Abstract
There is an increasing interest in rare earth (RE) doped nanoparticles (NPs) due to their sharp absorption and photoluminescence (PL) in the near infrared (NIR) spectral region. These NIR based nanoparticles (NPs) could allow biological imaging at substantial depths with enhanced contrast and high spatial resolution due to the absence of auto fluorescence in biological samples under infrared excitation. Here, we present the highly efficient infrared photoluminescence in GdF3:Nd3+ nanoparticles under 800 nm excitation within the hydrodynamic size limitations for bio-applications. The downconversion (Stokes emission) absolute quantum yield (QY) measurements in powder, poly maleic anhydride- alt-1- octadicene (PMAO) coated powder and colloidal solutions have been investigated. QY measurements have revealed that downconversion(Stokes emission)QY in an average 5 ± 2 nm sized GdF3: 1% Nd3+colloidalNPs are 2000 times higher than efficient upconversion (UC) particles NaYF4: 20 % Er/ 2% Yb of same size. Furthermore, the utility of these NIR emitting nanoparticles forbioimagingprobe has been demonstrated by confocal imaging and spectroscopic study.
1. Introduction
Fluorescence analysis is one of the most important procedures in biology and biomedicine due to its non-invasive mode and its extraordinary sensitivity.1–4 Generally speaking, a biomolecule probe is attached with certain markers to produce a quantifiable fluorescent signal.1–3 As requirements for bio-analysis, neither the bioactivity of the host probe nor the optical property of the marker is expected to be changed or damaged during imaging. In the past, dyes were extensively studied for their application as a biomarker.5, 6 However, to serve as optical markers, the rapid photobleaching of dyes limits the available detection time, and the short fluorescent lifetimes (on the order of nano and pico seconds) and broad emission do not benefit reducing the background interference to increase the signal to noise ratio.7, 8 As a result, the major marker system still relies on fluorescent proteins, whose manipulation can be combined with genetic techniques.1, 4, 9, 10 These engineered proteins can be endogenously expressed by organisms, but always suffer from weak luminescence.4, 11–13 These limitations create a strong need for another class of fluorescent markers.
Over the past decade, quantum dots (QDs) have attracted a great deal of attention from biologists.14–16 QDs are distinguished from all other materials by their characteristics of unique size and composition-dependent luminescence which can be tuned. As a fluorescent marker, QDs are gifted with many advantages such as relatively narrow emission bandwidth, considerable photo-stability, single-source- excitation for multiplex detection, and comparable size with that of biomolecules (below 10 nm).14–16 However, toxicity, and high synthesis expenditure in QDs are some of the drawbacks in using QDs for high throughput and in vivo applications.17
The demand for new markers is still on-going. In recent years, rare earth (RE) doped upconversion (UC) nanomaterials have been proposed as an alternative candidate to quantum dots and dyes.18–20 Being a crystalline host, negligibly low level of leaching of ions has been observed for these inorganic phosphors, which is essential for biological applications.21, 22 In our recent work, we have shown that rare earth fluorides and oxysulphides are not toxic below the concentration of 200 μg/mL and easily uptaken by cells.23, 24 The nontoxic nature enables these nanomaterials to act as contrast agents in biomedical imaging. After the first report of Er/Yb doped NaYF4 UC nanoparticles as bio probes, research has expanded quickly to include RE doped UC nanoparticle being used for immunoassays.19, 25–34 One advantage of these RE doped UC nanophosphors is background free images with high signal to noise ratio. Since the excitation wavelength (980 nm) lies within the biological transparent window of 680–1100 nm, where soft tissues do not strongly absorb or scatter, it is possible to excite these UC phosphors at a higher tissue depth than with a UV-VIS excitation source. However, recent study by Wang et al. have shown a temperature rise of 10 °C for samples (5 mg UCNP per mL of deionized water) under the 980 nm excitation for 10 min, in contrast to 2 °C under 808 nm excitation (both at a power density of 35 W/cm2).35 The same rise of temperature was observed for deionized water as well.35 In the same study, Wang et al. observed the death of all cells (HEK 293T) at the excitation power density of 400 mW/cm2 under 980 nm excitation, while most of the cells survived at 808 nm laser excitation for 5 minute.35 In our recent studies, downconversion (Stokes) emission at 1064 nm from fluoride based nanoparticles was detected through a thickness up to 4.93 mm of pig skin under 800 nm excitation compared to that of 1.3 mm for UC emission (red) under 980 nm excitation.36, 37 These effects may be due to the higher absorption coefficient of water (0.48 cm−1) at 980 nm compared to that of 0.02 cm−1 at 800 nm. More importantly, at 800 nm excitation, a larger portion of incident photons is absorbed by hemoglobin, instead of water. Consequently, the laser induced heating effect due to water absorption is much less when using an at 800 nm excitation source than a 980 nm source. Also, fast blood circulation ensures heat dissipation which is beneficial for minimizing the local overheating with laser excitation at 800 nm especially for in vivo setup.
In addition, the quantum yield (QY) of these as prepared UC nanophosphors is low due to the multiphoton photon process involved in the fluorescence mechanism at 980 nm excitation. Above all, UC fluorescence of these Er/Yb doped phosphors lies in visible (red + green) region where soft tissues show strong absorption or scattering.
On the other hand, the hydrodynamic diameter of the functionalized particles should be less than 50 nm to be useful for bio applications. Within this limitation, the QY for as prepared UC nanophosphors has to be enhanced drastically to make them useful as biomarkers. Despite recent progress in the synthetic approach for RE-doped UC nanocrystals, it is still very challenging to obtain an efficient UC with QY higher than 0.1% within the size limitation discussed above. The reason for the lower UC QY in RE doped nanocrystals is mainly due to the multiphoton absorption process necessary for UC emission. As the nanoparticles size decreases, the concentration of the surface dopants increases leading to the suppression of emission intensity due to quenching and increased concentration of crystal defects. On the other hand, Nd3+ has a NIR excitation band at wavelengths shorter than 980 nm, such as 800, corresponding to transitions from 4F5/2.38 The stokes emission process in Nd3+:GdF3 under 800 nm excitation further ensures the higher QY at 800 nm excitation compared to that of UC based nanoparticles at 980 nm excitation.
With this in mind, this work explores the absolute downconversion QYs of these Nd3+ doped GdF3 phosphors in comparison with efficient UC nanoparticles NaYF4: Yb/Er. Since the QY for UC photo luminescence has been shown to be much lower than that of the downconversion emission, here we propose and demonstrate the efficient downconversion emission in as prepared Nd3+ doped GdF3 with an average size of 5 ± 2 nm under 800 nm excitation. The absolute UC (anti-Stokes) QYs for the commonly available NaYF4: 20 %Er/2%Yb are compared with the downconversion quantum yield of GdF3:Nd3+ of similar size. In addition, the synthesis and morphology of GdF3:Nd3+ nanophosphors are reported. Based on the downconversion QY obtained even at this nano size, we propose these downconverting efficient infrared-based GdF3: Nd3+ phosphors as more suitable candidate for deep tissue bioimaging application over the UC (UC) particles with the added benefit of minimum absorption and heating of the biological medium. To the best of our knowledge, this is the first report on the extensive analysis of the downconversion QY in a phosphor comparing with UC QY in β-NaYF4: 20 % Er/2% Yb of same size in the infrared region. Furthermore, cellular uptake and distribution of these NIR emitting nanoparticles within the cell were demonstrated by confocal imaging.
2. EXPERIMENTAL SECTION
2.1 Synthesis of Nanoparticles
The GdF3:Nd3+ nanoparticles were synthesized by the thermal decomposition (TD) route using trifluroacetate precursors and a three neck flask heated to 345°C in a nitrogen atmosphere. The Nd3+ doping concentration was varied to yield 0.1, 0.5, 1.0, and 2.0 mol% of the solution.39 After synthesis, the solution was sonicated with ethanol, centrifuged to separate the product, and freeze dried. The nanoparticles were coated with poly maleic anhydride- alt-1-octadicene (PMAO) as described in the literature to provide a platform for further bioconjugation.40
The X-ray powder diffraction (XRD) measurements were done using the RIGAKU Ultima IV X-ray diffractometer with Cu Kα (λ = 1.5 A) to determine the phase and structure of the particles. The morphology of the GdF3 doped with Nd3+ nanoparticles were characterized by using transmission electron microscope (TEM) and high resolution transmission electron microscopy (HRTEM 2010F) operated at 200 kV. The Scanning Transmission Electron Microscope (STEM) images were recorded in probe Cs-corrected JEOL JEM-ARM 200F operated at 200 kV. High Angular Annular Dark Field (HAADF) STEM images were obtained with a convergence angle of 26 mrad and the collection semi-angles from 50 to 180 mrad. These variations in semi-angles satisfy the conditions set forth for the detectors to eliminate contributions from unscattered and low-angle scattered electron beams. The probe size used was about 0.09 nm with the probe current of 22 pA. In addition, bright field (BF) STEM images were recorded by using a collection semi-angle of 11 mrad. Electron dispersive x-ray (EDX) spectrum was obtained using a probe size of 0.13 nm with the probe current 86 pA. Furthermore, high resolution TEM analyses were also performed using a JEOL JEM-2010F TEM equipped with a field emission gun operated at 200 kV. Finally, the digital micrograph software GATAN was used to analyze the collected TEM images.
2.2 Optical Measurements
Fluorescence characterization of the synthesized nanocrystals was done using the near infrared (NIR) 800 nm power tunable fiber coupled Fabry Perot continuous laser diode (Thorlab, Model LM14S2) and the emission of the sample was measured by the QuantaMaster 51 spectrofluorimeter from Photon Technology International Inc. (PTI) with a InGAS detector using the appropriate settings. The integrating sphere setup was built for the QY measurement as shown in ESI†-S1.
2.3 Confocal Microscopy
Confocal imaging was done using customized Zeiss 710 multiphoton system. The cells were imaged by exciting the DAPI at 560 nm, the Alexa fluor at 647 nm. Emission at 532 nm from the particles was used to trace the particles within the cells under 488 nm excitation.
3. RESULT AND DISCUSSION
3.1 Structural Characterization
Since the XRD data for the hexagonal GdF3 have not been reported and show slight deviations from the orthorhombic GdF3 crystal (PDF No. 12-0788), this phase was compared with hexagonal SmF3 (PDF No. 05-0563) and all the diffraction peaks in Fig. 1 was indexed to the hexagonal structure. The X-ray peak positions and intensities for GdF3: × % Nd3+ (x = 1% and 2%) nanocrystals were matching very well with PDF No. 05-0563 and PDF 72-1435 reported for hexagonal SmF3 and LaF3 nanoparticles respectively.41 The XRD results reveal that the well-crystallized GdF3: Nd3+ phosphors is in hexagonal phase with cell parameters a = b = 0.6998, and c = 0.6813 nm. It can be seen that (111) peak of sample is sharper compared to other peaks. Again, the broadened peaks are due to the nanometer size of the particles.
Fig. 1.
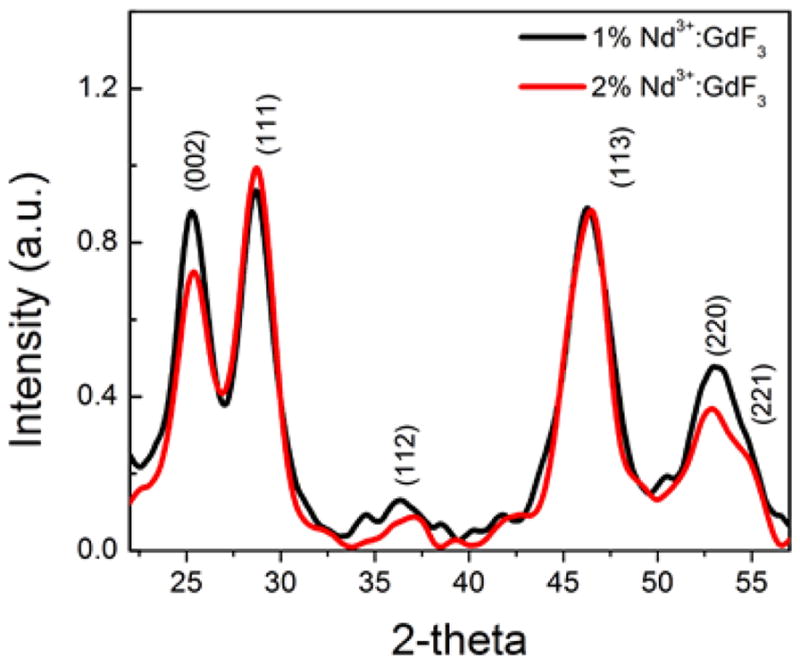
XRD patterns of the GdF3: 1% Nd3+ and 2% Nd3+ nanoparticles synthesized through thermal decomposition
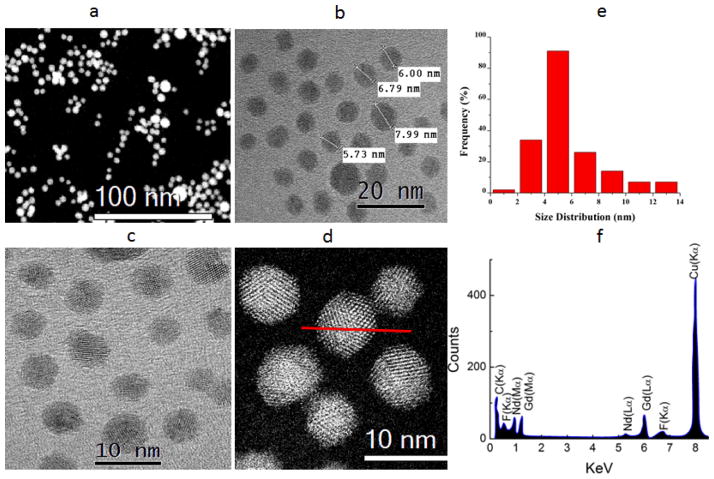
Figure 2 (a, b, and c) shows the HAADF and bright field STEM images of the GdF3:Nd3+ nanoparticles. Fig. 2 (d) show the magnified bright field STEM image of the nanoparticles confirming the two different morphology of the synthesized particles with size distribution of about 2–14 nm as shown in histogram in the inset of Fig. 2(e). Statistically, nearly all of the particles were found to be quasi-spherical with roughly 2 % of the nanoparticles being octahedral. The quasi-spherical morphology of the particles can be seen in the Fig. 2 (a, b, and c). The compositional distribution of each element in the nanoparticles was revealed by EDX line-scanning analysis. Line-scan EDX spectrum of Gd, F doped with Nd, as shown in Fig. 2 (f) was recorded through the center of an individual nanoparticle marked by a red line as shown in Fig. 2(d). The Gd-L, M and F-K, and Nd-L, M; EDX signals were clearly traced across the region of the nanoparticles as shown in Fig. 2(f).
Fig. 2.
(a, b) HAADF STEM image of the Nd3+: GdF3 nanoparticles synthesized through thermal decomposition, (b, c) bright field images of the GdF3:Nd3+ nanoparticles, (d) High resolution HAADF image of the GdF3:Nd3+ nanoparticles at atomistic level, (e) Particle size distribution of GdF3:Nd3+ nanoparticles (f) EDX pattern confirming the doping of Nd3+.
The HRTEM images as shown Fig. 3 (a, b, and c) with the clear lattice fringes clearly indicate the crystallinity of the GdF3: Nd3+ nanoparticles. A high resolution HRTEM image as shown in Fig. 3 (a) also elucidates the octahedral morphology of the synthesized particles.
Fig. 3.
(a) Octahedral morphology of the particles, (b, c) lattice fringes for (111) plane from the selected area as shown in (a), (d) selected area electron diffraction pattern of GdF3 nanocrystals, (e) HAADF STEM Z-contrast image of the GdF3: Nd3+ nanocrystals, (f) Z-contrast HAADF image of the GdF3:Nd3+ nanoparticles at atomistic level with disparity of intensity indicating that nanoparticles is composed of different atoms of Gd, Nd, and F.
High-resolution TEM images of a single octahedral GdF3: Nd3+ nanocrystal with (111) lattice fringes are shown in Fig. 3 (b, c). The selected area electron diffraction pattern as shown in Fig. 3 (d) shows the diffraction rings of GdF3 nanocrystals, which further confirms the hexagonal phase of the nanocrystal with majority oriented in (111) direction. The presence of clear spots in every ring confirms the higher order crystallinity of GdF3:Nd3+ nanoparticles. Additionally, HAADF images were collected as shown in Fig. 3 (e) and Fig. 3 (f) to study the dopants profile in the nanocrystals. Although atomistic level Z- contrast HAADF STEM images are usually found to be useful to identify the elements within a nanoparticle, it was not helpful in this case due to the similar contrast observed in the HAADF images since the atomic number of Nd (60) and Gd (64)only differs by four. However, we have observed the variation in the intensity profile as shown in Fig. 3(f), which is an indication that nanoparticles are composed of different atoms of Gd, Nd and F.
3.2 Quantum Yield for GdF3: Nd3+
First, near-infrared fluorescence for GdF3: Nd3+ phosphors powders was collected as a function of the Nd3+ concentration with identical experimental conditions under 800 nm excitation at the excitation power density of 12.74 W/cm2 as shown in Fig. 4 (a). The slits for the fluorescence measurement were opened significantly to allow enough photons into the spectrofluorometer. The saturation of the detector was controlled using optical density filters. Since the NIR detector of the spectrofluorometer saturated faster with excitation source than the emission spectra, neutral density filters with OD = 2.0 were used. Corrected spectra were generated for the final calculation. The emission spectrum was taken between 850–1400 nm with the peak emission intensity at 1064 nm. Among three primary NIR emission bands at 891, 1064, and 1344 nm, 1064 nm band was found to have the largest intensity. The GdF3:Nd3+ with a 1.0% doping of Nd3+ yielded the highest 1064 nm emission intensity while samples with higher doping of Nd3+ have shown weaker emission possibly due to quenching by the high volume of surface defects (See ESI†-S2 for GdF3: 2 % Nd3+). Based on this fluorescence analysis, GdF3: 1% Nd3+ was selected for the downconversion QY measurement.
Fig. 4.
Fluorescence profile at the excitation power density of 12.74 ± 2.0 W cm−2 under 800 nm excitation, (a) GdF3:Nd3+ at 0.1%, 0.5%, 1.0%, and 2.0% doping concentrations (b) GdF3:1% Nd3+ at various excitation power densities as shown in inset of figure, (c) laser profile under the ref (black) and with sample (red), (d) fluorescence profile for GdF3:1% Nd3+ with PMAO coating.
Due to the experimental limitations, measurements were carried out at three different excitation power densities under 800 nm excitation for the GdF3: 1% Nd3+ powders as shown in Fig.4 (b). Fig. 4 (c) shows the diffuse reflectance of the excitation spectra for the sample and the reference. The difference in area of the diffuse reflectance was used to calculate the number of photon absorbed (see ESI†-S3 and ESI†-S4). The maximum total absolute downconversion QY of 10.2 ± 1.5 % was measured for the GdF3:Nd3+ nanophosphor powder at the excitation power density of 12.74 ± 2.0 W cm−2 at 800 nm excitation. Similarly, downconversion QY of 5.02 ± 0.75 % and 2.2 ± 0.33 % were measured at the excitation power density of 5.3 ± 0.8 and 1.4 ± 0.2 W/cm2 respectively. Any emission and absorption by nanoparticles was not observed during the indirect excitation. This may be due to narrow absorption and emission cross sections of the trivalent REs ions. This shows that reflectivity of the reference, particle size effects, and diffuse reflectance from the front part of the quartz window of the sample holder are the primary source for the error in this measurement but not reabsorption.
With the known quantum yield of 10 % for IR-140 dye at the spectral range of 862–1013 nm range at 150 mW of excitation power under 800 nm excitation, a comparison method was also implemented to check the accuracy of the measurement as discussed in ESI†-S5.5 Comparison measurement for 1% Nd3+ :GdF3 powder shows the downconversion QY in GdF3:1% Nd3+ is 5.8 ± 0.87 % at 150 mW (4.77 W/cm2) of excitation under 800 nm, which is very close to the measured QY at 5.3 W/cm2 using integrating sphere for GdF3:1% Nd3+ powder. This clearly validates our QY measurement setup with integrating sphere. Details about the QY setup, calibration and measurements have been discussed in section ESI†-S1, ESI†-S3, ESI†-S4, and ESI†-S5. Scaling of the downconversion emission spectra revealed that the 1064 nm emission from Nd3+ represents around 90 % of the overall downconversion emission intensity. In addition, comparing with the UC QY of 0.005 ± 0.0005% (at 150 W/cm2) reported by Van Veggel et al. for β-NaYF4: 20% Yb3+/2% Er3+ of 8–10 nm size particles, downconversion QY for GdF3:1% Nd3+ nanophosphor powder is 2000 times higher even at the excitation power density of 12.74 ± 2.0 W cm−2 at 800 nm excitation.42 This shows that these particles have a higher QY within the biological window which yields more photon counts (information density) for bioimaging applications compared to UC nanoparticles.
3.3 Heating Effect Evaluation
It is expected that lowering the excitation power density will minimize the laser induced heating in the cells. The excitation power density used in this work (12.74± 2.0 W cm−2) was one third of the power density used by Wang and et al. (35 Wcm−2) in their study.35 Therefore, we expect the temperature change due to laser induced heating at 800 nm excitation should be less than 1°C based on the 2 °C rise wang et al. observed. We expect that the downconversion (Stokes) QY in these phosphors are efficient enough for in vitro and in vivo applications.
3.4 Quantum Yield for PMAO Coated GdF3:Nd3+ powder
Fig 4 (d) shows the NIR emission spectra obtained with and without the additional PMAO coating for the GdF3:1% Nd3+ at the excitation power density of 12.74 ± 2.0 W/cm2. The measured downconversion QY for GdF3:1% Nd3+ coated with PMAO does not show significant change for these particles, which is important since the polymer coating is essential in making the particles biocompatible. The PMAO was expected to further increase the stability of the particles and water dispersibility. The long suspension time in water for the PMAO coated particles compared to that of uncoated particles ensures the increased water dispersibility of the particles (see ESI† S6).
3.5 Quantum Yield for Colloidal GdF3:Nd3+
The comparison method was implemented to measure the downconversion QY for colloidal GdF3: 1% Nd3+ with respect to reported QY for the dye IR-140 to mimic the experimental condition.5 Using the QY of 10 % reported for the IR-140 at 150 mW (4.77 W/cm2) of excitation under 800 nm, downconversion QY for GdF3: 1% Nd3+ at the concentration of 0.05 mg/mL was measured to be 1 ± 0.05 %. Similarly, QY of 1.5 ± 0.075 % was measured for GdF3:1% Nd3+ at 255 mW (8.28 W/cm2). This verifies that QY for collodial GdF3: 1% Nd3+ is concentration and excitation power density dependent. The spectra collected for colloidal GdF3: 1% Nd3+ at different powers is shown in Fig. S7 with respect to the spectra for IR-140 under 800 nm excitation.
3.6 Chemical Stability and Imaging
The results shown in ESI† S8 suggest that the fluorescence at 1064 nm is not significantly affected over a large variation in the pH. The pH independent fluorescence QY in GdF3: 1% Nd3+ nanoparticles shows that these nanoparticles (GdF3: 1% Nd3+) are chemically stable. These results further confirm that there is no significant increase in defects within the nanoparticles when these particles were introduced in different medium. Being a crystalline host, we expect significantly low level of leaching of ions in these medium as observed by Lux and Passuello for an un-doped GdF3 nanoparticles in their recent in vivo study.21, 22
3.7 Cytotoxicity and Cell Viability
Cytotoxicity tests were performed using MDA-MB-231 cells which were incubated with concentrations ranging from 50 to 200 μg/mL of uncoated and PMAO coated GdF3:Nd3+ nanoparticles as shown in Fig. 5. After 24 hours, an MTT assay was used to assess the toxicity of nanoparticles at these concentrations. The PMAO coating was chosen to increase water solubility, cellular uptake, and provide a carboxyl group for ease of further functionalization. For the lower concentrations, the cell viability was ~ 70% and there was no significant difference between the PMAO coated and uncoated nanoparticles. For the highest concentration of the PMAO coated nanoparticles, they actually promoted cell proliferation as is evidenced by the large increase in cell population.
Fig. 5.
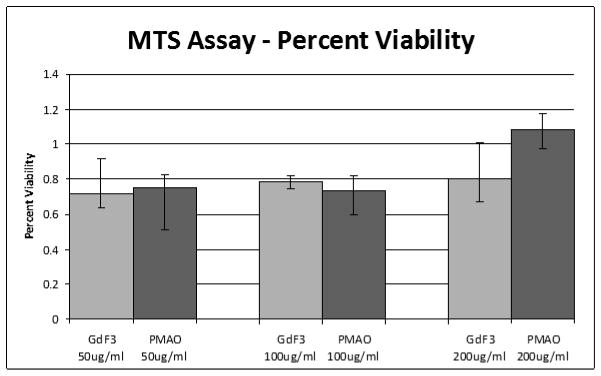
Toxicity study of GdF3:Nd3+ nanoparticles using MDA-MB-231 cells obtained by using MTT assay after 24 hrs. Both original and PMAO coated nanoparticles were used at different concentrations with minimal cell death occurring at all the concentrations studied.
Higher cell viability rate was also observed for these present phosphors in fibroblast cell line.36 The higher cell viability rate for these particles confirms that these PMAO coated nanophopshors (GdF3: 1% Nd3+) are not toxic even at the high concentration of 200 μg/ml.
Finally, the suitability of these nanoparticles for bioimaging applications was investigated using the confocal mode in multiphoton/confocal microscope. Fibroblast cells (L929) were incubated with the PMAO coated GdF3: 1% Nd3+ phosphors for 24 hours, stained, and imaged with a confocal microscope (ESI† S9). Optical slices of the cells were collected by exciting the DAPI at 358 nm, the Alexa Fluor at 647 nm.
The nanoparticles were excited with at 488 nm and their emission at 532 nm was used to create the nanoparticle channel in the multiphoton microscopy since the current detectors do not respond to 1064 nm. In this paper, the confocal microscopy study was included to investigate how the nanoparticles were being internalized within the cells. It can be clearly seen that nanoparticles have been internalized by the fibroblasts within 24 hours as shown in Fig. 6. The nanoparticles were found well dispersed within the cytoplasm and the nuclei. In addition, we did not observe any clustering of the nanoparticles or deformation of the cells due to the nanoparticles during imaging. Although, 532 nm fluorescence from particle was used to locate the nanoparticles within the cells, the primary goal is to utilize these nanoparticles for NIR-to-NIR imaging applications, and the visible emission was merely used to locate the nanoparticles within cells under confocal microscopy.
Fig. 6.
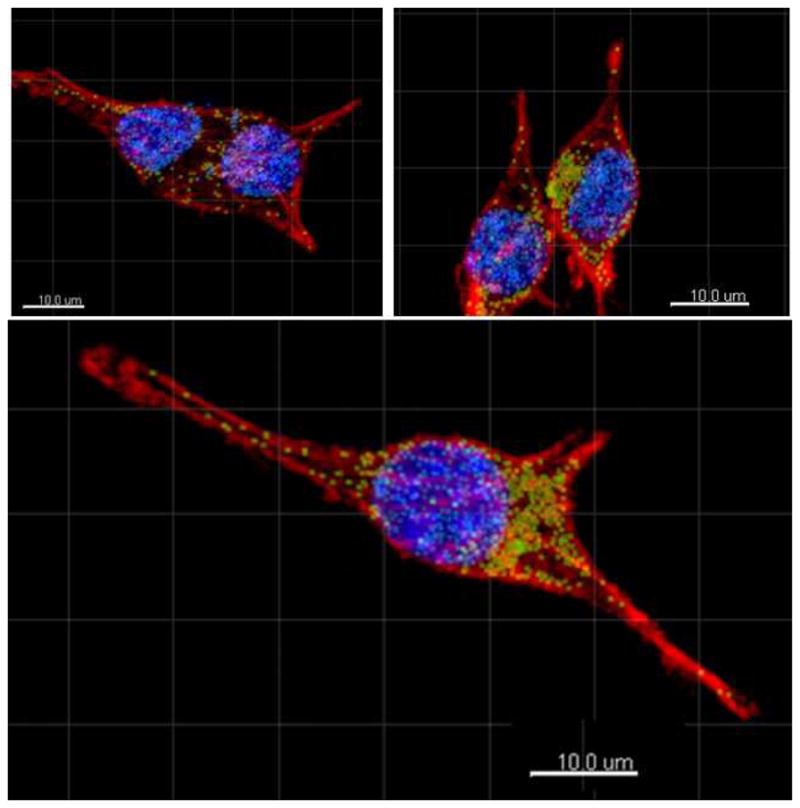
Multiphoton microscope images of fibroblast cells with PMAO coated GdF3:Nd3+ nanoparticles. Figure shows that nanoparticles were uptaken by the cytoplasm and the nuclei without clustering. The green dots denote emission correlated to nanoparticles within the cytoplasm, and the light blue denotes emission from nanoparticles correlated to within the nuclei. Image was obtained by staining nuclei with DAPI (blue channel) and cytoplasm with phalloidin (red channel).
In fact, our spectral analysis show that Nd3+ possesses higher absorption cross section (5.06 × 10−20 cm2) at 800 nm excitation compared to that of absorption cross section (0.09 × 10−20 cm2) at 488 nm excitation. Furthermore, higher emission cross-section (3.91 × 10−19 cm2) at the emitting wavelength 1064 nm under 800 nm excitation compared to that of emission cross section (0.08 × 10−20 cm2) at 532 ensures the higher QY at 1064 nm (see ESI† S10 and ESI† S11). Although, water has slightly higher absorption coefficient approximately 0.25 cm−1 at 1064 nm compared to that of absorption coefficient (0.00447 cm−1) at 532 nm, there are number of other tissue chromophores such as melalin, cytochrome, c oxidase, myoglobin, those can be largely ignored in the near infrared but not in the visible.43 In addition, taking into account the large increase in scattering by biological tissues at shorter (visible) wavelengths compared to NIR, the ‘useful’ ranges for biological probes can be narrow down as 650–930 nm and 1040–1100 nm.43 The measured QY for powder GdF3: 1% Nd3+ and colloidal GdF3: 1% Nd3+ also demonstrated the potential for NIR imaging in the second biological window (1040–1100) compared to that of visible regime. In vivo imaging using fluorescence within the NIR region will be conducted in future to show the importance of these particles as a contrast agent in the NIR (1040–1100) regime.
4. CONCLUSIONS
In summary, we have demonstrated that GdF3: Nd3+ nanoparticles exhibit very efficient downconversion (Stokes) emission under 800 nm excitation and are within the hydrodynamic size limitation for bioimaging applications. We demonstrate the highest downconversion QY of 10.2 ± 1.6 % in GdF3: 1% Nd3+ powder at the excitation power density of 12.74 ± 2 W/cm2. More importantly, we have found that downconversion QY in GdF3: 1% Nd3+ phosphors is 2000 times higher than that of UC β-NaYF4: 20% Yb3+/2% Er3+ of the same size. Furthermore, downconversion QY in colloidal GdF3: 1% Nd3+ phosphors were also found to be excitation power and concentration dependent. This shows that RE doped downconverting nanoparticles yields more photon counts (information density) for bioimaging applications compared to that of UC nanoparticles. In addition, we have shown that having both excitation and emission within the biological window, these GdF3: 1% Nd3+ nanoparticles have superior optical properties for NIR based imaging compared to that of RE doped UC nanoparticles. Finally, well dispersed uptake of these NIR emitting nanoparticles by fibroblast cells (L929) have been demonstrated by confocal imaging. In vivo imaging using fluorescence within the NIR ((1040–1100) region is underway and will be followed as a future communication.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the financial support from the National Science Foundation Partnerships for Research and Education in Materials (NSF-PREM) grant NO-DMR-0934218. We also would like to acknowledge the partial funding from the NIGMS MBRS-RISE GM060655, the National Center for Research Resources (G12RR013646-12), and the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health. The authors would also like to thank Dr. Colleen Witt and Dr. Annette Rodriguez in the UTSA Biophotonics Core for their help with the two photon imaging.
Footnotes
Electronic Supplementary Information (ESI) available: [Quantum Yield (QY) setup, TEM images showing defects, QY calibration and calculation, QY calculation using comparison method, pH dependent fluorescence, cell viability and cytotoxicity, absorption spectra with absorption and emission cross-sections table for the excitation and emission bands]. See DOI: 10.1039/b000000x
Author Contributions
*Madhab Pokhrel, L.C. Mimum, Gangadharan Ajithkumar, Brian Yust, and Dhiraj K Sardar, Department of Physics and Astronomy, University of Texas at San Antonio, One UTSA Cirlce, San Antonio, Texas 78249, United States, ekf012@my.utsa.edu
Ashish Dhanale, Liang Tang, Department of Biomedical Engineering University of Texas at San Antonio One UTSA Circle, San Antonio, Texas 78249, United States
Notes and references
- 1.Niswender K, Blackman S, Rohde L, Magnuson M, Piston D. Journal of microscopy. 1995;180:109–116. doi: 10.1111/j.1365-2818.1995.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DJ, Allan VJ. Science Signaling. 2003;300:82. doi: 10.1126/science.1082160. [DOI] [PubMed] [Google Scholar]
- 3.Weissleder R, Pittet MJ. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakowicz JR. Principles of fluorescence spectroscopy. Springer; 2009. [Google Scholar]
- 5.Leduc M, Weisbuch C. Optics Communications. 1978;26:78–80. [Google Scholar]
- 6.Wu C, Mino K, Akimoto H, Kawabata M, Nakamura K, Ozaki M, Ohmiya Y. Proceedings of the National Academy of Sciences. 2009;106:15599–15603. doi: 10.1073/pnas.0908594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggeling C, Widengren J, Rigler R, Seidel C. Analytical Chemistry. 1998;70:2651–2659. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 8.Patterson GH, Piston DW. Biophysical journal. 2000;78:2159. doi: 10.1016/S0006-3495(00)76762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Shyy JYJ, Chien S. Annu Rev Biomed Eng. 2008;10:1–38. doi: 10.1146/annurev.bioeng.010308.161731. [DOI] [PubMed] [Google Scholar]
- 10.Hardman R. Environmental health perspectives. 2006;114:165. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakowicz JR. Topics in Fluorescence Spectroscopy: Volume 4: Probe Design and Chemical Sensing. Plenum Publishing Corporation; 1994. [Google Scholar]
- 12.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Nature biotechnology. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 13.Shaner NC, Steinbach PA, Tsien RY. Nature methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 14.Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Current opinion in biotechnology. 2002;13:40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 15.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 16.Michalet X, Pinaud F, Bentolila L, Tsay J, Doose S, Li J, Sundaresan G, Wu A, Gambhir S, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derfus AM, Chan WC, Bhatia SN. Nano letters. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer JC, Johnson NJJ, van Veggel FCJM. Chemistry of Materials. 2009;21:2010–2012. [Google Scholar]
- 19.Pansare VJ, Hejazi S, Faenza WJ, Prud’homme RK. Chemistry of Materials. 2012;24:812–827. doi: 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Shao Q, Deng R, Wang C, Teng X, Cheng K, Cheng Z, Huang L, Liu Z, Liu X, Xing B. Angewandte Chemie International Edition. 2012;51:3125–3129. doi: 10.1002/anie.201107919. [DOI] [PubMed] [Google Scholar]
- 21.Lux F, Roux S, Perriat P, Tillement O. Current Inorganic Chemistry. 2011;1:117–129. [Google Scholar]
- 22.Passuello T, Pedroni M, Piccinelli F, Polizzi S, Marzola P, Tambalo S, Conti G, Benati D, Vetrone F, Bettinelli M. Nanoscale. 2012;4:7682–7689. doi: 10.1039/c2nr31796f. [DOI] [PubMed] [Google Scholar]
- 23.Kumara MPGA. Brian Yusta Chris Mimuna, Francisco Pedrazaa, Ashish Dhanaleb, Liang Tangb, Ai-Ling Linc, and V. P. D. a. D. K. Sardara. Advance Materials. 2013 Submitted. [Google Scholar]
- 24.Mimun LC, Ajithkumar G, Pokhrel M, Yust BG, Elliott ZG, Pedraza F, Dhanale A, Tang L, Lin AL, Dravid VP, Sardar DK. Journal of Materials Chemistry B. 2013;1:5702–5710. doi: 10.1039/C3TB20905A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Wang C, Ma X, Wang Q, Cheng Y, Wang H, Li Y, Liu Z. Advanced Functional Materials. 2013;23:272–280. [Google Scholar]
- 26.Shen J, Sun LD, Zhu JD, Wei LH, Sun HF, Yan CH. Advanced Functional Materials. 2010;20:3708–3714. [Google Scholar]
- 27.Chen G, Ohulchanskyy TY, Kumar R, Ågren H, Prasad PN. ACS Nano. 2010;4:3163–3168. doi: 10.1021/nn100457j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Shen J, Ohulchanskyy TY, Patel NJ, Kutikov A, Li Z, Song J, Pandey RK, Ågren H, Prasad PN, Han G. ACS Nano. 2012;6:8280–8287. doi: 10.1021/nn302972r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y, Achilefu S, Gu Y. ACS Nano. 2012;7:676–688. doi: 10.1021/nn304872n. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Peng J, Sun L, Li F. ACS Nano. 2011;5:8040–8048. doi: 10.1021/nn202620u. [DOI] [PubMed] [Google Scholar]
- 31.Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN. Nano letters. 2008;8:3834–3838. doi: 10.1021/nl802223f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhan Q, Qian J, Liang H, Somesfalean G, Wang D, He S, Zhang Z, Andersson-Engels S. ACS Nano. 2011;5:3744–3757. doi: 10.1021/nn200110j. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Ohulchanskyy TY, Liu S, Law WC, Wu F, Swihart MT, Ågren H, Prasad PN. ACS Nano. 2012;6:2969–2977. doi: 10.1021/nn2042362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim SF, Riehn R, Tung C-k, Ryu WS, Zhuo R, Dalland J, Austin RH. Nanotechnology. 2009;20:405701. doi: 10.1088/0957-4484/20/40/405701. [DOI] [PubMed] [Google Scholar]
- 35.Wang YF, Liu GY, Sun LD, Xiao JW, Zhou JC, Yan CH. ACS Nano. 2013;7:7200–7206. doi: 10.1021/nn402601d. [DOI] [PubMed] [Google Scholar]
- 36.Mimun LC, Gangadharan A, Pokhrel M, Yust BG, Elliott Z, Pedraza F, Dhanale A, Tang L, Lin A-L, Dravid V, Sardar D. Journal of Materials Chemistry B. 2013 doi: 10.1039/C3TB20905A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajithkumar G, Yoo B, Goral DE, Hornsby PJ, Lin AL, Ladiwala U, Dravid VP, Sardar DK. Journal of Materials Chemistry B. 2013;1:1561–1572. doi: 10.1039/C3TB00551H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pokhrel M, Ray N, Kumar GA, Sardar DK. Opt Mater Express. 2012;2:235–249. [Google Scholar]
- 39.Chatterjee DK, Rufaihah AJ, Zhang Y. Biomaterials. 2008;29:937–943. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 40.Moros M, Pelaz B, López-Larrubia P, García-Martin ML, Grazú V, Jesus M. Nanoscale. 2010;2:1746–1755. doi: 10.1039/c0nr00104j. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Hayakawa T, Nogami M, Ishikawa Y. Journal of Nanomaterials. 2010;2010:68. [Google Scholar]
- 42.Boyer JC, van Veggel FCJM. Nanoscale. 2010;2:1417–1419. doi: 10.1039/c0nr00253d. [DOI] [PubMed] [Google Scholar]
- 43.Tsai CL, Chen JC, Wang WJ. Journal of Medical and Biological Engineering. 2001;21:7–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.