Abstract
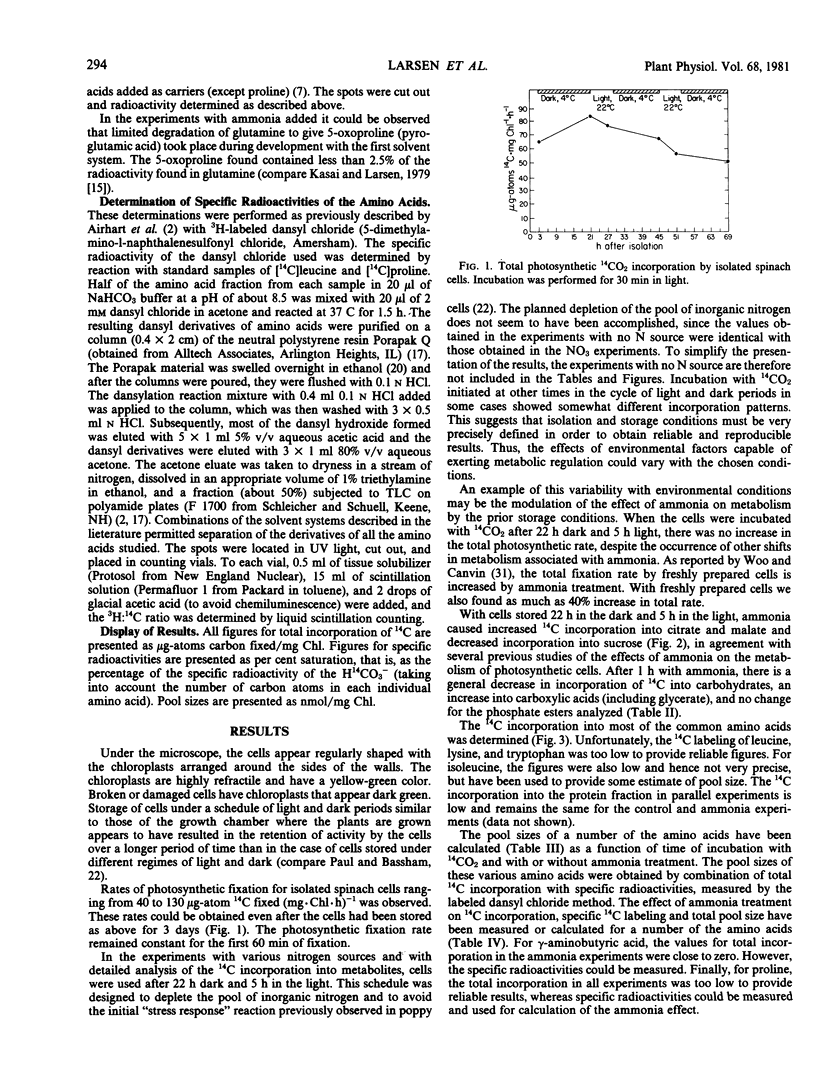
Isolated cells from leaves of Spinacia oleracea have been maintained in a state capable of high rates of photosynthetic CO2 fixation for more than 60 hours. The incorporation of 14CO2 under saturating CO2 conditions into carbohydrates, carboxylic acids, and amino acids, and the effect of ammonia on this incorporation have been studied. Total incorporation, specific radioactivity, and pool size have been determined as a function of time for most of the protein amino acids and for γ-aminobutyric acid. The measurements of specific radio-activities and of the approaches to 14C “saturation” of some amino acids indicate the presence and relative sizes of metabolically active and passive pools of these amino acids.
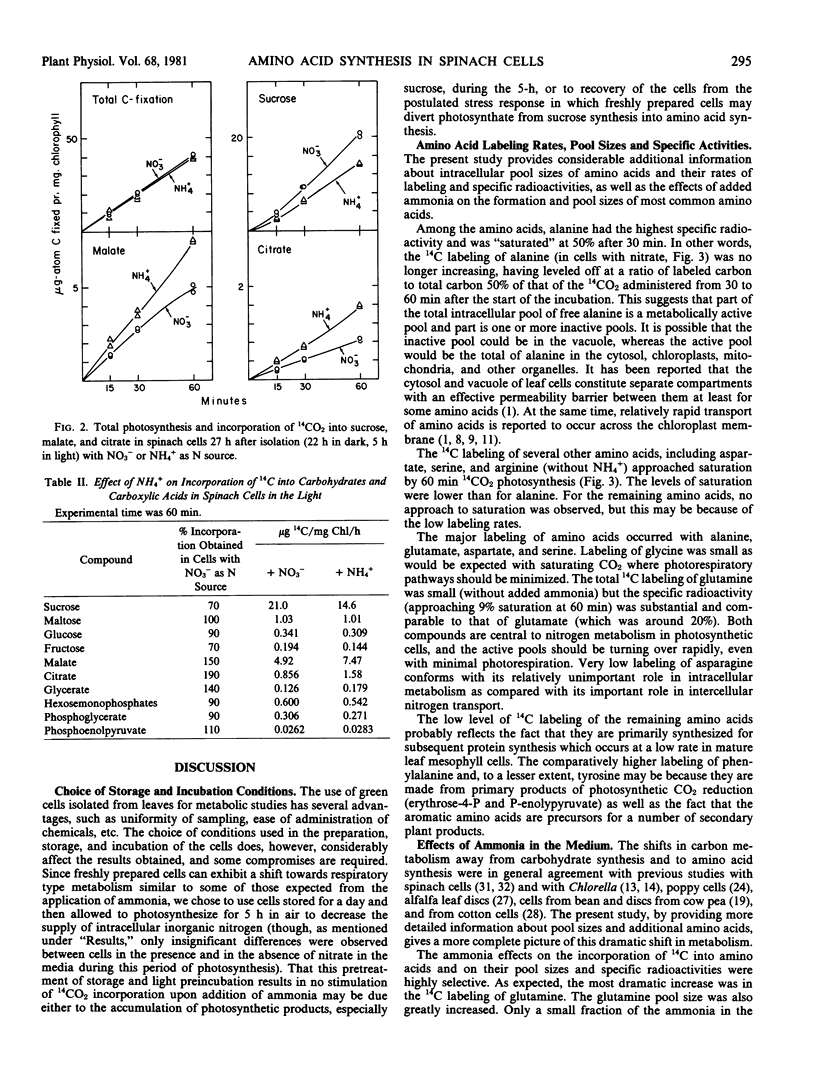
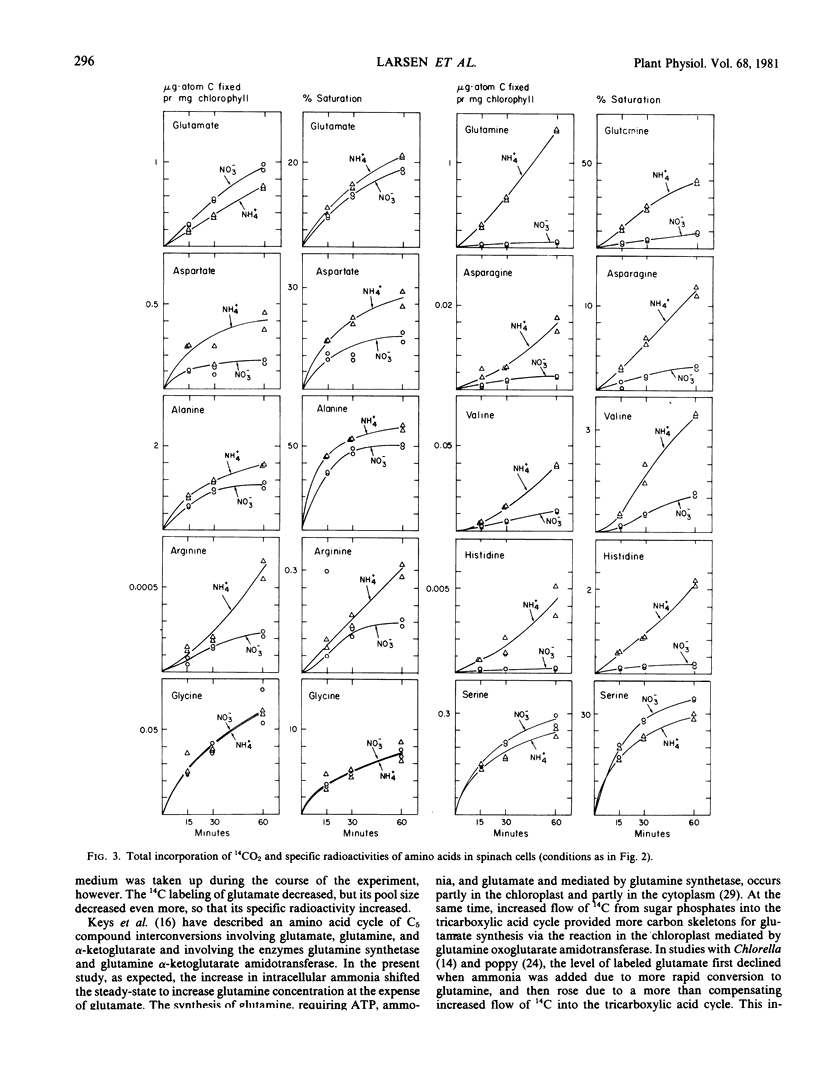
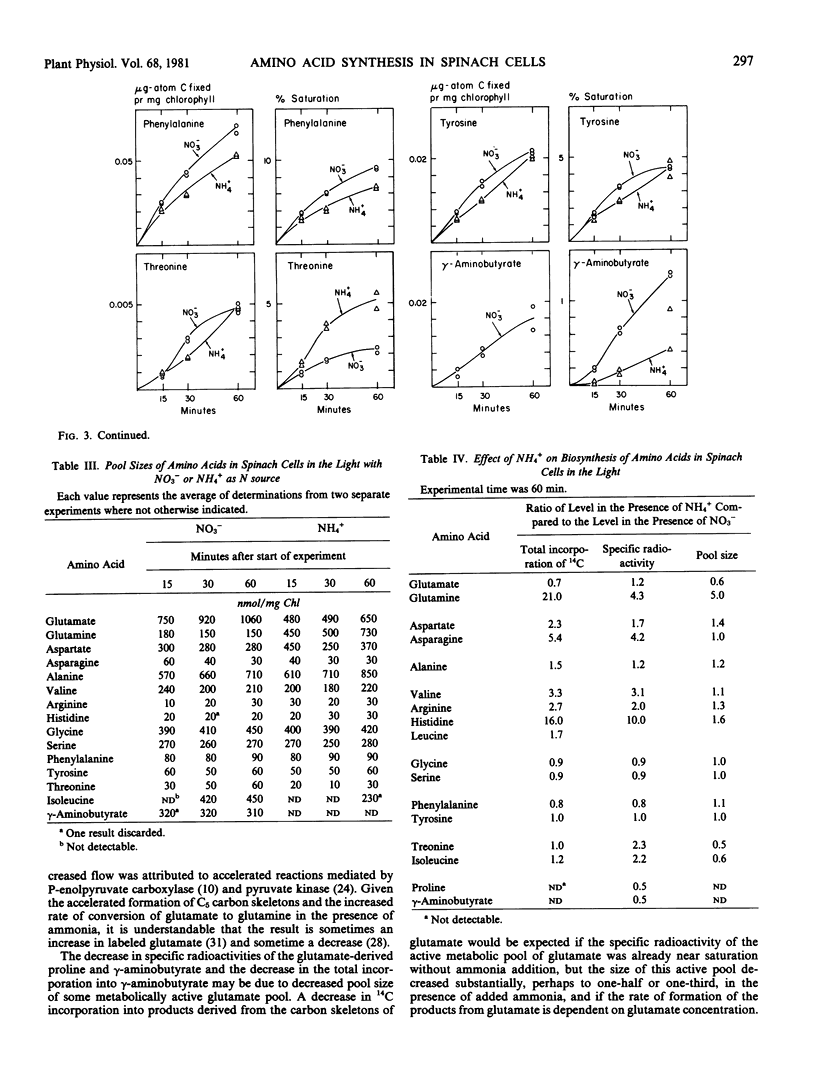
Added ammonia decreased carbon fixation into carbohydrates and increased fixation into carboxylic acids and amino acids. Different amino acids were, however, affected in different and highly specific ways. Ammonia caused large stimulatory effects in incorporation of 14C into glutamine (a factor of 21), aspartate, asparagine, valine, alanine, arginine, and histidine. No effect or slight decreases were seen in glycine, serine, phenylalanine, and tyrosine labeling. In the case of glutamate, 14C labeling decreased, but specific radioactivity increased. The production of labeled γ-aminobutyric acid was virtually stopped by ammonia.
The results indicate that added ammonia stimulates the reactions mediated by pyruvate kinase and phosphoenolpyruvate carboxylase, as seen with other plant systems. The data on the effects of added ammonia on total labeling, pool sizes, and specific radioactivities of several amino acids provides a number of indications about the intracellular sites of principal synthesis from carbon skeletons of these amino acids and the selective nature of effects of increased intracellular ammonia concentration on such synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Kanazawa T., Kanazawa K., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in Chlorella pyrenoidosa during photosynthesis and respiration. Biochim Biophys Acta. 1972 Mar 16;256(3):656–669. doi: 10.1016/0005-2728(72)90201-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Macnicol P. K. Determination of specific radioactivity of plant amino acids using dansylation. Anal Biochem. 1978 Mar;85(1):71–78. doi: 10.1016/0003-2697(78)90275-0. [DOI] [PubMed] [Google Scholar]
- Mills W. R. Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 1980 Jun;65(6):1166–1172. doi: 10.1104/pp.65.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H., Gnanam A. A possible mechanism of ammonium ion regulation of photosynthetic carbon flow in higher plants. Plant Physiol. 1979 Aug;64(2):263–268. doi: 10.1104/pp.64.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederwieser A. Adsorption of neutral polystyrene resin. A simple method for extraction of 2,4-dinitrophenyl derivatives from aqueous solution and for decoloration of protein hydrolysates. J Chromatogr. 1971 Jan 20;54(2):215–223. doi: 10.1016/s0021-9673(01)80267-3. [DOI] [PubMed] [Google Scholar]
- Paul J. S., Bassham J. A. Effects of Sulfite on Metabolism in Isolated Mesophyll Cells from Papaver somniferum. Plant Physiol. 1978 Aug;62(2):210–214. doi: 10.1104/pp.62.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. S., Bassham J. A. Maintenance of High Photosynthetic Rates in Mesophyll Cells Isolated from Papaver somniferum. Plant Physiol. 1977 Nov;60(5):775–778. doi: 10.1104/pp.60.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Jensen R. G. Metabolism of Separated Leaf Cells: III. Effects of Calcium and Ammonium on Product Distribution During Photosynthesis with Cotton Cells. Plant Physiol. 1973 Jul;52(1):17–22. doi: 10.1104/pp.52.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]



