Abstract
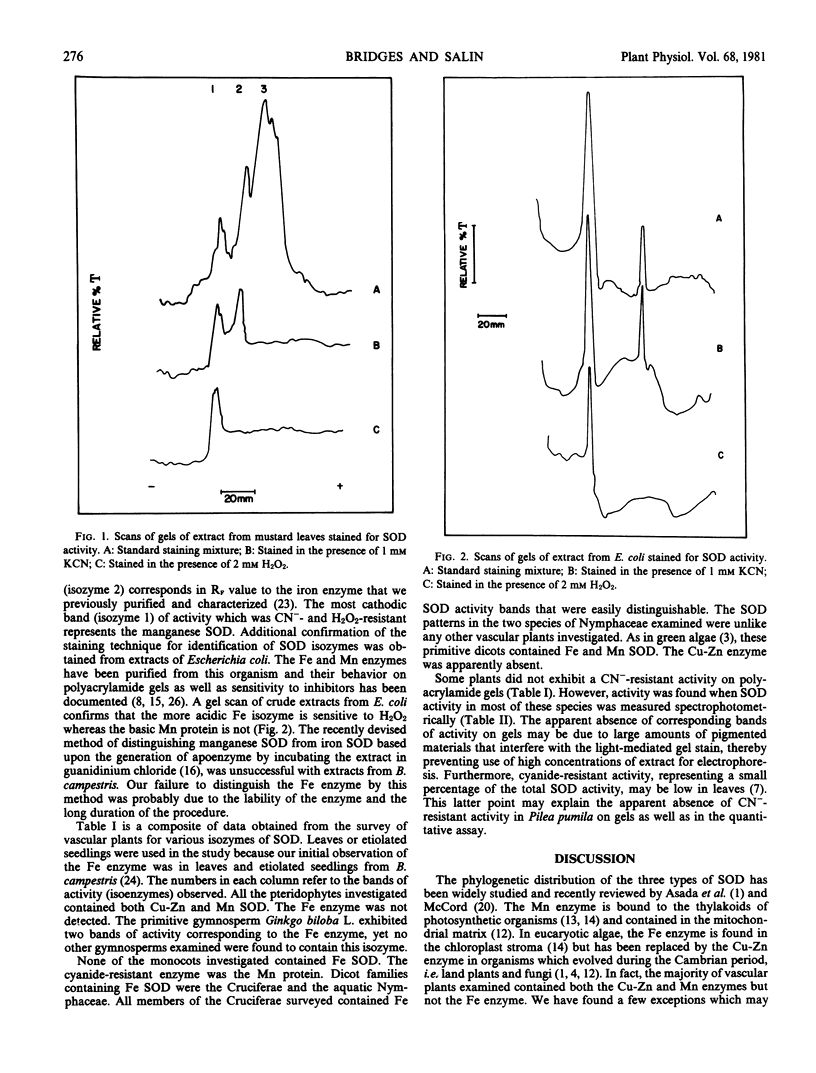
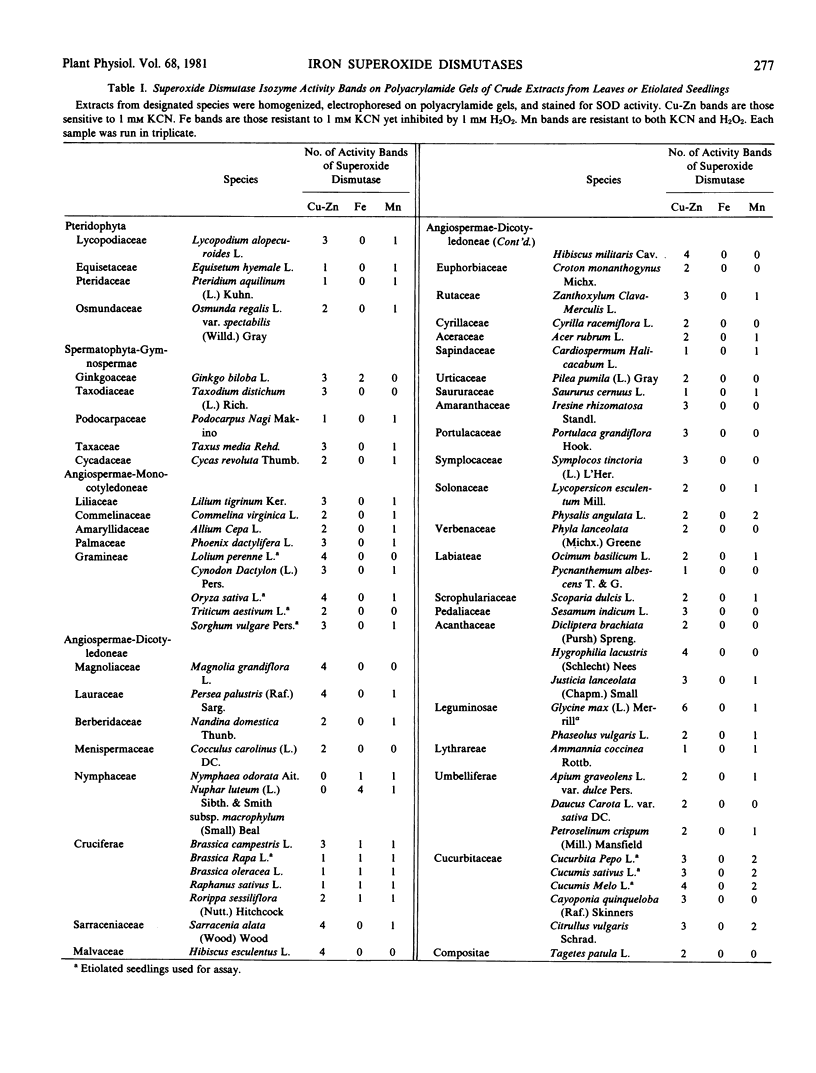
Superoxide dismutases (EC 1.15.1.1) in vascular plants representing different evolutionary levels were characterized using polyacrylamide gel electrophoresis. The three forms of the enzyme were distinguished from each other based on the following criteria: a) the Cu-Zn enzyme is sensitive to cyanide wherease the Fe and Mn enzymes are not; and b) the Cu-Zn and Fe enzymes are inhibited by H2O2 whereas the Mn enzyme is H2O2-resistant. Of the 43 plant families investigated, the Fe-containing superoxide dismutase was found in three families: Gingkoaceae, Nymphaceae, and Cruciferae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Kanematsu S., Takahashi M., Kona Y. Superoxide dismutases in photosynthetic organisms. Adv Exp Med Biol. 1976;74:551–564. doi: 10.1007/978-1-4684-3270-1_46. [DOI] [PubMed] [Google Scholar]
- Asada K., Kanematsu S., Uchida K. Superoxide dismutases in photosynthetic organisms: absence of the cuprozinc enzyme in eukaryotic algae. Arch Biochem Biophys. 1977 Feb;179(1):243–256. doi: 10.1016/0003-9861(77)90109-6. [DOI] [PubMed] [Google Scholar]
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Giannopolitis C. N., Ries S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977 Feb;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L. E., Cammack R., Schwitzguebel J. P., Palmer J. M., Hall D. O. Intracellular localization, isolation and characterization of two distinct varieties of superoxide dismutase from Neurospora crassa. Biochem J. 1980 May 1;187(2):321–328. doi: 10.1042/bj1870321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Kanematsu S., Asada K. Ferric and manganic superoxide dismutases in Euglena gracilis. Arch Biochem Biophys. 1979 Jul;195(2):535–545. doi: 10.1016/0003-9861(79)90380-1. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Lengfelder E., Elstner E. F. Cyanide insensitive iron superoxide dismutase in Euglena gracilis. Comparison of the reliabilities of different test systems for superoxide dismutases. Z Naturforsch C. 1979 May-Jun;34C(5-6):374–380. doi: 10.1515/znc-1979-5-609. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Cammack R., Hall D. O. Purification and physicochemical properties of superoxide dismutase from two photosynthetic microorganisms. Biochim Biophys Acta. 1976 Jul 8;438(2):380–392. doi: 10.1016/0005-2744(76)90255-2. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Isolation and characterization of an iron-containing superoxide dismutase from a eucaryote, Brassica campestris. Arch Biochem Biophys. 1980 May;201(2):369–374. doi: 10.1016/0003-9861(80)90524-x. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]



