggesting that impairment in recognition memory during abstinence may be specific to depression-prone smokers. Our data also suggest that DP+ females exhibited greater abstinence-induced deficits in working memory compared with females with no history of depression. This is consistent with evidence that females report higher rates of depressive symptoms and a stronger relationship between depression and smoking (Pratt & Brody, 2010; Weinberger et al., 2012). Perhaps smoking improves the ability of depression-prone smokers to allocate resources to cognitively demanding tasks, particularly among females and this, in turn, reinforces smoking behavior. This difficulty sustaining concentration or remembering new information may partially explain why cognitive–behavioral strategies for smoking cessation show limited success among depression-prone smokers (Brown et al., 2007). Therefore, cognitive training strategies to improve short-term recognition memory may enhance the ability of depression-prone smokers to access and implement the strategies taught in cognitive-behavioral smoking cessation treatment.
The cognitive decrements observed during abstinence may accompany abstinence-induced cognitive biases common in depression. In the current study, we observed that depression-prone smokers exhibited a stronger abstinence-induced attentional bias for positive stimuli when compared with neutral stimuli. Specifically, after overnight abstinence from smoking, the DP+ group responded more slowly to positive compared with neutral stimuli, which is thought to reflect the allocation of resources to emotionally salient stimuli (Epp et al., 2012). These data are also consistent with those from the larger study that found that smoking abstinence increased negative affect across all smokers, but the DP+ group also showed greater changes in positive affect (Audrain-McGovern et al., in press). In addition, depression-prone smokers experienced a greater loss of pleasure from other reinforcers during nicotine withdrawal suggesting that smoking may regulate positive affect, rather than simply reducing negative affect for depression-prone smokers (Audrain-McGovern et al., in press). Perhaps the reduction in the rewarding effects of other reinforcers during nicotine withdrawal contributed to the abstinence-induced attentional bias to positive stimuli in the DP+ group. In the current study, abstinence-induced attentional bias to negative stimuli was only evident among males. This is partially consistent Audrain-McGovern and colleagues (in press) suggesting that abstinence alters the salience of negative stimuli, regardless of depression status. These findings highlight, for the first time, a more complex model of the relationship between depression status and the increased risk of smoking relapse. Cognitive intervention strategies, such as attentional retraining techniques, have been successful in modifying attentional bias and reducing anxiety and depression symptoms (Beard, Sawyer, & Hofmann, 2012; Hakamata et al., 2010; Wells & Beevers, 2010) and improving alcohol dependence treatment outcome (Cox, Fadardi, Intriligator, & Klinger, 2014; Schoenmakers et al., 2010; Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011). Perhaps these strategies can be adapted to address the disproportionate loss of positive affect depression-prone smokers during abstinence by translating the attentional bias toward positive stimuli to enhance engagement in positively reinforcing behaviors.
Cognitive deficits associated with smoking abstinence and depression may share common neural substrates and can be explained from a neurobiological perspective. For example, depression is associated with hippocampal atrophy (Sheline, Sanghavi, Mintun, & Gado, 1999; Sheline, Wang, Gado, Csernansky, & Vannier, 1996), a brain structure responsible for the transfer of short-term associations into long-term memory storage (Scoville & Milner, 1957).
Research in rodents has shown that withdrawal from nicotine is associated with deficits in hippocampal-dependent learning (Davis, James, Siegel, & Gould, 2005; Gould et al., 2012; Kenney & Gould, 2008) suggesting that nicotine may reduce deficits in STM associated with hippocampal damage. Thus, abstinence effects in depression-prone smokers may be more pronounced on tasks that rely on hippocampal function and this may partially explain why the observed abstinence effect on short-term recognition memory was specific to depression-prone smokers. Procognitive medications that target nicotinic acetylcholine receptors (nAChRs) in the hippocampus may be a useful strategy for attenuating these memory deficits that may reduce relapse rates among depression-prone smokers. Moreover, hypoactivation in brain regions important for cognitive function (e.g., the right and left dorsolateral prefontal cortices [DLPFC]) has been linked with major depressive disorder (Foland-Ross & Gotlib, 2012; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007) and deficits in cognitive function during smoking abstinence (Falcone et al., 2013; Sweet et al., 2010). Recent evidence suggests that using noninvasive brain stimulation methods, such as transcranial DC stimulation (tDCS), to stimulate the DLPFC may reduce craving for cigarettes (Fregni et al., 2008) and reduce attentional bias for emotional stimuli in depressed patients (Brunoni et al., 2014). It remains to be seen whether tDCS represents an effective strategy for treating nicotine dependence among depressed smokers.
As the first study to examine whether depression-prone smokers experience unique cognitive deficits during smoking abstinence, there are several strengths including the assessment of multiple aspects of cognition. Previously, deficits in cognitive functioning associated with depression, such as attention and memory, have been studied independent from attentional biases (Gotlib & Joor-mann, 2010). By including measures of cognitive function and attentional bias, we were able to ascertain whether certain cognitive deficits are specific to depression-prone smokers whereas others are common across all smokers. Nevertheless, several limitations of the present study warrant mention. Because we did not assess for a current diagnosis of depression, our findings may not extend to smokers who meet criteria for current major depression. The E-Stroop task used in the current study utilized a blocked design and it possible that the observed differences in response time to positive versus neutral stimuli reflect a semantic priming effect from the previous trial, rather than attentional bias (Cox, Fadardi, & Pothos, 2006; Williams et al., 1996). In addition, although we did not include smoking words, it is possible that depressive symptoms moderate abstinence effects on the processing of smoking-related stimuli. With respect to the N-back task, we did not find significant abstinence effects on RT, which is in contrast to previous studies in our lab (Patterson et al., 2010; Patterson et al., 2009). It is possible that the visuospatial working memory task used in the current study was not sensitive to abstinence effects and this highlights the need for more standardized measures of cognition such as the National Institute of Health Toolbox for Assessment of Neurological and Behavioral Function (http://www.nihtoolbox.org). Finally, we examined nontreatment seeking smokers after overnight abstinence and the only biochemical measure to verify abstinence was CO. Therefore, we cannot determine whether these findings generalize to the larger population of smokers who want to quit smoking or whether these effects persist after longer periods of abstinence.
The present study provides initial evidence that, in addition to mild deficits in working memory, depressive symptoms may moderate abstinence-induced deficits in specific domains of cognitive function. Specifically, smokers with a history of depression exhibited deficits in short-term recognition memory and a shift in attentional bias for positive stimuli during abstinence, compared with smoking-as-usual. The greater cognitive burden during abstinence among depressed smokers may prompt relapse in an effort to restore functioning to precessation levels that may partially explain the high prevalence of smoking and increased risk of relapse. Thus, these findings may help identify novel targets for nicotine dependence treatment aimed at attenuating these specific deficits to improve cessation rates.
Acknowledgments
This study was supported by National Institute on Drug Abuse R21 DA031946 (JAM). The NIH had no role in the study beyond financial support. All authors made substantial contributions and all have approved the final manuscript. The authors have no conflicts of interest to report.
Footnotes
Analyses were conducted with the full sample of 87 and were not substantially different from the subset of matched groups. The DP+ group showed declines in STM during abstinence compared with smoking, whereas the DP− group did not (interaction p = .026). There were small decrements in working memory accuracy during abstinence (p = .05), but this did not interact with depression status. During the Emotional Stroop task, all smokers showed an attentional bias toward negative stimuli during abstinence (p = .05), but the DP+ group also showed an attentional bias toward positive stimuli (interaction p = .09).
Contributor Information
Rebecca Ashare, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania.
Andrew A. Strasser, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania
E. Paul Wileyto, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania.
Jocelyn Cuevas, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania..
Janet Audrain-McGovern, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania..
References
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76(Pt B):581–591. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Wileyto EP, Ashare RL, Cuevas J, Strasser AA. Reward and affective regulation in depression-prone smokers. Biological Psychiatry. doi: 10.1016/j.biopsych.2014.04.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43:724–740. doi: 10.1016/j.beth.2012.01.002. doi:10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: A meta-analysis. Psychological Medicine. 2013;43:2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. doi:10.1016/S0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, Miller IW. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research. 2007;9:721–730. doi: 10.1080/14622200701416955. doi:10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Zanao TA, Vanderhasselt MA, Valiengo L, de Oliveira JF, Boggio PS, Felipe F. Enhancement of affective processing induced by bifrontal transcranial direct current stimulation in patients with major depression. Neuromodulation. 2014;17:138–142. doi: 10.1111/ner.12080. doi:10.1111/ner.12080. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, Baile WF. The effects of depressed mood on smoking cessation: Mediation by postcessation self-efficacy. Journal of Consulting and Clinical Psychology. 2003;71:292–301. doi: 10.1037/0022-006x.71.2.292. doi: 10.1037/0022-006X.71.2.292. [DOI] [PubMed] [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Progress in Brain Research. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. doi:10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Intriligator JM, Klinger E. Attentional bias modification for addictive behaviors: Clinical implications. CNS Spectrums. 2014:1–10. doi: 10.1017/S1092852914000091. doi:10.1017/S1092852914000091. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-Stroop test: Theoretical considerations and procedural recommendations. Psychological Bulletin. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. doi:10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. The Journal of Neuroscience. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. doi:10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I–effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. doi:10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: Effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. doi:10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Elibero A, Evans DE. Attentional bias for smoking and affective stimuli: A Stroop task study. Psychology of Addictive Behaviors. 2006;20:490–495. doi: 10.1037/0893-164X.20.4.490. doi:10.1037/0893-164X.20.4.490. [DOI] [PubMed] [Google Scholar]
- Ehlis AC, Bahne CG, Jacob CP, Herrmann MJ, Fallgatter AJ. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: A functional near-infrared spectroscopy (fNIRS) study. Journal of Psychiatric Research. 2008;42:1060–1067. doi: 10.1016/j.jpsychires.2007.11.011. doi:10.1016/j.jpsychires.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Ellis HC, Ashbrook PW. Resource allocation model of the effects of depressed mood states on memory. In: Fiedler K, Forgas JP, editors. Affect, cognition, and social behavior: New evidence and integrative attempts. Hogrefe & Huber Pub.; Göttingen: 1988. pp. 25–43. [Google Scholar]
- Epp AM, Dobson KS, Dozois DJA, Frewen PA. A systematic meta-analysis of the Stroop task in depression. Clinical Psychology Review. 2012;32:316–328. doi: 10.1016/j.cpr.2012.02.005. doi:10.1016/j.cpr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Evins AE, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh A, Maurizio F. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. Journal of Clinical Psychopharmacology. 2008;28:660–666. doi: 10.1097/JCP.0b013e31818ad7d6. doi:10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, Laprate L, Detre JA, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addiction Biology. 2013 doi: 10.1111/adb.12051. doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Frontiers in Psychology. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. doi:10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE. Where's the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. doi:10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. Journal of Clinical Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. doi:10.4088/JCP.v69n0105. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3:389–395. doi:10.1037/1064-1297.3.4.389. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. doi:10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. doi:10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacology Biochemistry and Behavior. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. doi:10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. doi:10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. doi:10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. doi:10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neuro-circuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. doi:10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Smith SS, Jorenby DE, Piper ME, Fiore MC, Baker TB. Depression predicts smoking early but not late in a quit attempt. Nicotine & Tobacco Research. 2007;9:677–686. doi: 10.1080/14622200701365301. doi:10.1080/14622200701365301. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould T. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular Neurobiology. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. doi:10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. doi:10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addictive Behaviors. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nature Reviews Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. doi:10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: Effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15:21–36. doi: 10.1037/1064-1297.15.1.21. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. doi:10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levine MD, Marcus MD, Perkins KA. A history of depression and smoking cessation outcomes among women concerned about post-cessation weight gain. Nicotine & Tobacco Research. 2003;5:69–76. doi: 10.1080/1462220021000060455. doi:10.1080/1462220021000060455. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addictive Behaviors. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. doi:10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiology of Learning and Memory. 2011;96:553–563. doi: 10.1016/j.nlm.2011.06.006. doi:10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor R, Moolchan E, Heishman S. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser A, Siegel S, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. doi:10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper N, Rodu B. Evidence of sex differences in the relationship between current tobacco use and past-year serious psychological distress: 2005–2008 National Survey on Drug Use and Health. Social Psychiatry and Psychiatric Epidemiology. 2013;48:1261–1271. doi: 10.1007/s00127-012-0644-0. doi:10.1007/s00127-012-0644-0. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58:193–198. doi: 10.1037/h0049234. doi:10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Phaf RH, Kan K-J. The automaticity of emotional Stroop: A meta-analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:184–199. doi: 10.1016/j.jbtep.2006.10.008. doi:10.1016/j.jbtep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive Behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. doi:10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief, April. 2010:1–8. [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug and Alcohol Dependence. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. doi:10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11. doi: 10.1136/jnnp.20.1.11. doi:10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. The Journal of Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. doi:10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. doi:10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. doi:10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne SC, Nunes EV, Jiang H, Tyson C, Rotrosen J, Reid MS. The relationship between depression and smoking cessation outcomes in treatment– seeking substance abusers. The American Journal on Addictions. 2010;19:111–118. doi: 10.1111/j.1521-0391.2009.00015.x. doi:10.1111/j.1521-0391.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- SRNT Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. doi:10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Mulligan RC, Finnerty CE, Jerskey BA, David SP, Cohen RA, Niaura RS. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Research. 2010;183:69–74. doi: 10.1016/j.pscychresns.2010.04.014. doi:10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Carter BL, Robinson JD, Wetter DW, Lam CY, Kerst W, Cinciripini RM. Attentional bias is associated with incentive-related physiological and subjective measures. Experimental and Clinical Psychopharmacology. 2009;17:247–257. doi: 10.1037/a0016658. doi:10.1037/a0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. doi:10.1037/0893-164X.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. doi:10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of major depressive disorder and gender to changes in smoking for current and former smokers: Longitudinal evaluation in the US population. Addiction. 2012;107:1847–1856. doi: 10.1111/j.1360-0443.2012.03889.x. doi: 10.1111/j.1360-0443.2012.03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TT, Beevers CG. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition & Emotion. 2010;24:719–728. doi:10.1080/02699930802652388. [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science. 2011;22:490–497. doi: 10.1177/0956797611400615. doi:10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Wedgwood L, Niven H, Kay-Lambkin F. Smoking cessation and depression: Current knowledge and future directions. Drug and Alcohol Review. 2006;25:97–107. doi: 10.1080/09595230500459560. doi:10.1080/09595230500459560. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. doi:10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Riley WT. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10:1691–1715. doi: 10.1080/14622200802443569. doi:10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatrica Scandinavica. 1987;75:495–499. doi: 10.1111/j.1600-0447.1987.tb02824.x. doi:10.1111/j.1600-0447.1987.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Corenthal C, Wilson S. A self-report scale to diagnose major depressive disorder. Archives of General Psychiatry. 1986;43:1076–1081. doi: 10.1001/archpsyc.1986.01800110062008. doi:10.1001/archpsyc.1986.01800110062008. [DOI] [PubMed] [Google Scholar]