Abstract
Acute kidney injury (AKI) is a major kidney disease associated with poor clinical outcomes both in short- and long-term. Autophagy is a cellular stress response that plays important roles in the pathogenesis of various diseases. Autophagy is induced in proximal tubules during AKI. A renoprotective role of autophagy in AKI has been demonstrated by pharmacological and genetic inhibitory studies. The role of autophagy in kidney recovery and repair from AKI, however, remains largely unknown. A dynamic change of autophagy during the recovery phase of AKI seems to be important for tubular proliferation and repair. In renal fibrosis, autophagy may either promote it through inducing tubular atrophy and decomposition or prevent it by mediating intracellular degradation of excessive collagen. Further research is expected to gaining understanding of the regulation of autophagy in kidney injury and repair, elucidate the pathological roles of autophagy in renal fibrosis, and discover therapeutic targets for treating AKI and preventing its progression to chronic kidney disease.
Keywords: autophagy, acute kidney injury, kidney repair, apoptosis, fibrosis
Introduction
Autophagy is an ‘auto-digestive’ process in a cell that promotes the delivery of intracellular components from the cytoplasm to lysosomal or vacuolar compartments for terminal degradation and recycling[1]. In the past decade, there has been an intense interest in elucidating the basic molecular mechanism of autophagy and defining its roles in human health and disease [2]. In renal systems, both in vitro cell culture and in vivo animal models and human kidney studies suggest that autophagy plays important roles in the maintenance of renal function under both physiological and pathological conditions [3, 4]. This review summarizes the current understanding of the role and regulation of autophagy in acute kidney injury (AKI) and repair.
Autophagy in AKI
Acute kidney injury (AKI) is a major renal disease that is associated with significant mortality and the development of chronic kidney disease and end stage renal disease. The pathogenesis of acute kidney injury is multifactorial. Pathologically, AKI is characterized by tubular cell injury and death. Recent studies have demonstrated that autophagy is rapidly induced during AKI to protect tubular cells from injury and death [5].
Renal ischemia–reperfusion injury (IRI) is a main cause of AKI that is frequently associated with cardiac surgery, renal vascular obstruction, and kidney transplantation. In 2007, Chien et al. showed that autophagic genes, namely beclin-1 and LC3, were induced during renal IRI in rats [6]. Interestingly, beclin-1 and LC3 expression was shown along with apoptosis in injured renal tubules and Bcl-XL could block their expression as well as tubular apoptosis [6]. Subsequent work by Suzuki et al. further showed autophagosome-like structures in renal tubular cells during renal IRI in mice and notably, also in human kidney transplants [7]. In 2010, we demonstrated autophagy induction during hypoxia incubation of renal tubular cells and renal IRI in mice. The induction was rapidly, prior to tissue damage or tubular apoptosis [8]. Consistently, later studies using GFP-LC3 transgenic mice showed an increased formation of GFP-LC3 puncta in proximal tubules during renal IRI [9, 10].
Autophagy is also induced in experimental models of nephrotoxic AKI. This has been shown during cisplatin treatment of various renal proximal tubular cell lines [11–13]. Autophagy induction was indicated by autophagosome formation and LC3-II accumulation. Similar to hypoxic incubation, autophagy was induced by cisplatin within hours preceding the occurrence of apoptosis. Importantly, detailed examination by electron microscopy revealed a time-dependent increase of autophagic vacuoles in kidney proximal tubular cells after cisplatin treatment of C57BL/6 mice [11]. Cisplatin-induced autophagy was further monitored in kidney tissues of GFP-LC3 mice, consistently unveiling autophagy induction in proximal tubules [13, 14].
The role of autophagy in renal IRI was originally suggested to participate in tubular cell death [6, 7]. For example, Suzuki et al. showed that autophagy inhibitors suppressed H2O2-induced cell death in HK2 cells [7]. However, recent studies using pharmacological, and particularly genetic approaches, have provided compelling evidence for a renoprotective role of autophagy in several experimental models of AKI [5]. We showed that in cultured renal tubular cells, pharmacological inhibitors of autophagy as well as autophagy gene knockdown enhanced apoptosis during hypoxia incubation and anoxia-reoxygenation treatment. Importantly, inhibition of autophagy by chloroquine or 3-methyladenine worsened renal IRI in mice, as indicated by renal function, histology, and tubular apoptosis, supporting a protective role of autophagy in ischemic AKI [8]. In a conditional knockout mouse model, Atg5-deficiency in proximal tubules dramatically sensitizes the kidneys to ischemic injury, resulting in impaired kidney function, accumulation of damaged mitochondria as well as increased tubular cell apoptosis and necrosis. These findings further highlight a critical role autophagy plays in maintaining tubular cell integrity and promoting cell survival during ischemic AKI [9, 10]. A renoprotective role of autophagy has also been shown in cisplatin-induced AKI. Under this condition, inhibition of autophagy by chloroquine aggravated, whereas up-regulation of autophagy by rapamycin attenuated renal function loss and tubular damage. Consistently, proximal tubule-specific Atg7 knockout mice exhibited increased renal injury compared to their wild-type littermates [15]. Furthermore, in proximal-tubule specific Atg5 knockout mice, cisplatin induced more severe kidney injury, accompanied by a massive accumulation of protein aggregates as well as enhanced DNA damage and p53 activation [14]. In addition, autophagy has been suggested to protect kidneys against septic AKI. In rats subjected to cecal ligation and puncture (CLP), autophagy increased transiently in kidneys at the early stage of sepsis and then went down toward to the basal level at the late stage. Interestingly, this decline of autophagic response is associated with the development of kidney injury induced by sepsis. Moreover, knockdown of ATG7 exaggerated, whereas preincubation of rapamycin diminished tumor necrosis factor α-induced cell death in cultured renal tubular cells [16].
Autophagy in recovery from AKI
a. Dynamic changes of autophagy in renal tubules during injury and repair
It is noteworthy that in the aforementioned studies induction of autophagy in proximal tubular cells and in kidneys is mainly examined at a certain time point of AKI. Using a novel autophagy reporter mouse model in which a tandem RFP-EGFP-LC3 fusion protein is expressed ubiquitously under the CAG promoter, a recent study has revealed the dynamics and flux of autophagy in proximal tubules under stress conditions including starvation and ischemic AKI [17]. While the EGFP fluorescence is sensitive to the acidic environment in the lysosome, the RFP signal is more stable and remained. Therefore, colocalization of both EGFP and RFP fluorescence indicates early autophagic vesicles such as phagophores and autophagosomes. In contrast, autolysosomes appear red (RFP only) due to quenching of the EGFP fluorescence in the low pH environment [18]. Under normal conditions, nephrons expressed few EGFP and RFP puncta; however, renal IRI led to an increase in the numbers of both RFP and EGFP puncta at 1 day after IRI. The number of EGFP puncta returned to control levels at 3 days after IRI, whereas the high levels of RFP puncta persisted till 7 days after reperfusion. These findings suggest that autophagy was initiated at 1 day after IRI with increased formation of autophagosomes. As kidneys were recovering from injury at day 3, autophagosomes proceeded to fuse with lysosomes for clearance, indicating that resolution of autophagy was predominant during the recovery phase. mTOR was activated in proximal tubules after renal IRI and facilitated autophagy resolution. Of note, in the cells with inhibited mTOR and persistent RFP puncta, tubular proliferation was inhibited, suggesting that autophagic cells are less likely to divide for tubular repair [17].
b. Autophagy in renal fibrosis
Although renal tubular cells have the ability to regenerate after injury, tubular repair after severe or episodic AKI is mostly incomplete, resulting in renal interstitial fibrosis [19, 20]. Induction of autophagy and its contribution to fibrotic diseases has been suggested in the lung, liver and heart [21–23]. The pathological roles of autophagy in fibrosis in those organs vary greatly depending on the type of cells or tissues and pathological conditions [24]. Evidence thus far regarding autophagy in kidney fibrosis is mainly from the studies using UUO and TGF-β models [25]. Under these conditions, involvement of autophagy in either tubular atrophy or degradation of collagen has been suggested, which apparently contribute oppositely to the pathogenesis of renal fibrosis.
Autophagy in tubular atrophy during fibrosis
The role of atrophic changes in renal tubules in peritubular fibrosis has been well-documented. Earlier studies in rat models of microembolism- and renal ablation-induced fibrosis showed that focal fibrotic lesions with clusters of atrophic proximal tubules alternated with nonfibrotic areas containing normal tubules [26, 27]. The close spatial relationship between atrophic tubules and the development of interstitial fibrosis was further demonstrated in rat kidneys following IRI [28]. Importantly, proximal tubules in areas that had been previously damaged failed to differentiate. In contrast to surrounding tubules that did recover normally, these atrophic tubules showed significantly decreased expression of differentiation markers such as Na+-K+-ATPase, ksp-cadherin, and meprin [28]. Myofibroblasts greatly proliferated in the interstitium adjacent to injured and regenerating tubules by 3 days of reperfusion, and notably, continued to proliferate around the atrophic tubules with undifferentiated tubular epithelium. As a result, by 14 days of reperfusion, foci of tubulointerstitial fibrosis formed around the atrophic tubules [28]. Interestingly, there was a persistent increase of PDGF-B in atrophic tubules and this phenotype change of tubular cells was associated with an increased expression of PDGFR-β in adjacent interstitial fibroblasts, suggesting a paracrine ligand-receptor couple that may have been responsible for fibroblast proliferation and ECM protein deposition in these renal fibrosis models [26–28].
The connection of autophagy and tubular atrophy during renal fibrosis was initially suggested in mice subjected to UUO [29]. In this study, autophagy was activated in obstructed tubules, as indicated by accumulation of autophagosomes, increased expression of Beclin1, and conversion of LC3-I to LC3-II. These changes were accompanied by an increased lysosomal activity, further suggesting induction of autophagic flux in obstructed tubules. Along with autophagy, tubular apoptosis was also induced in obstructed tubules. Importantly, the development of tubular atrophy correlated with autophagy and apoptosis in a time-dependent manner, suggesting that autophagy may act in concert with apoptosis to induce tubular atrophy and nephron loss in this obstructive uropathy [29]. Koesters et al. further suggested the involvement of autophagy in tubular degeneration and fibrosis using a tetracycline-controlled transgenic mouse model that overexpresses TGF-β1 in renal tubules [30]. They showed that sustained expression of TGF-β1 induced autophagy in tubular cells, as indicated by strong immunostaining of LC3 and formation of autophagic vacuoles under EM. Importantly, the tubules with autophagic features were collapsed, with cell debris filling in remnant lumina. These tubular changes were associated with widespread peritubular fibrosis. The nuclei of such degenerating cells displayed a normal chromatin pattern without positive TUNEL staining for apoptosis. Together, these results suggest that autophagy may be a main force driving tubular dedifferentiation and decomposition in TGF-β1-induced fibrosis [30].
Autophagic degradation of collagen I during fibrosis
Intracellular degradation of ECM proteins including collagen by autophagy has been suggested in alveolar epithelial cells and pulmonary fibroblasts of fibrotic lung tissues as well as cardiac fibroblasts in cardiac fibrosis [24]. The negative regulation of matrix protein by autophagy was recently showed in primary cultured mouse kidney mesangial cells (MMC) [31]. Both protein and mRNA levels of collagen I was induced by TGF-β1 in MMC. Bafilomycin A, an autophagy inhibitor that blocks autolysosomal degradation, further enhanced the protein level of collagen I without affecting the mRNA expression. Under this condition, colocalization of collagen I with LC3 and LAMP1was also increased. Consistent with the pharmacological findings, genetic knockdown of Beclin1 accelerated collagen I protein accumulation by TGF- β1. Compared to wild-type cells, MMC obtained from heterozygous beclin1 knockout (beclin1−/−) mice had increased levels of collagen aggregates even under normal conditions, and treatment of TGF-β1 further enhanced aggregated collagen I. Together, these results have revealed a role of autophagy as a protective mechanism to prevent excess collagen accumulation in the kidney [31].
Conclusions
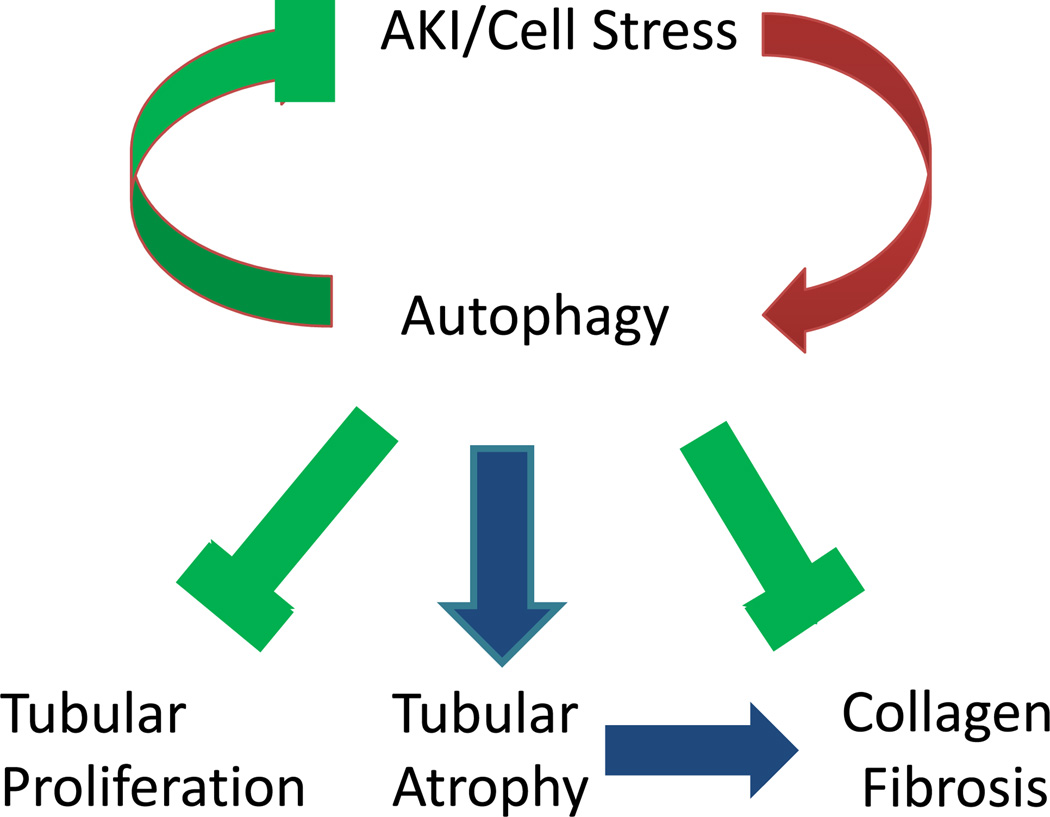
In conclusion, autophagy is induced in kidneys in response to AKI and protects against kidney injury. During the recovery phase of AKI, resolution of autophagy may promote cell proliferation for tubular regeneration and repair. The role of autophagy in renal fibrosis after AKI is poorly understood. Evidence obtained from recent studies using UUO and TGF-β models have demonstrated dual roles of autophagy. On one hand, persistent activation of autophagy may contribute to tubular atrophy and thereby promote kidney fibrosis. On the other hand, autophagy can prevent fibrosis by mediating intracellular degradation of excessive collagen (Figure 1). Further research should focus on the regulation of autophagy in kidney injury and repair as well as the role of autophagy in renal fibrosis following AKI. A comprehensive understanding of the regulation and pathological roles of autophagy in AKI and its recovery will facilitate the discovery of genetic and pharmacologic approaches for treating AKI and preventing AKI progression.
Figure 1.
Diagram depicting the roles of autophagy in AKI and its recovery
Acknowledgements
The work was supported in part by grants from National Natural Science Foundation of China [81370791] and the National Institutes of Health and Department of Veterans Administration of USA.
Footnotes
Disclosure: The authors declared no competing interests.
Reference
- 1.Crotzer VL, Blum JS. Autophagy and adaptive immunity. Immunology. 2010;131:9–17. doi: 10.1111/j.1365-2567.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20:519–537. doi: 10.1089/ars.2013.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8:1009–1031. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston MJ, Dong Z. Autophagy in Acute Kidney Injury. Semin Nephrol. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien CT, Shyue SK, Lai MK. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation. 2007;84:1183–1190. doi: 10.1097/01.tp.0000287334.38933.e3. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 8.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–837. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 11.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–F787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Kuwana H, Shimamura Y, Ogata K, Taniguchi Y, Kagawa T, et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin Exp Nephrol. 2010;14:112–122. doi: 10.1007/s10157-009-0254-7. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Wang ZV, Hill JA, Lin F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J Am Soc Nephrol. 2014;25:305–315. doi: 10.1681/ASN.2013040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–155. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan HM, et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–292. doi: 10.1016/j.ajpath.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Aranguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, et al. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta. 2011;1812:23–31. doi: 10.1016/j.bbadis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Del Principe D, Lista P, Malorni W, Giammarioli AM. Fibroblast autophagy in fibrotic disorders. J Pathol. 2013;229:208–220. doi: 10.1002/path.4115. [DOI] [PubMed] [Google Scholar]
- 25.Ding Y, Choi ME. Regulation of Autophagy by TGF-beta: Emerging Role in Kidney Fibrosis. Semin Nephrol. 2014;34:62–71. doi: 10.1016/j.semnephrol.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Asano M, Abe K, Miyazaki M, Suzuki T, Hishida A. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dial Transplant. 2005;20:1559–1565. doi: 10.1093/ndt/gfh872. [DOI] [PubMed] [Google Scholar]
- 28.Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, et al. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol. 2009;174:1291–1308. doi: 10.2353/ajpath.2009.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem. 2012;287:11677–11688. doi: 10.1074/jbc.M111.308460. [DOI] [PMC free article] [PubMed] [Google Scholar]