Abstract
OBJECTIVE
Persons with diabetes have accelerated muscle loss compared with their counterparts. The relationship of hyperglycemia per se to declines in muscle function has not been explored yet has implications for developing appropriate intervention strategies to prevent muscle loss.
RESEARCH DESIGN AND METHODS
We examined 984 participants aged 25–96 years in the Baltimore Longitudinal Study of Aging (2003–2011) with HbA1c, knee extensor strength (isokinetic dynamometer), and lean body mass (DEXA) measured at baseline. Participants had repeated measurements up to 7.5 years later. Muscle quality was defined as knee extensor strength/leg lean mass. Participants were categorized by HbA1c quartile (<5.5, 5.5–5.79, 5.8–6.09, and ≥6.1% or <37, 37–40, 40–43, and ≥43 mmol/mol). Mixed-effects regression models were used to examine the regression of muscle outcomes on HbA1c.
RESULTS
Muscle strength and quality were significantly lower across HbA1c quartiles (both P < 0.001), without differences in muscle mass at baseline. Comparing highest versus lowest HbA1c quartiles and adjusting for age, race, sex, weight, and height, strength was significantly lower (−4.70 ± 2.30 N · m; P value trend = 0.02) and results were unchanged after adjustment for physical activity (P value trend = 0.045) but of borderline significance after additional adjustment for peripheral neuropathy (P value trend = 0.05). Adjusting for demographics, muscle quality was significantly lower (−0.32 ± 0.15 N · m/kg; P value trend = 0.02) in the highest versus lowest HbA1c quartiles, but differences were attenuated after adjusting for weight and height (−0.25 ± 0.15 N · m/kg; P value trend = 0.07). Muscle mass measures were similar across HbA1c quartiles.
CONCLUSIONS
Hyperglycemia is associated with persistently lower muscle strength with aging, but this effect may be mediated, at least in part, by peripheral neuropathy. Future studies should explore if better glycemic control can preserve muscle function in diabetes.
Introduction
Persons with diabetes are more likely to experience accelerated loss of muscle mass, strength, and quality over time, particularly in the lower extremities, compared with those without diabetes (1,2). Such effects of diabetes on muscle may explain why patients with diabetes are at high risk of developing functional disability and mobility limitations (3–6). However, the reason for this accelerated decline of muscle function in persons with diabetes is still unclear. As the prevalence of diabetes continues to rise, and persons with diabetes live longer (7–9), diabetes will become a major contributor to sarcopenia in the elderly, with increased burden of disability and rising health care costs (10). Thus, understanding factors related to the loss of muscle in persons with diabetes has implications for future preventive efforts and has broad, significant public health implications.
A possible hypothesis is that hyperglycemia, which is an early manifestation in the development of diabetes, damages muscle and results in loss of strength and mass. Previous observational studies have described the cross-sectional association of fasting and 2-h post–75-g oral glucose tolerance test levels (and insulin levels) with loss of muscle mass and strength in persons with and without diabetes (11–14). However, no studies to date have examined the relationship of hyperglycemia with longitudinal changes in muscle mass, strength, or quality. Glycemic status may also be altered in prediabetic states, and poor diabetes control may cause wide fluctuations of glycemia over time, which may further impact muscle function.
Of note, longitudinal studies of aging have shown that declines in muscle strength exceed what is expected on the basis of the decline in muscle mass alone, especially after the age of 60–70 years (15,16). This progressive mismatch probably occurs because of change in muscle composition and progressive denervation (17,18). When these age-related changes occur, it is possible that the effect of hyperglycemia or diabetes on muscle function may be moderated (17,18). Unfortunately, previous studies did not address the question of whether the association of diabetes with loss of muscle function is affected by age.
In the current study, we used data from the Baltimore Longitudinal Study of Aging (BLSA) to investigate the hypothesis that the severity of hyperglycemia (assessed by HbA1c) is associated with decreased muscle strength, mass, and quality, and these associations are not affected by age and other potential confounders such as physical activity or the presence of peripheral neuropathy. In addition, we verified whether hyperglycemia affects muscle characteristics over the entire adult and older age lifespan.
Research Design And Methods
Study Population
The BLSA is a longitudinal cohort study conducted by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, since 1958. BLSA participants are community-dwelling men and women recruited primarily from the Baltimore–Washington, DC, area with above-average education, income, and access to medical care (19). Participants underwent extensive evaluations at regular, predefined intervals (on average, intervals were every 2.5 ± 1.2 years). Participants in the BLSA currently return for evaluations based on age. Participants under age 60 years are assessed every 4 years, those aged 60–79 years are assessed every 2 years, and participants aged 80 years and older are assessed annually.
A total of 984 participants (aged 26–96 years) who had HbA1c measured at least once (baseline) and assessment of both quadriceps strength and DEXA between the years 2003 and 2011 was included in this study. The mean length of follow-up was ∼1.9 ± 2.2 years for all subjects (range 0–7.5 years).
The research protocol was approved by the Intramural Research Program of the National Institute on Aging and the institutional review board of the MedStar Health Research Institute, Baltimore, MD. All participants provided written informed consent.
Assessment of HbA1c
HbA1c was assessed using the automated DiaSTAT analyzer between the years 2003 and 2006 (Bio-Rad, Hercules, CA) and the Dimension Vista System (Siemens, Camberley, U.K.) from 2007 onwards. The values from both instruments were standardized such that the results were comparable.
There were 1,720 visits with a measure of HbA1c, and many participants had repeated visits where HbA1c was assessed (one visit, n = 495; two visits, n = 308; three visits, n = 124; four visits, n = 48; and five visits, n = 9). Participants also had information on both knee extensor and DEXA assessment at all of these visits. For analytical purposes, HbA1c assessments were divided into quartiles as follows: quartile 1 (HbA1c <5.5% or <37 mmol/mol, n = 262); quartile 2 (HbA1c 5.5–5.79% or 37–40 mmol/mol, n = 246); quartile 3 (HbA1c 5.8–6.09% or 40–43 mmol/mol, n = 216); and quartile 4 (HbA1c ≥6.1% or ≥43 mmol/mol, n = 260).
Assessment of Knee Extensor Strength
Knee extensor strength was assessed using the Kinetic Communicator (Kin-Com, model 125E; Chattecx, Chattanooga, TN) isokinetic dynamometer. Maximal voluntary torque was measured in the dominant knee extensor at an angular velocity of 0.52 rad/s (30°/s). Gravity corrections to torque were performed (20). Three graded submaximal practice repetitions preceded the test. These were followed by three maximal efforts, separated by 30 s of rest intervals. Knee extensor strength was considered as the maximum of three trials. Peak torque was assessed by using the Kin-Com computer software (version 3.2). Reliability of strength testing by the Kin-Com dynamometer has been reported elsewhere (21). Mean coefficient of variation was 5% (20). The total number of knee extensor measurements available for 984 subjects across all visits was n = 1,720.
Assessment of Muscle Mass Using DEXA
Total and leg lean body mass were assessed using DEXA (model DPX-L; Lunar Radiation, Madison, WI) to determine fat mass, fat-free mass, and bone mineral content for the total body and lower extremities (22). All scans were analyzed by one investigator using the Lunar version 1.2i DPX-L program for body-composition analyses. These scans were considered reliable, with <1% difference between repeated scans a few weeks apart (20). The scanner was calibrated daily before testing. The total number of DEXA measurements available for 984 subjects across all visits was n = 1,720.
Assessment of Muscle Quality
To assess muscle quality of the lower extremity, knee extensor strength was divided by DEXA-derived leg lean body mass, similar to other studies (2).
Assessment of Thigh Cross-sectional Area by Computed Tomography
Ten-millimeter (120 kVp, 200–250 mA) cross-sectional images were obtained at the midfemur level by the Somatom Sensation 10 CT scanner (Siemens, Malvern, PA), considered to be the midpoint between the medial edge of the greater trochanter and the intercondyloid fossa in scout-view images. A single cross-sectional image of the midthigh was analyzed by BonAlyse (BonAlyse Oy, Jyväskylä, Finland), a software for processing computed tomography (CT) images that identifies muscle tissue, fat, and bone. Further details regarding assessment of CT thigh cross-sectional area (CSA) have been previously described (14). Overall, 726 participants had one or more measures of CT thigh CSA, distributed as follows: one visit, n = 511; two visits, n = 178; three visits, n = 29; and four visits, n = 8.
Covariates
Demographics including age, sex, and race were assessed by questionnaire. Height and weight were measured objectively by standard methods. Diabetes was defined as fasting glucose ≥126 mg/dL, 2-h oral glucose tolerance test ≥200 mg/dL, self-reported history, or current use of oral hypoglycemic agents or insulin.
Physical activity level was determined using a standardized questionnaire, modeled from the well-validated Minnesota Leisure-Time Physical Activity Questionnaire (23). An overall physical activity score (in kcal/week) was calculated for each participant (24). A total of 1,327 visits had information on physical activity available; thus, 23% of visits had physical activity data imputed. Data were imputed using an expectation maximum algorithm in combination with a bootstrap approach with the assumption of missing at random and a multivariate normal distribution. Ten imputed sets of data were created using the data from all of the models being considered. The variables with missing data were then examined for distribution of these variables. Models were run on each individual imputed data set, and then separate results from these 10 data sets were averaged and variance estimated as described by Rubin (25).
Peripheral nerve function was evaluated by measuring peroneal motor nerve conduction velocity (NCV) using standard techniques (26) (Nicolet Viking Select, Madison, WI). Testing was performed on the right leg if no contraindications were present. Contraindications included amputation, ulcer, trauma, knee replacement, or surgery. Details of neuropathy assessment have been previously described (14). A total of 1,024 visits had information on peripheral neuropathy available; 40% of visits had peripheral neuropathy data imputed using methods similar to those described for physical activity.
Statistical Analysis
Differences in baseline characteristics by quartile of HbA1c were summarized as means ± SD and tested by ANOVA for continuous variables and by χ2 tests for categorical variables.
Knee extensor strength, leg lean mass, total body lean mass, thigh CSA, and knee extensor strength/leg lean mass (muscle quality) were plotted against age for each HbA1c quartile using a local regression (loess) function in figures. The loess curves represent an exploratory data analysis with smoothing lines to depict the general shape of the response data across all of the data. Data plotted in figures were truncated below 38 years of age for comparability since HbA1c quartile 4 did not have any participants younger than this age. We tested the interaction of HbA1c quartiles with slope of muscle decline using a linear regression model.
Mixed-effects regression models (Supplementary Data) were used to examine the regression of knee extensor strength, leg lean mass, total lean mass, CT thigh CSA, and knee extensor strength/leg lean mass (muscle quality) on HbA1c. Follow-up time was entered into each regression model in addition to age at time of entry into the study as a continuous variable given the unbalanced study design of BLSA (fixed effect). In the final mixed-effects regression model used for analyses, a random parameter for intercept only was included. The maximum likelihood estimation procedure was used. Four models were fitted, which sequentially included covariates of interest: model 1 was adjusted for age, race, and sex; model 2 was model 1 + height + weight; model 3 was model 2 + physical activity; and model 4 was model 3 + peripheral NCV. We chose to use both height and weight in our models, instead of BMI, given that higher BMI can also be due to greater total body lean mass, similar to previous studies suggesting that both height and weight are independently related to muscle outcomes (21).
We used a likelihood ratio test comparing models with HbA1c to models without HbA1c for each muscle outcome, including variables known to be associated with muscle outcomes such as age, follow-up time, sex, and race in these models, and found results to be statistically significant (P < 0.05). These results suggested that HbA1c was related to observed differences in muscle outcomes between participants even after accounting for these covariates. Differences in the rate of decline in muscle outcomes (slope) by HbA1c quartile were explored using a likelihood ratio test comparing models with an interaction term for HbA1c × time to models without an interaction term. In addition, β-coefficients ± SEs for the difference in intercept for each muscle outcome were calculated comparing each of the higher HbA1c quartiles (quartiles 2–4) to reference quartile 1 in sequential regression analyses. Tests of trend were also performed across HbA1c quartiles. Interactions of HbA1c with sex were not significant (P > 0.05) for any of the muscle outcomes examined; thus, analyses for the overall study cohort are displayed.
Sensitivity analyses were performed in persons with measured (nonimputed) data only. In additional sensitivity analyses, subjects with an established clinical diagnosis of diabetes were excluded. In sensitivity analyses, we also adjusted for weight-training activities (total minutes over 2 weeks) in models 3 and 4.
A two-tailed P value < 0.05 was used to indicate statistical significance. Analyses were done using packages lme4 and lmerTest in R version 3.0.2.
Results
The participant characteristics according to HbA1c quartile at baseline are shown in Table 1. The mean HbA1c values for each quartile were as follows: quartile 1 (mean HbA1c = 5.2%), quartile 2 (mean HbA1c = 5.6%), quartile 3 (mean HbA1c = 5.9%), and quartile 4 (mean HbA1c = 6.6%). Participants in the higher HbA1c quartiles (P < 0.001) were significantly older, heavier, and taller than those in the lower quartiles. There were also significant differences in race by HbA1c quartile, with less white participants in the higher HbA1c quartiles (P < 0.001). There were no differences in sex by HbA1c. Physical activity levels were similar across HbA1c quartiles. NCV, a measure of peripheral nerve function, was significantly lower across HbA1c quartiles (P < 0.001). Not surprisingly, the percentage of participants with known diabetes was significantly greater in the higher HbA1c quartiles compared with the lowest quartile (P < 0.001).
Table 1.
Selected baseline characteristics of the study participants, according to HbA1c quartile at baseline
| HbA1c level | |||||
|---|---|---|---|---|---|
| Quartile 1 (<5.5% or <37 mmol/mol) | Quartile 2 (5.5–5.79% or 37–40 mmol/mol) | Quartile 3 (5.8–6.09% or 40–43 mmol/mol) | Quartile 4 (≥6.1% or ≥43 mmol/mol) | P value | |
| Demographics | |||||
| Age (years) | 58.82 ± 15.10 | 64.60 ± 13.98 | 67.75 ± 12.48 | 68.29 ± 10.69 | <0.001 |
| Sex (% female) | 49.2 | 50.8 | 49.1 | 48.1 | 0.94 |
| Race (%) | <0.001 | ||||
| White | 75.3 | 69.5 | 66.6 | 54.9 | |
| Black | 21.0 | 24.9 | 26.2 | 35.6 | |
| Other | 3.7 | 5.6 | 7.2 | 9.5 | |
| History and examination | |||||
| Physical activity (kcal/day)* | 2,223.46 ± 2,552.38 | 2,178.25 ± 2,914.55 | 1,789.16 ± 1,576.43 | 1,959.97 ± 3,002.87 | 0.50 |
| NCV (m/s)* | 46.9 ± 6.3 | 46.4 ± 6.3 | 46.1 ± 6.5 | 44.9 ± 6.3 | <0.001 |
| Known diabetes (%) | 6.5 | 9.3 | 11.1 | 31.9 | <0.001 |
| Body composition | |||||
| Weight (kg) | 77.27 ± 15.47 | 75.42 ± 16.04 | 76.37 ± 15.18 | 80.91 ± 16.06 | <0.001 |
| Height (cm) | 171.04 ± 9.10 | 169.10 ± 9.79 | 168.30 ± 9.09 | 168.90 ± 9.15 | 0.007 |
| Knee extensor strength (N · m) | 151.76 ± 51.29 | 142.11 ± 53.10 | 134.48 ± 43.27 | 132.30 ± 40.91 | <0.001 |
| Knee extensor/leg lean mass (N · m/kg) | 9.48 ± 2.3 | 9.15 ± 2.2 | 8.82 ± 2.11 | 8.53 ± 2.26 | <0.001 |
| Leg lean mass (kg) | 16.06 ± 3.7 | 15.58 ± 4.1 | 15.36 ± 3.7 | 15.78 ± 3.8 | 0.23 |
| Total lean mass (kg) | 49.17 ± 3.7 | 47.93 ± 4.1 | 47.43 ± 3.7 | 49.03 ± 3.8 | 0.19 |
| Thigh CSA (mm2) | 12,057 ± 3,030 | 11,446 ± 3,510 | 11,135 ± 3,184 | 11,847 ± 3,116 | 0.26 |
Data are mean ± SD unless otherwise indicated.
*Only original data (nonimputed) shown.
Muscle strength (knee extensor strength) and muscle quality (knee extensor strength/leg lean mass) were all significantly decreased from lower to higher HbA1c quartiles (both P < 0.001). In contrast, measures of muscle mass including leg lean mass, total lean mass, and thigh CSA did not significantly differ by HbA1c quartiles.
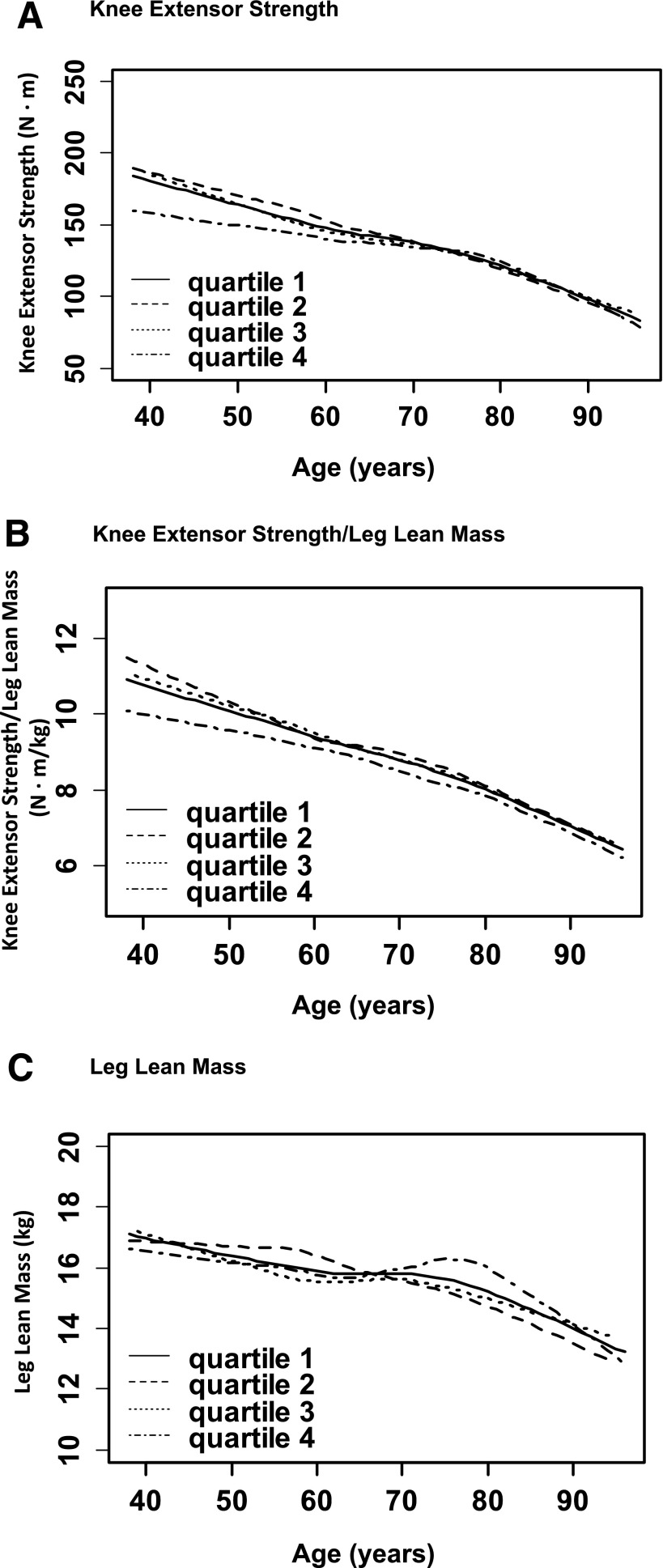
We next performed analyses to examine the average decline in muscle strength, mass, and quality with aging for participants categorized according to time-varying quartile of HbA1c (Fig. 1). In Fig. 1A, knee extensor strength generally remains lower for participants in the highest HbA1c category (quartile 4) compared with the lower HbA1c categories (quartiles 1–3) with older ages. We did not find any evidence for a statistically significant difference in linear slopes across HbA1c quartiles in Fig. 1A (P > 0.05). To further examine the relationship of HbA1c with the average decline in knee extensor strength during aging, we next included a break point at age 65 years in regression models and found that results were unchanged. Of note, this model assumes that an actual break point is present at this age that we could not confirm in these exploratory data analyses.
Figure 1.
The relationship of age with average muscle strength, muscle quality, and muscle mass is shown for participants categorized by time-varying quartile of HbA1c. For muscle strength (A), participants in HbA1c quartile 4 generally have lower values than those in HbA1c quartiles 1–3 with older ages. For muscle quality (B), participants in HbA1c quartile 4 generally have lower values than those in HbA1c quartiles 1–3 with older ages. For muscle mass (C), there are no consistent differences in values across HbA1c quartiles with older ages. There was no significant interaction of HbA1c with slopes for any of the muscle outcomes (all P > 0.05).
In Fig. 1B, knee extensor strength/leg lean mass generally remains lower for participants in HbA1c quartile 4 compared with all other quartiles (quartiles 1–3) with older ages. We did not find any evidence for a statistically significant difference in slopes across HbA1c quartiles in Fig. 1B (P > 0.05).
In Fig. 1C, leg lean mass overall declines with older age, though less dramatically than muscle strength (Fig. 1A). However, differences by HbA1c quartile are quite variable, with no consistent patterns found for participants in higher versus lower HbA1c quartiles with older ages. We did not find any evidence for a statistically significant difference in linear slopes across HbA1c quartiles in Fig. 1C (P > 0.05). To further examine the relationship of HbA1c with the average decline in leg lean mass during aging, we next included a quadratic term in regression models and found that results were unchanged in exploratory data analyses.
To further explore the relationship of HbA1c and muscle characteristics, we used mixed-effects regression models adjusting for potential confounders (Table 2). The P values for the likelihood ratio test comparing model 1 with and without an interaction term for HbA1c × time were as follows: knee extensor strength (P = 0.38); knee extensor strength/leg lean mass (P = 0.40); and leg lean mass (P = 0.55). These findings show that rates of decline (slopes) for all muscle outcomes were not significantly different across HbA1c quartiles.
Table 2.
Mixed-effects regression models exploring differences in muscle outcomes among BLSA participants, according to HbA1c quartile at time of visit§
| Model 1 | Model 2 | Model 3‡ | Model 4‡ | |
|---|---|---|---|---|
| Knee extensor strength (N · m) | ||||
| Age at first visit (years) | −1.78 ± 0.08* | −1.48 ± 0.09* | −1.47 ± 0.09* | −1.41 ± 0.09* |
| Time since first visit (years) | −0.99 ± 0.32* | −0.76 ± 0.31* | −0.79 ± 0.29* | −0.72 ± 0.29* |
| HbA1c quartile** | ||||
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 1.04 ± 2.08 | 0.86 ± 2.05 | 0.64 ± 2.05 | 0.69 ± 2.05 |
| Q3 | 0.03 ± 2.19 | −0.27 ± 2.15 | −0.12 ± 2.17 | −0.004 ± 2.17 |
| Q4 | −3.40 ± 2.33 | −4.70 ± 2.30† | −4.67 ± 2.32† | −4.47 ± 2.32 |
| P value for trend | 0.10 | 0.02 | 0.045 | 0.05 |
| Knee extensor strength/leg lean mass (N · m/kg) | ||||
| Age at first visit (years) | −0.07 ± 0.004* | −0.07 ± 0.005* | −0.07 ± 0.005* | −0.07 ± 0.005* |
| Time since first visit (years) | −0.09 ± 0.02* | −0.09 ± 0.02* | −0.09 ± 0.02* | −0.09 ± 0.02* |
| HbA1c quartile** | ||||
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | −0.01 ± 0.13 | −0.002 ± 0.13 | −0.001 ± 0.13 | 0.001 ± 0.13 |
| Q3 | −0.06 ± 0.14 | −0.04 ± 0.14 | −0.03 ± 0.14 | −0.02 ± 0.14 |
| Q4 | −0.32 ± 0.15† | −0.25 ± 0.15 | −0.24 ± 0.15 | −0.23 ± 0.15 |
| P value for trend | 0.02 | 0.07 | 0.22 | 0.24 |
| Leg lean mass (kg) | ||||
| Age at first visit (years) | −0.08 ± 0.005* | −0.04 ± 0.004* | −0.04 ± 0.004* | −0.04 ± 0.004* |
| Time since first visit (years) | 0.02 ± 0.01 | 0.04 ± 0.01* | 0.05 ± 0.01* | 0.05 ± 0.01* |
| HbA1c quartile** | ||||
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 0.13 ± 0.09 | 0.08 ± 0.08 | 0.08 ± 0.09 | 0.09 ± 0.09 |
| Q3 | 0.18 ± 0.10 | 0.09 ± 0.09 | 0.09 ± 0.09 | 0.09 ± 0.09 |
| Q4 | 0.20 ± 0.11 | 0.01 ± 0.10 | 0.02 ± 0.10 | 0.02 ± 0.10 |
| P value for trend | 0.07 | 0.99 | 0.63 | 0.63 |
| Total body lean mass (kg) | ||||
| Age at first visit (years) | −0.18 ± 0.01* | −0.07 ± 0.009* | −0.07 ± 0.009* | −0.07 ± 0.01* |
| Time since first visit (years) | −0.29 ± 0.02* | −0.23 ± 0.02* | −0.23 ± 0.02* | −0.22 ± 0.02* |
| HbA1c quartile** | ||||
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 0.11 ± 0.17 | −0.05 ± 0.16 | −0.02 ± 0.16 | −0.02 ± 0.16 |
| Q3 | 0.23 ± 0.18 | 0.03 ± 0.17 | 0.04 ± 0.17 | 0.04 ± 0.17 |
| Q4 | 0.40 ± 0.20 | 0.04 ± 0.18 | 0.06 ± 0.19 | 0.06 ± 0.19 |
| P value for trend | 0.04 | 0.76 | 0.96 | 0.96 |
| Thigh CSA (mm2) | ||||
| Age at first visit (years) | −118.2 ± 5.3* | −96.7 ± 4.9* | −96.5 ± 4.9* | −93.5 ± 5.0* |
| Time since first visit (years) | −113.3 ± 15.9* | −96.9 ± 14.5* | −99.3 ± 13.9* | −95.5 ± 13.8* |
| HbA1c quartile** | ||||
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 132.0 ± 92.5 | 110.4 ± 84.6 | 147.9 ± 83.2 | 148.1 ± 82.6 |
| Q3 | 146.4 ± 107.6 | 103.4 ± 98.1 | 126.4 ± 96.9 | 127.2 ± 96.3 |
| Q4 | 264.8 ± 116.7† | 83.2 ± 106.8 | 142.05 ± 105.5 | 138.4 ± 104.9 |
| P value for trend | 0.03 | 0.53 | 0.34 | 0.34 |
§β-Coefficients ± SE shown.
*P < 0.05.
**Model 1: adjusted for age at first visit, race, sex, and time since first visit (only age and time coefficients are shown above). Model 2: adjusted for variables in model 1 and weight and height. Model 3: adjusted for variables in model 2 and physical activity. Model 4: adjusted for variables in model 3 and NCV.
†P < 0.05 compared with reference quartile (Q1).
‡Physical activity and NCV were imputed.
Participants in HbA1c quartile 4 compared with reference (HbA1c quartile 1) had decreased knee extensor strength after accounting for age, race, and sex (−3.40 ± 2.33 N · m, model 1), with a trend across HbA1c categories approaching statistical significance (P = 0.10). After further adjustment for weight and height (model 2), knee extensor strength was significantly decreased in the highest versus lowest HbA1c quartile (−4.70 ± 2.30 N · m) with a significant trend of lower knee extensor strength across higher HbA1c quartiles (P = 0.02). In model 3, after accounting for physical activity, knee extensor strength was significantly lower in the highest versus lowest HbA1c quartile (−4.67 ± 2.32 N · m), and the trend for knee extensor strength across HbA1c categories remained significant (P value for trend = 0.045). In the fully adjusted model further accounting for peripheral neuropathy, knee extensor strength was lower in the highest versus lowest HbA1c quartile (−4.47 ± 2.32 N · m) but such difference was no longer significant, and the trend across HbA1c categories was of only borderline significant (P value for trend = 0.05). Interestingly, the difference in knee extensor strength comparing HbA1c quartile 2 to reference was positive, but such a difference was not statistically significant in any of the models.
Differences in muscle quality (knee extensor strength/leg lean mass) were also explored in mixed regression models. Participants in the highest HbA1c quartile had significantly poorer muscle quality compared with those in the lowest HbA1c quartile (−0.32 ± 0.15 N · m/kg), and the trend of decreasing muscle quality across higher HbA1c categories was statistically significant (P = 0.02) in model 1 (Table 2). After accounting for weight and height (model 2), the association of decreased muscle quality across HbA1c categories was attenuated and only approached statistical significance (P value for trend = 0.07) while in the fully adjusted model was no longer significant (model 4; P value for trend = 0.24).
In regards to leg lean mass, there were no significant differences in higher versus lower HbA1c quartiles in models 1–4. Interestingly, for total body lean mass, participants in higher versus lower quartiles had greater values after accounting for demographics (P value = 0.04 for trend, model 1). However, after further adjustment for weight and height (model 2), these differences were no longer significant (P value for trend >0.05). Similar results were observed for thigh CSA with participants in HbA1c quartile 4 compared with those in reference category (quartile 1) having significantly higher values (264.8 ± 116.7 mm2) in model 1 (P value = 0.03 for trend). However, after adjusting for weight and height in model 2, this trend was not significant.
In sensitivity analysis, using only nonimputed data, participants in the highest versus lowest HbA1c quartile had significantly decreased muscle strength in model 3 (−6.74 N · m ± 2.59; P = 0.01) with a trend toward significance after further accounting for peripheral neuropathy (−5.68 ± 3.03 N · m; P = 0.06; model 4). For muscle quality, those in the highest versus lowest HbA1c quartile had significantly decreased muscle quality in model 3 (−0.40 ± 0.17 N · m/kg; P = 0.01) and also after further accounting for peripheral neuropathy (−0.41 ± 0.19 N · m/kg; P = 0.03; model 4). These results reinforce the robustness of the findings obtained including imputed data, with an even more significant association for muscle quality using only measured data.
In additional sensitivity analyses, excluding participants with history of known diabetes, participants in the highest HbA1c quartile tended to have decreased knee extensor strength than those in the lowest quartile accounting for age, race, sex, weight, and height, although the difference was not statistically significant (−3.18 ± 2.53 N · m; P = 0.20). Similarly, decreased muscle quality (knee extensor strength/leg lean mass) was observed in HbA1c quartile 4 versus quartile 1, but the difference was not statistically significant (−0.16 ± 0.16 N · m/kg; P = 0.20). We found no significant differences of muscle mass across higher HbA1c quartiles (leg lean mass, total body lean mass, and thigh CSA). The inclusion of HbA1c as a diagnostic criterion for diabetes did not change results of sensitivity analyses.
In sensitivity analyses that additionally accounted for weight-training activities, we found that results were unchanged compared with primary analyses.
Conclusions
In this study, we found that elevated HbA1c, a marker of chronic hyperglycemia, was associated with persistently lower muscle strength compared with normoglycemia. The significant associations of hyperglycemia with lower muscle strength over time were independent of potential confounders, including demographics, anthropometrics, and physical activity, and were potentially accounted for, at least in part, by peripheral neuropathy. The robustness of these findings was confirmed in analyses that accounted for changing glycemic status during follow-up for persons with repeated HbA1c measures, which appropriately characterize the relationship of hyperglycemia with longitudinal changes in muscle outcomes.
To our knowledge, there have been no previous studies that related hyperglycemia with longitudinal changes in muscle strength or quality. Interestingly, studies looking at the effect of diabetes noted similar differences in knee extensor strength (∼4 N · m) in persons with versus without diabetes over 3 years (2) as those reported in our study for persons in the highest versus lowest quartile of HbA1c, which is in a range of potential clinical significance (15,27). The declines in muscle function were also greater in those with undiagnosed diabetes and more dramatic with longer diabetes duration or higher HbA1c in other studies (28). These findings suggested a potential role for hyperglycemia and/or insulin resistance in the accelerated loss of muscle observed in persons with diabetes. Yet previous studies were limited to a shorter follow-up period (<6 years) and restricted to older adults. Further, the effects of comorbidities that may be present in both impaired glucose states and diabetes (29), such as peripheral neuropathy, were not consistently taken into account. Our study further adds to this literature by specifically demonstrating that high HbA1c, a marker of sustained hyperglycemia, predicts persistently lower muscle strength and, potentially, muscle quality even after accounting for age-related declines. Our participants spanned a wide range of ages and follow-up was up to 7.5 years later. We also had availability of nerve conduction velocities, a well-validated measure of peripheral neuropathy, and found that the relationship of hyperglycemia with loss of muscle strength was partially accounted for by the presence of peripheral neuropathy.
Interestingly, in our cohort, hyperglycemia was not related to decreased skeletal muscle mass over time, which is in contrast with previous cross-sectional studies (21). Our findings also differ from previous reports of excessive loss of skeletal muscle mass in older adults with diabetes compared with those without diabetes (1). It is possible that factors distinct from hyperglycemia may relate to loss of skeletal muscle mass in persons with diabetes. Of note, we did find that muscle quality was lower in persons with hyperglycemia compared with those who had normoglycemia with aging in our study, although the results were not significant after accounting for confounders. Our results are similar to previous studies that have reported accelerated decline of muscle quality in persons with diabetes compared with those without diabetes (2), but more specifically characterizes the role of hyperglycemia in this process.
The effects of hyperglycemia and/or insulin resistance on mitochondrial dysfunction, protein degradation, and autophagy pathways in skeletal muscle have been described by other authors (30–32) and may be related to our observations of persistently decreased muscle strength and, potentially, muscle quality in persons with elevated levels of HbA1c. Further, reduced peripheral nerve function has been related to poorer lower extremity muscle strength in older adults (33); in our study, the association of hyperglycemia with decreased muscle strength was in part accounted for by peripheral neuropathy.
The relationship of hyperglycemia with longitudinal impairments in muscle function may represent the initial stage in the development of clinical phenotypes such as disability, frailty, and potentially early mortality in persons with diabetes. HbA1c is a relatively long-term measure of hyperglycemia that is subject to less measurement error compared with other biomarkers of glycemia (34). We and others have reported an association of HbA1c with functional disability (4,6) and mortality (35); muscle loss may mediate this association similar to older adults (27). HbA1c in higher ranges is also related to the development of frailty, a geriatric condition in which muscle weakness is a key criterion (3). Thus, the relationship of hyperglycemia with muscle impairment has potential wide-ranging consequences on both quality of life and mortality for persons with diabetes.
Interestingly, therapies that lower blood glucose or improve insulin sensitivity have been reported to improve skeletal muscle function by some authors. In observational studies, skeletal muscle mass loss is attenuated by use of insulin sensitizers in older men with diabetes (36). In interventional studies of animals and humans, the use of peripheral insulin sensitizers can lead to improved mitochondrial activity and less protein degradation in skeletal muscle, with measurable increases in lean body mass (30,37–40). However, these are preliminary findings that need to be better investigated in future studies. Also, the role of weight-bearing physical activities that could impact both glycemic control and quadriceps strength warrants further exploration. Well-controlled exercise interventions may improve both insulin sensitivity and mitochondrial function in skeletal muscle (41–44).
The strengths of our study are the comprehensive testing that was performed in BLSA and the assessment of both skeletal muscle strength and mass, allowing for characterization of muscle quality. Whereas past studies examining the association of diabetes with accelerated loss of muscle used only baseline glucose status (1,2), we were able to update glucose status with HbA1c at successive visits using rigorous analytic methods (mixed-effects regression models), allowing more robust assessment of glycemic exposure. Given the availability of valid information on peripheral neuropathy using NCVs, we were able to account for the contribution of neuropathy to our findings. We were also able to explore effects of hyperglycemia over and above age-related declines in muscle outcomes given the wide age range of our study participants.
The limitations of our study include the use of a single measure to assess hyperglycemia. However, HbA1c has the least variability of the glucose biomarkers available (34). While we had repeated HbA1c measures on many participants, this measure was not available for all participants included in this study, potentially leading to misclassification of glycemic status for some participants over time. Further, muscle quality was assessed using knee extensor strength divided by leg lean body mass; the assessment of leg muscle mass was not necessarily specific to the quadriceps muscle. Yet, this has been a commonly used as a measure of muscle quality in other studies (2). In our cohort, information on physical activity and peripheral neuropathy was not available for all visits, but we were able to impute results on visits with missing measurements. However, this may have still led to limited power in detecting associations particularly in the fully adjusted models, yet, compared with analyses with measured data only, resulted in more conservative estimates. Further, not having this information on peripheral neuropathy and physical activity in all participants could have impacted the results of our study, particularly those regarding older individuals who are more likely to be inactive. The length of follow-up was variable among participants. It is possible that longer follow-up may have resulted in different findings, and the effect of hyperglycemia may diminish after a few years.
We chose to use HbA1c quartiles based on our study population so that we could explore potential nonlinear relationships of hyperglycemia with muscle outcomes. However, results for muscle strength were similar when we defined HbA1c quartiles using clinically defined cutoffs (<5.7, 5.7–6.4, and ≥6.5%), though were limited by fewer participants in the HbA1c ≥6.5% group (data not shown). Despite moderately strong correlation, HbA1c levels may still underestimate postprandial hyperglycemia and its impact on skeletal muscle (45). We also could not account for the effects of glucose-lowering medications or diabetes duration on muscle outcomes given the relatively small proportion of patients with undiagnosed diabetes, particularly in lower HbA1c quartiles. It is possible that the definition of diabetes used also included participants who had prediabetes on glucose-lowering medications, but this would have likely underestimated the degree of muscle loss in participants with diabetes. The use of self-reported measures for physical activity may have led to residual confounding in our study in comparison with objective measures (46–48). Our observational study provides limited insight into possible mechanisms that could underlie the association of hyperglycemia with persistently decreased muscle strength, but this should be investigated in future studies.
In summary, our study demonstrates that hyperglycemia predicts persistently lower muscle strength after accounting for age-related effects but may be mediated, at least in part, by peripheral neuropathy. It remains unclear if hyperglycemia at different times of day (i.e., postprandial versus fasting) may have relatively greater impact on the accelerated loss of muscle; this should be explored in future studies. Further interventional studies are needed to better investigate if improving hyperglycemia and/or insulin resistance can impact loss of muscle strength or quality over time. Our findings may inform preventive efforts targeted at potentially preserving muscle function in older persons and, ultimately, can facilitate strategies to reduce the burden of disability for persons with diabetes.
Supplementary Material
Article Information
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK-093583), the Johns Hopkins University Older Americans Independence Center (P30-AG-021334), and the Intramural Research Program of the National Institute on Aging.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.R.K., J.E., S.H.G., and L.F. were responsible for study concept and design, analysis and interpretation of the data, and drafting and critical revision of the manuscript for important intellectual content. E.J.M. was responsible for study concept and design, acquisition of data, analysis and interpretation of data, and drafting and critical revision of the manuscript for important intellectual content. E.J.M. and L.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-1166/-/DC1.
References
- 1.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study . Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study . Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes Care 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 3.Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc 2012;60:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower-extremity disability in older women with diabetes: the Women’s Health and Aging Study. Diabetes Care 2003;26:70–75 [DOI] [PubMed] [Google Scholar]
- 5.Ryerson B, Tierney EF, Thompson TJ, et al. Excess physical limitations among adults with diabetes in the U.S. population, 1997-1999. Diabetes Care 2003;26:206–210 [DOI] [PubMed] [Google Scholar]
- 6.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care 2010;33:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 8.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 9.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–85 [DOI] [PubMed] [Google Scholar]
- 11.Lazarus R, Sparrow D, Weiss ST. Handgrip strength and insulin levels: cross-sectional and prospective associations in the Normative Aging Study. Metabolism 1997;46:1266–1269 [DOI] [PubMed] [Google Scholar]
- 12.Barzilay JI, Cotsonis GA, Walston J, et al. Health ABC Study . Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care 2009;32:736–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care 2005;28:2541–2542 [DOI] [PubMed] [Google Scholar]
- 14.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci 2012;67:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum K, Hildebrandt U, Edel K, et al. Comparison of skeletal muscle strength between cardiac patients and age-matched healthy controls. Int J Med Sci 2009;6:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 17.Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc 2014;62:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 19.Shock NW, Greulich RC, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC, U.S. Govt Printing Office, 1984 (publ. no. 84-2450) [Google Scholar]
- 20.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol 1997;83:1581–1587 [DOI] [PubMed] [Google Scholar]
- 21.Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999-2002. J Am Geriatr Soc 2013;61:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–1112 [DOI] [PubMed] [Google Scholar]
- 23.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755 [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80 [DOI] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, John Wiley & Sons, Inc., 1987 [Google Scholar]
- 26.Aminoff M. Clinical Electromyography in Clinical Practice. 3rd ed. Aminoff M, Ed. New York, Churchill-Livingstone, 1998, p. 488–489 [Google Scholar]
- 27.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2002;50:897–904 [DOI] [PubMed] [Google Scholar]
- 28.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes 2006;55:1813–1818 [DOI] [PubMed] [Google Scholar]
- 29.Rajabally YA. Neuropathy and impaired glucose tolerance: an updated review of the evidence. Acta Neurol Scand 2011;124:1–8 [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006;147:4160–4168 [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 32.Kaushik S, Rodriguez-Navarro JA, Arias E, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab 2011;14:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. Health ABC Study . Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2009;57:2004–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007;167:1545–1551 [DOI] [PubMed] [Google Scholar]
- 35.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011;34:1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CG, Boyko EJ, Barrett-Connor E, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group . Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011;34:2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P. Improved skeletal muscle oxidative enzyme activity and restoration of PGC-1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 2007;31:1302–1310 [DOI] [PubMed] [Google Scholar]
- 38.Rabøl R, Boushel R, Almdal T, et al. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab 2010;12:806–814 [DOI] [PubMed] [Google Scholar]
- 39.Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 2005;288:E930–E934 [DOI] [PubMed] [Google Scholar]
- 40.Skov V, Glintborg D, Knudsen S, et al. Pioglitazone enhances mitochondrial biogenesis and ribosomal protein biosynthesis in skeletal muscle in polycystic ovary syndrome. PLoS ONE 2008;3:e2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care 1998;21:1353–1355 [DOI] [PubMed] [Google Scholar]
- 42.Praet SF, Jonkers RA, Schep G, et al. Long-standing, insulin-treated type 2 diabetes patients with complications respond well to short-term resistance and interval exercise training. Eur J Endocrinol 2008;158:163–172 [DOI] [PubMed] [Google Scholar]
- 43.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 44.van Tienen FH, Praet SF, de Feyter HM, et al. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab 2012;97:3261–3269 [DOI] [PubMed] [Google Scholar]
- 45.van Dijk JW, Manders RJ, Hartgens F, Stehouwer CD, Praet SF, van Loon LJ. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res Clin Pract 2011;93:31–37 [DOI] [PubMed] [Google Scholar]
- 46.Westerterp KR. Assessment of physical activity: a critical appraisal. Eur J Appl Physiol 2009;105:823–828 [DOI] [PubMed] [Google Scholar]
- 47.Hamer M, Stamatakis E, Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. Med Sci Sports Exerc 2014;46:1946–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James BD, Boyle PA, Bennett DA, Buchman AS. Total daily activity measured with actigraphy and motor function in community-dwelling older persons with and without dementia. Alzheimer Dis Assoc Disord 2012;26:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.