Abstract
OBJECTIVE
Understanding the effect of diabetes as well as of alternative treatment strategies on cerebral structure is critical for the development of targeted interventions against accelerated neurodegeneration in type 2 diabetes. We investigated whether diabetes characteristics were associated with spatially specific patterns of brain changes and whether those patterns were affected by intensive versus standard glycemic treatment.
RESEARCH DESIGN AND METHODS
Using baseline MRIs of 488 participants with type 2 diabetes from the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) study, we applied a new voxel-based analysis methodology to identify spatially specific patterns of gray matter and white matter volume loss related to diabetes duration and HbA1c. The longitudinal analysis used 40-month follow-up data to evaluate differences in progression of volume loss between intensive and standard glycemic treatment arms.
RESULTS
Participants with longer diabetes duration had significantly lower gray matter volumes, primarily in certain regions in the frontal and temporal lobes. The longitudinal analysis of treatment effects revealed a heterogeneous pattern of decelerated loss of gray matter volume associated with intensive glycemic treatment. Intensive treatment decelerated volume loss, particularly in regions adjacent to those cross-sectionally associated with diabetes duration. No significant relationship between low versus high baseline HbA1c levels and brain changes was found. Finally, regions in which cognitive change was associated with longitudinal volume loss had only small overlap with regions related to diabetes duration and to treatment effects.
CONCLUSIONS
Applying advanced quantitative image pattern analysis methods on longitudinal MRI data of a large sample of patients with type 2 diabetes, we demonstrate that there are spatially specific patterns of brain changes that vary by diabetes characteristics and that the progression of gray matter volume loss is slowed by intensive glycemic treatment, particularly in regions adjacent to areas affected by diabetes.
Introduction
Findings from many cross-sectional and longitudinal population studies have shown that diabetes is associated with higher prevalence of global cognitive impairment and an increased risk of dementia (1–4). The underlying mechanisms of the relationship between diabetes and cerebral disease are still unclear (5). However, brain imaging studies using MRI have provided important insights into structural correlates of cognitive dysfunction in people with type 2 diabetes pointing to a convincing evidence for an association between diabetes and structural brain abnormalities such as cerebral atrophy and lacunar infarcts (6–10).
Besides studying the possible physiological contribution of diabetes to late-age brain pathology, another important research topic, which has received much less attention, is the influence of diabetes disease management strategies on brain structure. The Memory in Diabetes (MIND) substudy, embedded within the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (11,12), was designed to test the effects of early glycemic intervention on brain outcomes in older people with type 2 diabetes. Previously published baseline results from the ACCORD-MIND trial have shown that lower levels of baseline cognitive performance are associated with higher levels of HbA1c and diabetes duration (13). However the primary MRI analyses of the trial showed that although people in a intensive glycemic treatment group had less longitudinal decline in total brain volume at 40 months compared with those receiving a standard treatment strategy (P = 0.0007), no significant differences were found in the cognitive outcomes between the two treatment groups (14). This difference in findings may reflect the effect of several factors with a basis ranging from methodological to biological. Previous analyses investigated relatively global measures of brain volume changes, and possibly these changes were relatively restricted to regions mediating cognitive functions other than those tested in the trial. Also possible is that treatment-related changes in brain volume loss precede cognitive changes and are therefore detected first.
The current study investigated whether there are spatially heterogeneous patterns of structural brain changes that vary by diabetes characteristics and by glycemic treatment strategy. Advanced computer-based imaging pattern analysis methods, including volume-preserving spatial normalization that allows for accurate quantification of very localized brain changes (regional analysis of volumes examined in normalized space [RAVENS]) (15–17) and a new voxel-based analysis framework (optimally-discriminative voxel-based analysis [ODVBA]) (18), were applied on longitudinal MRI data from 488 ACCORD-MIND participants to detect imaging patterns that highlight potential spatial differences in the relationship between images and subject groups.
Our general hypothesis is that specific areas of the brain are particularly more vulnerable to diabetes effects. In this report we investigate diabetes duration and HbA1c levels, two main clinical variables relevant to diabetes and shown to be related to cognition (13). We also hypothesize that there are spatially specific patterns of longitudinal change that differ between the intensive and standard glycemic treatment arms and that those regions displaying longitudinal treatment effects will show similarities with those vulnerable to diabetes effects.
Research Design and Methods
Participants
ACCORD, described in detail elsewhere (11), was a randomized, multicenter, double two-by-two factorial parallel-treatment trial that tested the effect of treatment strategies to control blood glucose, blood pressure, and blood lipid concentrations on cardiovascular disease events. Participants targeted by ACCORD were aged 45–79 years and had type 2 diabetes, high HbA1c concentrations (>7.5% [>58 mmol/mol]), and a high risk for cardiovascular disease events suggested by significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease. Key exclusion criteria were frequent or recent serious hypoglycemic events, unwillingness to monitor glucose at home or inject insulin, BMI greater than 45 kg/m2, serum creatinine level greater than 1.5 mg/dL (133 μmol/L), or other serious illness. The exclusion criteria were applied before randomization. All ACCORD participants were randomly assigned to receive intensive glycemic treatment targeting HbA1c to less than 6.0% (42 mmol/mol) or standard glycemic treatment targeting HbA1c to 7.0–7.9% (53–63 mmol/mol).
The ACCORD-MIND study design has been described elsewhere (12). From within the overall ACCORD study population, 2,977 participants who had been randomly assigned to treatment groups were recruited into the ACCORD-MIND. A cognitive test battery was administered to MIND participants at baseline and at 20 months and 40 months after randomization. The cognitive battery tested for verbal memory (Rey Auditory Learning Test [RAVLT], mean number of recalled words over five trials), processing speed (Digit Symbol Substitution Test [DSST], number of cells correctly filled in), and executive function (modified Stroop Test (STROOP), time to finish the interference challenge).
The MRI substudy participants have been previously described (14). A total of 614 participants had a successful baseline scan, from which 503 had a successful 40-month follow-up scan. After processing of image data, 15 participants were excluded from the analysis based on visual quality control (QC) due to insufficient quality of the final image maps. Reasons for missing and excluded scans were similarly distributed across treatment groups (Supplementary Table 1). The final sample included data from 488 ACCORD-MIND participants. The treatment groups had similar baseline characteristics and cardiovascular risk factors (Supplementary Table 2).
MRI Scan Protocol
The standardized MRI scan protocol (12) included axial, coronal, and sagittal gradient echo scout views that served as localizers; a three-dimensional fast spoiled gradient-echo T1-weighted sequence (repetition time [TR], 21; flip angle, 30; echo time [TE], 8) to image brain structure, and a two-dimensional axial fast spin-echo (FSE) fluid-attenuated inversion recovery (TR, 8,000; inversion time, 2,000; TE, 100) and proton-density/T2-weighted (TR, 3,200; TE1, 27; TE2, 120) sequences to image pathology. Voxel size was 1.5 × 0.9 × 0.9 mm for the three-dimensional T1 sequence and 3.0 × 0.9 × 0.9 mm for the two-dimensional sequences. Scanners at the four MRI sites had identical field strength (1.5 Tesla) but were from three different manufacturers.
Monthly MRI QC was managed by the University of Pennsylvania Department of Radiology. The procedures followed the American College of Radiology’s (ACR) MRI QC Program, which is based on the analysis of data acquired from scanning an ACR–National Electrical Manufacturers Association QC phantom. Each field center was responsible for keeping its ACCORD scanners within ACR performance specifications and for sending monthly digital images to the MRI QC center for in-house review. According to ACR phantom analyses, MRI scanner performance was stable across the MRI sites and over the duration of the study. In vivo confirmation of scanner performance over time is reflected by the stability of subject intracranial volumes (ICV) over time (baseline mean ICV, 1,132.34 cm3; follow-up mean ICV, 1,132.32 cm3; P = 0.47 by paired t test).
Image Processing
The MRIs were first preprocessed using previously validated and published techniques (16). The preprocessing steps included 1) alignment to the anterior commissure–posterior commissure plane; 2) removal of extracranial material (skull-stripping) and cerebellum; 3) N3 bias correction (19); 4) tissue segmentation into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), and lateral ventricles (VN) (20); 5) nonlinear image warping (21) to a common brain atlas using a Montreal Neurological Institute template, as previously described (22); and 6) formation of regional volumetric maps, called RAVENS maps (15–17), using tissue-preserving image warping to enable comparative analysis of tissue volumes in the common template space.
RAVENS map intensity values quantify the regional distribution of GM, WM, and ventricular CSF, with one RAVENS map for each tissue type. In particular, RAVENS values in the template’s (stereotaxic) space are directly proportional to the volume of the respective structures in the original brain scan. Therefore, regional volumetric measurements and comparisons are performed by measurements and comparisons of the respective RAVENS maps. For example, patterns of relatively lower regional GM volume in the temporal lobe are quantified by patterns of RAVENS decrease in the temporal lobe in the template space. The RAVENS algorithm has been extensively validated and applied to a variety of studies (15,23).
The RAVENS maps were normalized by individual ICV to adjust for global between-person differences in intracranial size and down-sampled to 2 × 2 × 2 mm. Estimates of region-specific longitudinal volumetric change were obtained from the baseline and follow-up RAVENS maps by calculating the intensity difference at each voxel between the two time points. These rates of change are denoted as dRAVENS.
Statistical Analyses
For the pattern analysis of the imaging data, we used the diabetes duration and HbA1c levels at baseline as dependent variables for the cross-sectional analysis, and the glycemic intervention arm (i.e., standard or intensive treatment) as the dependent variable for the longitudinal analysis. To investigate regional patterns of brain change associated with longitudinal change in cognition, an additional analysis was done using cognitive test scores as dependent variables.
In our initial statistical analysis on baseline data using the standard voxel-based morphometry (VBM) approach, we identified trends for diabetes duration (e.g., regions with significance P < 0.01), but no regions showed significance after false discovery rate (FDR) correction (24) using a significance threshold of q ≤ 0.05. Accordingly, we applied a new voxel-based analysis methodology, ODVBA (18), taking into account the superiority of ODVBA to the classical VBM approach in sensitivity and spatial specificity, which has been demonstrated on both structural and functional MRI (25,26).
To leverage the strengths of ODVBA, which is a discriminative method, the values for diabetes duration and HbA1c were dichotomized and used in the analysis as class variables by grouping the subjects with values lower than the 20th percentile and with values higher than the 80th percentile of each variable into the “low” and “high” groups, respectively (27). The low diabetes duration group included subjects with 0–4 years of diabetes (average 2.5 ± 1.3), compared with 15–40 years (average 21 ± 6.3) for the high diabetes duration group. Participants in the low HbA1c group had HbA1c values between 5.5% (37 mmol/mol) and 7.4% (57 mmol/mol), with an average 7.1% ± 0.3% (54 ± 3 mmol/mol), whereas the high HbA1c group had values between 8.8% (73 mmol/mol) and 11.8% (105 mmol/mol), with an average 9.6% ± 0.7% (81 ± 8 mmol/mol).
In the cross-sectional analysis, ODVBA was applied on baseline imaging values of GM, WM, and VN RAVENS maps. Before applying ODVBA, RAVENS maps were corrected for age, sex, and systolic blood pressure by fitting a voxel-by-voxel generalized linear model to the RAVENS values against the covariates and by calculating the residual of the regression at each voxel. ODVBA constructed a voxel-by-voxel image of significance derived from the optimized spatial adaptive filtering applied to the data, which highlights potential spatial differences in the relationship between images and subject groups. Because many statistical tests (i.e., one on each individual voxel) were being conducted, the final maps were corrected for multiple comparisons using FDR correction. The clusters that exceeded a significance threshold of q ≤ 0.05 after correction for multiple comparisons are reported.
In the longitudinal analysis, ODVBA was applied on GM, WM, and VN dRAVENS maps, which capture longitudinal rate of regional change in tissue volume in a given subject’s brain at each voxel, for detecting imaging patterns of volume change associated with group differences between subjects in intensive and standard glycemic treatment arms. FDR-corrected values are reported at a significance level of q ≤ 0.05.
For the analysis of cognition, the longitudinal rate of change of each participant in each cognitive test was estimated by linear regression using baseline, 20-month, and 40-month test scores for DSST, RAVLT, and STROOP. The subjects were then stratified into “declining” and “nondeclining” cognition categories based on the 20th and 80th percentiles, similar to what was done for diabetes duration and HbA1c. ODVBA was applied using GM, WM, and VN dRAVENS maps of participants in the two groups for each test.
Results
Patterns of Regional Volume Change Associated With Diabetes Characteristics
Regional pattern analysis using ODVBA was applied on baseline GM, WM, and VN RAVENS maps, which were corrected for age, sex, and systolic blood pressure, to identify group differences between subject categories for low and high values of diabetes duration and HbA1c.
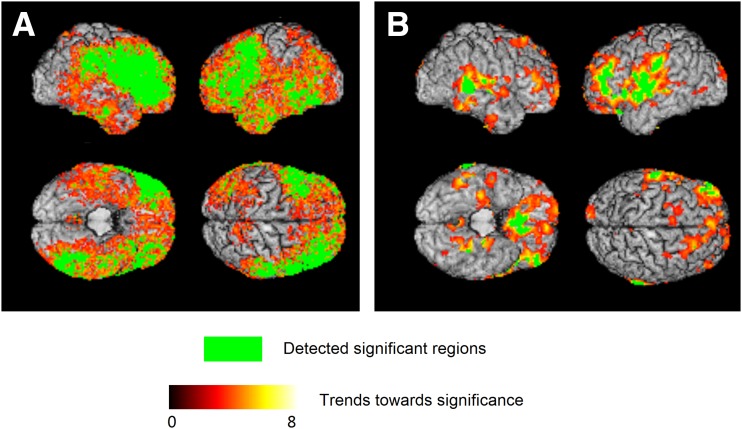
The baseline characteristics of participants with low and high diabetes duration are given in Table 1. Longer diabetes duration (more than 14 years), compared with relatively short duration of diabetes (less than 5 years), was correlated with a relatively lower regional GM volume, particularly in the frontal lobes, left temporal lobe, right parietal lobe, and limbic cortex. The specific regions that were affected were the middle frontal gyrus, precentral gyrus, and inferior frontal gyrus left and right; middle temporal gyrus, inferior temporal gyrus, and lateral occipitotemporal gyrus left; angular gyrus right; and parietal cingulate region right and left. The areas of significant group differences between GM RAVENS maps and short versus long diabetes duration after FDR correction (q < 0.05) are shown in Fig. 1A. A trend of positive association (P < 0.05) of lateral ventricular enlargement and diabetes duration was detected, but no voxels survived the FDR correction (Supplementary Fig. 1).
Table 1.
Baseline characteristics comparing low diabetes duration and high diabetes duration groups
| Diabetes duration |
|||||
|---|---|---|---|---|---|
| Overall (N = 488) | Short (n = 100) | Middle (n = 288) | Long (n = 100) | P value* | |
| Age (years) | 62.2 ± 5.6 | 60.7 ± 4.9 | 62.1 ± 5.8 | 63.9 ± 5.5 | <0.0001 |
| Women | 223 (45.7) | 53 (53.0) | 121 (42.0) | 49 (49.0) | 0.1247 |
| Education | 0.6944 | ||||
| Less than high school graduate | 45 (9.2) | 6 (6.0) | 27 (9.4) | 12 (12.0) | |
| High school graduate or GED | 119 (24.4) | 24 (24.0) | 70 (24.3) | 25 (25.0) | |
| Some college/technical school | 163 (33.4) | 39 (39.0) | 91 (31.6) | 33 (33.0) | |
| College graduate or more | 161 (33.0) | 31 (31.0) | 100 (34.7) | 30 (30.0) | |
| Clinical Center Network | 0.1374 | ||||
| 3-CCN-Minn/Iowa | 230 (47.1) | 40 (40.0) | 145 (50.3) | 45 (45.0) | |
| 4-CCN-Ohio/Mich | 68 (13.9) | 13 (13.0) | 39 (13.5) | 16 (16.0) | |
| 5-CCN-Northeast | 64 (13.1) | 13 (13.0) | 32 (11.1) | 19 (19.0) | |
| 6-CCN-Southeast | 126 (25.8) | 34 (34.0) | 72 (25.0) | 20 (20.0) | |
| Race/ethnicity | 0.3223 | ||||
| White only | 331 (67.8) | 71 (71.0) | 200 (69.4) | 60 (60.0) | |
| Spanish/Hispanic | 31 (6.4) | 5 (5.0) | 15 (5.2) | 11 (11.0) | |
| Black, non-Hispanic | 84 (17.2) | 17 (17.0) | 46 (16.0) | 21 (21.0) | |
| Asian, not-Hispanic or black | 7 (1.4) | 1 (1.0) | 6 (2.1) | 0 (0.0) | |
| Other | 35 (7.2) | 6 (6.0) | 21 (7.3) | 8 (8.0) | |
| Smoking status | 0.1547 | ||||
| Never | 226 (46.4) | 41 (41.0) | 131 (45.6) | 54 (54.0) | |
| Former | 200 (41.1) | 45 (45.0) | 115 (40.1) | 40 (40.0) | |
| Current | 61 (12.5) | 14 (14.0) | 41 (14.3) | 6 (6.0) | |
| Blood pressure (mmHg) | |||||
| Systolic | 134.6 ± 17.8 | 137.5 ± 18.6 | 133.4 ± 17.9 | 135.3 ± 16.7 | 0.3878 |
| Diastolic | 74.7 ± 10.2 | 78.4 ± 9.8 | 74.6 ± 10.3 | 71.3 ± 8.9 | <0.0001 |
| Diabetes duration (years) | 10.0 ± 7.1 | 2.5 ± 1.3 | 8.8 ± 2.8 | 21.0 ± 6.3 | <0.0001 |
| HbA1c (%) | 8.1 ± 0.9 | 8.0 ± 0.9 | 8.1 ± 0.9 | 8.3 ± 1.0 | 0.0631 |
| HbA1c (mmol/mol) | 65 ± 10 | 64 ± 10 | 65 ± 10 | 67 ± 11 | |
| Cholesterol (mg/dL) | |||||
| Total | 181.6 ± 40.4 | 190.1 ± 40.4 | 180.7 ± 41.2 | 176.0 ± 36.9 | 0.0135 |
| LDL | 101.1 ± 32.2 | 104.4 ± 32.3 | 101.2 ± 32.8 | 97.5 ± 30.1 | 0.1266 |
| HDL | |||||
| In women | 48.7 ± 12.4 | 47.8 ± 12.0 | 47.6 ± 11.8 | 52.6 ± 13.7 | 0.0570 |
| In men | 40.0 ± 10.3 | 38.0 ± 9.3 | 40.2 ± 10.7 | 41.1 ± 9.7 | 0.1493 |
| BMI (kg/m2) | 32.5 ± 5.1 | 33.4 ± 5.3 | 32.4 ± 5.1 | 31.7 ± 4.8 | 0.0235 |
| History of cardiovascular disease | 124 (25.4) | 22 (22.0) | 67 (23.3) | 35 (35.0) | 0.0458 |
| Total brain volume | 925.5 ± 96.2 | 928.0 ± 97.6 | 928.2 ± 95.1 | 915.3 ± 98.4 | 0.3512 |
| Depression** | 57 (11.7) | 14 (14.0) | 33 (11.5) | 10 (10.0) | 0.6673 |
| Baseline scores | |||||
| DSST | 54.6 ± 15.6 | 58.0 ± 15.3 | 54.6 ± 15.6 | 51.2 ± 15.0 | 0.0019 |
| RAVLT | 7.6 ± 2.5 | 8.1 ± 2.6 | 7.6 ± 2.4 | 7.1 ± 2.4 | 0.0028 |
| STROOP | 30.5 ± 15.6 | 29.7 ± 13.1 | 29.5 ± 15.8 | 34.2 ± 17.0 | 0.0441 |
| MMSE | 27.7 ± 2.3 | 27.6 ± 2.2 | 27.8 ± 2.4 | 27.5 ± 2.2 | 0.7612 |
Data are mean ± SD or n (%).
MMSE, Mini-Mental State Examination.
*Between short-duration and long-duration groups.
**Defined as patient health questionnaire score >10.
Figure 1.
Three-dimensional surface renderings of ODVBA results. A: GM RAVENS maps in relationship with short vs. long diabetes duration at baseline. Subjects with long diabetes duration (n = 100) had lower RAVENS values (i.e., lower regional GM volume, in the highlighted areas) compared with subjects with short diabetes duration (n = 100). B: GM dRAVENS maps in relationship with standard vs. intensive glycemic treatment arm. Subjects in the intensive treatment arm (n = 221) had lower longitudinal decrease in GM tissue volume in the highlighted areas compared with subjects in the standard treatment arm (n = 267). The green color indicates the detected significant regions with FDR-corrected q < 0.05. The hot color indicates the trends toward significance characterized by the −log (P) values shown in the color bar.
There was no statistically significant correlation of low versus high baseline HbA1c levels to the GM, WM, and VN RAVENS maps.
Patterns of Regional Volume Change Associated With Glycemia Treatment
The longitudinal analysis using ODVBA has been applied for identifying group differences in regional volume loss patterns between subjects in the intensive glycemic treatment arm and those in the standard treatment arm. Estimates of region-specific longitudinal volumetric change were obtained from the baseline and follow-up RAVENS maps by calculating the intensity difference at each voxel between the two time points.
Intensive treatment preserved GM volume more than the standard treatment (FDR-corrected q < 0.05) in certain cortical areas, specifically in the left and right superior temporal gyrus, left pre- and postcentral gyri, left and right medial front-orbital gyrus, and left and right frontal cingulate regions (Fig. 1B).
There was a partial overlap between the areas associated with treatment differences and those associated with diabetes duration. Importantly, the regions that showed longitudinal differences were mostly adjacent to regions displaying significant cross-sectional relationship with diabetes duration and tended to show just a trend at baseline.
Patterns of Regional Volume Change Associated With Longitudinal Change in Cognition
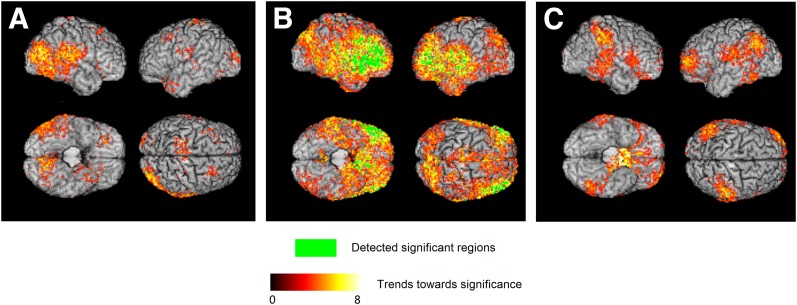
Summary statistics of cognitive decline and nondecline categories for each cognitive test are given in Table 2. A positive association between higher GM volume loss in certain cortical areas of the brain and cognitive decline was detected for all three cognitive tests; however, after FDR correction, significant group differences were found only for RAVLT (Fig. 2). ODVBA detected trends in right occipitotemporal regions for DSST, a global cortical trend for RAVLT with significant effects on frontotemporal regions, and trends mostly in parietal regions for STROOP.
Table 2.
Summary statistics of cognitive categories included in the pattern analysis
| Declining |
Nondeclining |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Rate of change* | Score at 40 months | Age (years) | N | Rate of change* | Score at 40 months | Age (years) | |
| DSST | 100 | −0.34 ± 0.14 | 48.15 ± 13.76 | 63.6 ± 6.06 | 100 | 0.18 ± 0.09 | 60.76 ± 12.07 | 61.42 ± 5.09 |
| RAVLT | 100 | −0.05 ± 0.02 | 6.80 ± 2.36 | 62.50 ± 5.88 | 100 | 0.08 ± 0.03 | 10.18 ± 2.09 | 60.70 ± 3.86 |
| STROOP** | 100 | 0.36 ± 0.40 | 41.5 ± 21.40 | 62.52 ± 5.20 | 100 | −0.29 ± 0.13 | 23.02 ± 7.3 | 62.19 ± 5.52 |
Data are mean ± SD.
*Change in score per month, estimated by linear regression to baseline, 20-month, and 40-month test scores.
**Contrary to DSST and RAVLT, a higher score in STROOP test means lower performance.
Figure 2.
Three-dimensional surface renderings of ODVBA results for longitudinal change in cognitive test scores. Group differences on GM dRAVENS maps between the group with declining cognition (n = 100) vs. nondeclining cognition (n = 100) are shown for DSST (A), RAVLT (B), and STROOP (C) tests. For each three tests, subjects in the group with declining cognition had a higher longitudinal decrease in GM volume in the highlighted areas compared with subjects in the group with nondeclining cognition. The green color indicates the detected significant regions with FDR-corrected q < 0.05. The hot color indicates the trends toward significance characterized by the −log (P) values shown in the color bar.
Conclusions
To the best of our knowledge, this is the first study to use pattern analysis methods to investigate the spatial specificity of patterns of brain volume loss in relation to diabetes duration and HbA1c. Moreover, this is the first study to apply these methods to investigate the spatial patterns of treatment effects on brain structure in a large diabetes clinical trial and using state-of-the-art pattern analysis methodology.
We applied a new VBM approach, ODVBA (18), in this analysis. In classical VBM methods, Gaussian smoothing of images, which is applied to account for registration errors and to integrate imaging signals from a region, has also become a limitation of these methods, because it is often chosen empirically and lacks spatial adaptivity to the shape and spatial extent of the region of interest, such as a region of volume loss. In contrast, ODVBA, using machine-learning techniques on local image neighborhoods, determines the spatially adaptive smoothing kernel whose coefficients define the optimally discriminative direction between two groups (e.g., patients and control subjects). Information from all neighborhoods that contain a given voxel is then composed to produce the statistic for each voxel, and permutation tests are used to obtain a statistical parametric map of group differences. Experimental results on three sets of previously published data from studies in schizophrenia, mild cognitive impairment, and Alzheimer disease suggest that ODVBA is considerably more sensitive in detecting group differences and performs better than classical VBM methods in the spatial extent of detected area and agreement of anatomical boundary (25).
We found, at baseline, that participants with longer diabetes duration had significantly reduced GM volumes in a number of brain regions. The frontal and temporal lobes were particularly more vulnerable to diabetes effects. The cortical patterns of decreased GM volume showed similarities with previously reported regions in MRI studies comparing diabetic and normal participants (8,9,28–36). In a similar approach, Brundel et al. (9) detected region-specific group differences between healthy controls and patients using cortical area and volume and thickness values, imaging measures complementary to those we used in our analysis. Consistent with our findings, they concluded that the cortical atrophy in type 2 diabetic patients was not equally distributed across the entire brain but showed spatially specific patterns. Also, detected patterns for the change, particularly in cortical area and volume, largely overlapped with those found in our analysis, showing large effects in middle temporal and frontal areas. In contrast to our findings, this study reported smaller cortical thickness values for diabetic patients in the hippocampal region. However, the authors noted that separating GM and WM in the hippocampal region with their present technique is difficult, which may affect the reliability of the cortical thickness and volume measurements.
Previous studies also reported enlarged VNs in diabetic patients compared with healthy controls (37,38). Although we lost significance after FDR correction, our uncorrected significance values (P < 0.05) showed a trend of positive association of lateral VN enlargement and diabetes duration (Supplementary Fig. 1). Detected regions showed similarities to the uncorrected group differences presented by Lee et al. (37).
An important finding is that intensive glycemic treatment displays relative preservation of some but not all brain regions. The finding of a differential treatment effect is consistent with previous findings (14) that preservation of the brain volume was significantly better in the participants in the intensive treatment arm during the 40-month follow-up period compared with the participants in the standard arm. The current analyses go beyond the volumetric measures by providing information on how the volume loss is spatially distributed and, hence, provides more information on how the disease and the intervention interact to produce the outcomes. We found a heterogeneous pattern affecting specific brain regions to a greater degree than others. In particular, the strongest treatment effects were found in brain regions around the Sylvian fissure as well in the medial-frontal cortex. Interestingly, the regions displaying a differential treatment effect of reduced GM volume loss were adjacent to but almost nonoverlapping with those regions that displayed significant association with diabetes duration at baseline. Therefore, intensive treatment appears to slow the spatial spreading of GM volume loss from regions mostly affected by diabetes duration toward adjacent brain regions. Additional studies must be performed, potentially by using individualized longitudinal analyses, to better measure this apparent “slowing of spatial progression” effect on an individual patient basis.
Interestingly, the areas that were associated to change in cognition were different from those affected from the treatment, which may explain the previous finding that there were no significant differences in the cognitive outcomes between the two treatment groups (14). Nonetheless, areas detected for RAVLT, although showing a global cortical effect, have also shown partial overlap with areas affected from the treatment as well as with areas affected from diabetes duration. Verbal learning tests have been considered a useful tool for the early diagnosis of cognitive decline and Alzheimer disease (39,40). As such, these overlapping regions might be particularly important for investigating the relationship between diabetes, its treatment, and dementia.
An important limitation of structural MRI is the lack of molecular specificity. Thus, interpretation of the measured volume changes in GM is challenging. Neuropathological correlates of the macroscopic volumetric reduction are heterogeneous and can include not only neuronal loss but also cortical thinning, subcortical vascular pathology, WM rarefaction, or other reasons (9). Results from animal models (41,42) have presented the associations of diabetes with neuronal changes in specific brain regions, with the aim of understanding underlying mechanisms of structural brain changes. However, these mechanisms are still unclear, as findings propose the involvement of multiple factors in the development of diabetes related brain changes (43). Consequently, the etiology of potentially relatively higher vulnerability of certain brain regions to diabetes duration cannot be elucidated by the current study and might relate to the differences in the underlying neuronal types and their sensitivity to insulin, underlying effects of diabetes in brain tissue perfusion, or might be effects of insulin resistance and its potential effect on brain connections. A growing body of work during the past decade has demonstrated that insulin-sensitive glucose transporters are localized to the same regions supporting memory, suggesting that insulin and its metabolism may contribute to normal cognitive functioning and that insulin abnormalities may exacerbate cognitive impairments, such as those associated with type 2 diabetes and even Alzheimer disease. The regional differences identified here in the cross-sectional and longitudinal analyses are similar to some of those brain regions associated with cognitive health (44,45).
No brain region was found to display significant correlation with low versus high HbA1c. That no brain region was significantly correlated with HbA1c levels was against our initial expectations, which were based on the central diagnostic/disease management role of HbA1c in diabetes and on earlier findings in the larger sample participating in the cognitive component of ACCORD-MIND (13). There, we found an association of increasing HbA1c and reduced performance on the cognitive tests of speed, memory, and executive function. Because the MRI sample was smaller than the cognitive sample, we may not have had enough power to detect differences in a measure such as HbA1c level, which only reflect blood glucose levels during the relatively short period of ∼3 months, whereas diabetes duration reflects the cumulative effect of the disease over years. However, we cannot rule out that other pathologic conditions associated with diabetes, such as hypertension, might have a relatively more deleterious effect on the brain than glucose levels. Finally, hypoglycemia might be associated with physiologic (e.g., perfusion) but not structural brain changes.
A limitation of this study is that the sample includes diabetic subjects only. Our findings on the relationship between diabetes duration and extent of brain volume loss are relatively conservative because they were obtained after correcting for age. Diabetes duration and age were somewhat correlated (r = 0.216), albeit not very strongly. The relationship between neurodegeneration and diabetes duration might therefore be even stronger. New studies, including nondiabetic control subjects, would be necessary to allow us to correct only for patterns of brain volume loss explained by normal aging and independently from diabetes duration.
Several factors might have contributed to the dissociation between the treatment effects on brain structure and on cognition (Launer et al. [14] found no treatment effects on cognition). Cognitive tests are known to be variable and subject to the learning effect; hence, imaging measurements, especially the pattern analysis adopted in this study, might be able to detect more subtle effects. Moreover, brain changes are likely to precede cognitive changes, which would further explain this dissociation. However, our results suggest a spatially heterogeneous effect of diabetes duration, as well as of its treatment, thereby pointing the way to future refinement of cognitive tests to tease out functions mediated by those brain regions that are more vulnerable to diabetes and more responsive to treatment.
Supplementary Material
Article Information
Funding. ACCORD-MIND was funded through an intra-agency agreement between the National Institute on Aging (NIA) and the National Heart, Lung, and Blood Institute (NHLBI) (AG-0002) and the NIA Intramural Research Program. ACCORD was funded by NHLBI (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, and N01-HC-95184). The image analysis was funded by National Institute of Biomedical Imaging and Bioengineering 5R01-EB-009234.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.E. wrote the manuscript. G.E., H.B., T.Z., and C.D. designed the imaging experiments and data analysis. H.B. and T.Z. performed the imaging experiments and data analysis. J.L., M.E.M., and J.D.W. were involved in quality control and data analysis. M.E.M., L.J.L., and R.N.B. designed the study. C.D. supervised the image analysis. All of the authors revised the manuscript. G.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-1196/-/DC1.
References
- 1.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol 2002;441:1–14 [DOI] [PubMed] [Google Scholar]
- 2.Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. Eur J Pharmacol 2004;490:169–175 [DOI] [PubMed] [Google Scholar]
- 3.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 4.Scheltens P, Fox N, Barkhof F, De Carli C. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol 2002;1:13–21 [DOI] [PubMed]
- 5.Launer LJ. Diabetes: vascular or neurodegenerative: an epidemiologic perspective. Stroke 2009;40(3 Suppl.):S53–S55 [DOI] [PMC free article] [PubMed]
- 6.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29:2539–2548 [DOI] [PubMed] [Google Scholar]
- 7.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2014;2:246–255 [DOI] [PubMed] [Google Scholar]
- 8.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013;36:4036–4042 [DOI] [PMC free article] [PubMed]
- 9.Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group . Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci 2010;299:126–130 [DOI] [PubMed] [Google Scholar]
- 10.Brundel M, Kappelle LJ, Biessels GJ. Brain imaging in type 2 diabetes. Eur Neuropsychopharmacol. 15 Mar 2014 [Epub ahead of print] [DOI] [PubMed]
- 11.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group . Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i [DOI] [PubMed] [Google Scholar]
- 12.Williamson JD, Miller ME, Bryan RN, et al. ACCORD Study Group . The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol 2007;99:112i–122i [DOI] [PubMed] [Google Scholar]
- 13.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators . Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial. Diabetes Care 2009;32:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators . Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage 2001;14:1361–1369 [DOI] [PubMed] [Google Scholar]
- 16.Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr 1998;22:827–837 [DOI] [PubMed] [Google Scholar]
- 17.Shen D, Davatzikos C. Very high-resolution morphometry using mass-preserving deformations and HAMMER elastic registration. Neuroimage 2003;18:28–41 [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Davatzikos C. ODVBA: optimally-discriminative voxel-based analysis. IEEE Trans Med Imaging 2011;30:1441–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97 [DOI] [PubMed] [Google Scholar]
- 20.Pham DL, Prince JL. Adaptive fuzzy segmentation of magnetic resonance images. IEEE Trans Med Imaging 1999;18:737–752 [DOI] [PubMed] [Google Scholar]
- 21.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 2002;21:1421–1439 [DOI] [PubMed] [Google Scholar]
- 22.Kabani NJ, Collins DL, Evans AC. A 3D neuroanatomical atlas. Fourth International Conference on Functional Mapping of the Human Brain, 7–12 June 1998, Montreal, Quebec, Canada [Google Scholar]
- 23.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosc 2003;23:3295–2301 [DOI] [PMC free article] [PubMed]
- 24.Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B Met 2002;64:479–498 [Google Scholar]
- 25.Zhang T, Davatzikos C. Optimally-discriminative voxel-based morphometry significantly increases the ability to detect group differences in schizophrenia, mild cognitive impairment, and Alzheimer’s disease. Neuroimage 2013;79:94–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Satterthwaite TD, Elliott M, Gur RC, Gur RE, Davatzikos C. Multivariate fMRI analysis using optimally-discriminative voxel-based analysis. Paper presented at the IEEE International workshop on Pattern Recognition in NeuroImaging, 2-4 July 2012, London, U.K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelman A, Park DK. Splitting a predictor at the upper quarter or third and the lower quarter or third. Am Stat 2009;63:1–8 [Google Scholar]
- 28.Akisaki T, Sakurai T, Takata T, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese Elderly Diabetes Intervention Trial (J-EDIT). Diabetes Metab Res Rev 2006;22:376–384 [DOI] [PubMed] [Google Scholar]
- 29.Manschot SM, Biessels GJ, de Valk H, et al. Utrecht Diabetic Encephalopathy Study Group . Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 2007;50:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musen G, Lyoo IK, Sparks CR, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006;55:326–333 [DOI] [PubMed] [Google Scholar]
- 31.Samaras K, Lutgers H, Wen W, et al. Glucose disorders exert a detrimental effect on total brain volume in the elderly: a 2-year prospective MRI study. Endocrine Abstracts 2012;29:OC3.6
- 32.Samaras K, Lutgers HL, Kochan NA, et al. The impact of glucose disorders on cognition and brain volumes in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2014;36:977–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaquist ER. The final frontier: how does diabetes affect the brain? Diabetes 2010;59:4–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiehuis AM, van der Graaf Y, Visseren FL, et al. SMART Study Group . Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 2008;39:1600–1603 [DOI] [PubMed] [Google Scholar]
- 35.van Elderen SG, de Roos A, de Craen AJ, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 2010;75:997–1002 [DOI] [PubMed] [Google Scholar]
- 36.Wessels AM, Simsek S, Remijnse PL, et al. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 2006;49:2474–2480 [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Yoon S, Renshaw PF, et al. Morphometric changes in lateral ventricles of patients with recent-onset type 2 diabetes mellitus. PLoS ONE 2013;8:e60515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmichael OT, Kuller LH, Lopez OL, et al., Acceleration of cerebral ventricular expansion in the Cardiovascular Health Study. Neurobiol Aging 2007;28:1316–1321 [DOI] [PMC free article] [PubMed]
- 39.Barzotti T, Gargiulo A, Marotta MG, et al. Correlation between cognitive impairment and the Rey auditory-verbal learning test in a population with Alzheimer disease. Arch Gerontol Geriatr Suppl 2004:57–62 [DOI] [PubMed] [Google Scholar]
- 40.Kuslansky G, Katz M, Verghese J, et al. Detecting dementia with the Hopkins Verbal Learning Test and the Mini-Mental State Examination. Arch Clin Neuropsychol 2004;19:89–104 [PubMed]
- 41.Toth C, Schmidt AM, Tuor UI, et al. Diabetes, leukoencephalopathy and RAGE. Neurobiol Dis 2006;23:445–461 [DOI] [PubMed] [Google Scholar]
- 42.Enhamre E, Carlsson A, Grönbladh A, Watanabe H, Hallberg M, Nyberg F. The expression of growth hormone receptor gene transcript in the prefrontal cortex is affected in male mice with diabetes-induced learning impairments. Neurosci Lett 2012;523:82–86 [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Choi Y, Jun C, et al. Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul) 2014;29:112–121 [DOI] [PMC free article] [PubMed]
- 44.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 2003;17:27–45 [DOI] [PubMed] [Google Scholar]
- 45.Schiöth HB, Craft S, Brooks SJ, Frey WH, 2nd, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol 2012;46:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.