Abstract
IMPORTANCE
Emerging data support bariatric surgery as a therapeutic strategy for management of type 2 diabetes mellitus.
OBJECTIVE
To test the feasibility of methods to conduct a larger multisite trial to determine the long-term effect of Roux-en-Y gastric bypass (RYGB) surgery compared with an intensive diabetes medical and weight management (Weight Achievement and Intensive Treatment [Why WAIT]) program for type 2 diabetes.
DESIGN, SETTING, AND PARTICIPANTS
A 1-year pragmatic randomized clinical trial was conducted in an academic medical institution. Participants included persons aged 21 to 65 years with type 2 diabetes diagnosed more than 1 year before the study; their body mass index was 30 to 42 (calculated as weight in kilograms divided by height in meters squared) and hemoglobin A1c (HbA1c) was greater than or equal to 6.5%. All participants were receiving antihyperglycemic medications.
INTERVENTIONS
RYGB (n = 19) or Why WAIT (n = 19) including 12 weekly multidisciplinary group lifestyle, medical, and educational sessions with monthly follow-up thereafter.
MAIN OUTCOMES AND MEASURES
Proportion of patients with fasting plasma glucose levels less than 126 mg/dL and HbA1c less than 6.5%, measures of cardiometabolic health, and patient-reported outcomes.
RESULTS
At 1 year, the proportion of patients achieving HbA1c below 6.5% and fasting glucose below 126 mg/dL was higher following RYGB than Why WAIT (58% vs 16%, respectively; P = .03). Other outcomes, including HbA1c, weight, waist circumference, fat mass, lean mass, blood pressure, and triglyceride levels, decreased and high-density lipoprotein cholesterol increased more after RYGB compared with Why WAIT. Improvement in cardiovascular risk scores was greater in the surgical group. At baseline the participants exhibited moderately low self-reported quality-of-life scores reflected by Short Form-36 total, physical health, and mental health, as well as high Impact of Weight on Quality of Life–Lite and Problem Areas in Diabetes health status scores. At 1 year, improvements in Short Form-36 physical and mental health scores and Problem Areas in Diabetes scores did not differ significantly between groups. The Impact of Weight on Quality of Life–Lite score improved more with RYGB and correlated with greater weight loss compared with Why WAIT.
CONCLUSIONS AND RELEVANCE
In obese patients with type 2 diabetes, RYGB produces greater weight loss and sustained improvements in HbA1c and cardiometabolic risk factors compared with medical management, with emergent differences over 1 year. Both treatments improve general quality-of-life measures, but RYGB provides greater improvement in the effect of weight on quality of life. These differences may help inform therapeutic decisions for diabetes and weight loss strategies in obese patients with type 2 diabetes until larger randomized trials are performed.
Despite substantial improvements in pharmaco-therapy for adults with type 2 diabetes mellitus, fewer than half attain the recommended goals for hemoglobin A1c (HbA1c) concentration, blood pressure, or cholesterol levels.1 These findings, as well as the considerable individual and public health burden of diabetes-related microvascular and macrovascular complications, demonstrate the continued need for new approaches to treat hyperglycemia and cardiovascular risk factors in patients with diabetes. Emerging data support substantial improvement in the management of diabetes, hypertension, and dyslipidemia for adults with diabetes following bariatric surgery. Few data are available for persons with lower-magnitude obesity, and very few randomized studies have measured patient-reported outcomes in this population.
We conducted the Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes (SLIMM-T2D) trial, a randomized, controlled, pragmatic, single-academic center study responding to an American Recovery and Reinvestment Act2 request for applications (05-DK-102) to assess the feasibility of methods to conduct a larger multisite trial comparing the long-term effect of bariatric surgery with that of medical management to improve glycemic control and cardiometabolic risk in obese patients with type 2 diabetes. We compared Roux-en-Y gastric bypass (RYGB) surgery with the intensive multidisciplinary medical diabetes and weight management program Weight Achievement and Intensive Treatment (Why WAIT), designed for application in real-world clinical practice. Why WAIT’s cognitive behavioral support is based on the Diabetes Prevention Program3 and Look AHEAD (Action for Health in Diabetes) study4,5 but the Why WAIT program differs importantly in medication adjustment plan, amount of caloric reduction and dietary composition, exercise type and duration, and diabetes education sessions, and is performed only in group sessions. A pragmatic design was selected to compare the effectiveness of Why WAIT using ongoing clinical care programs.
Methods
Trial Design
The study was a randomized, parallel-group, pragmatic trial stratified for body mass index (BMI) above or equal to 35 and below 35 (calculated as weight in kilograms divided by height in meters squared) with balanced randomization (1:1) (Figure 1). The study was conducted at an outpatient clinic and a hospital with shared academic affiliations to Harvard Medical School.
Figure 1. Enrollment, Randomization, and Retention of the Study Participants.
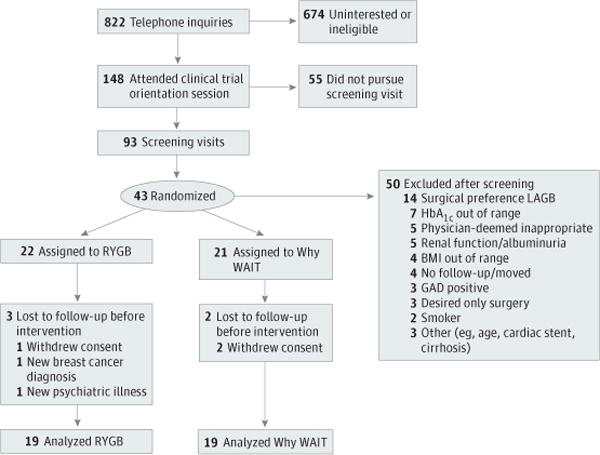
BMI indicates body mass index; GAD, antiglutamic acid decarboxylase antibody–positive; HbA1c, hemoglobin A1c; LAGB, laparoscopic adjustable gastric band; RYGB, Roux-en-Y gastric bypass; and WAIT, Weight Achievement and Intensive Treatment.
Setting and Participants
Participants were recruited from hospitals and clinics using electronic medical record review for identification or by advertisements. Eligible participants were aged 21 to 65 years with at least 1 year of type 2 diabetes, BMI 30 to 42, a strong desire for substantial weight loss, and a commitment to life-long medical and nutritional follow-up. They were free from active cardiovascular or other diseases prohibiting them from exercising safely or undergoing a bariatric surgical procedure. Additionally, potential participants had HbA1c levels above 7% (to convert to a proportion of total Hb, multiply by 0.01), regardless of ongoing treatment, or 6.5% or greater while receiving either 2 oral antihyperglycemic agents at greater than or equal to half-maximal dose or insulin, and with stable-dose treatment for more than 8 weeks. Individuals were excluded if they had detectable levels of antiglutamic acid decarboxylase antibody, a history of diabetic ketoacidosis, uncontrolled type 2 diabetes (HbA1c >12%), gastrointestinal disease, malignant disease within 5 years, significant cardiopulmonary or renal disease, active eating disorder, drug and/or alcohol abuse, impaired mental status, weight loss greater than 3% within the previous 3 months, participation in another weight-reduction program, or were using weight-reduction medications and/or supplements. Participants had to be nonsmoking for more than 2 months. Additional information on the full exclusion criteria are presented in the Supplement (eMethods).
Randomization and Interventions
The protocol was approved by Partner’s Healthcare human subject institutional review board and the US Food and Drug Administration. An independent data monitoring committee reviewed patient safety.
The study was described by telephone to the respondents. Potentially interested individuals attended in-person orientations, during which study design and medical and surgical interventions were reviewed. People with a preference for a bariatric procedure other than RYGB were not enrolled. Those interested in the trial were screened for appropriateness for the surgical and medical interventions. Randomization was computer-generated in centrally allocated blocks of 4, stratified by BMI above or equal to 35 and below 35.
The RYGB procedure was performed at Brigham and Women’s Hospital. All surgical patients were given routine antibiotic and venous thromboembolism prophylaxis and standardized anesthesia per routine hospital protocols. The RYGB procedure involved a 75-cm antecolic, antegastric Roux limb created with a 50-cm biliopancreatic limb. A 15- to 20-mL gastric pouch was created along the lesser curve of the stomach, and the lesser omentum was divided at that level. A gastrojejunostomy was constructed using a linear cutter stapler, and the gastroenterotomy was closed using a running polyglactin 910 suture (Vicryl 2.0; Ethicon Inc). Provocative leak tests were performed, including “blue dye” and “bubble” tests.
Participants randomized to the medical arm of the study enrolled in the Why WAIT program, which is designed for clinical practice6 and run quarterly at the Joslin Diabetes Center for groups of 10 to 15 patients. Why WAIT’s multidisciplinary approach includes an endocrinologist (O.H.), registered dietician, exercise physiologist, mental health provider (A.G.-F.), and certified diabetes nurse educator. Two-hour weekly group sessions are conducted during a 12-week initiation phase. Patients receive individual medication adjustments and participate in supervised group exercise and support/didactic sessions. Key aspects of Why WAIT include (1) weekly medication adjustments; (2) structured modified dietary intervention with hypocaloric (1500–1800 kcal) diet with carbohydrates (40%–45%), protein (20%–30%), and saturated fat intake reduced to less than 7%,7 with the 6 initial weeks including breakfast and lunch meal replacement (Boost Glucose Control; Nestle Health Science; nutrient content per 237 mL [8 fl oz] including calories, 190; protein, 16 g; carbohydrate, 16 g; fiber, 3 g; and fat, 7g), 2 snacks, and structured dinner menus; (3) up to 300 minutes per week of graded, balanced, and individualized exercise, with emphasis on strength training; (4) cognitive behavioral intervention; and (5) group education. A maintenance phase of individual monthly counseling follows for the remainder of the year. Additional information describing the Why WAIT program is provided in the Supplement, including the progression of exercise (eTable 1) and the didactic core curriculum (eTable 2).
Participants provided written informed consent first for screening for eligibility and again prior to randomization. Participants received compensation for the time and inconvenience associated with in-person study visits and local transportation or parking vouchers. Participants in the Why WAIT program also receive meal replacement nutritional drinks (Boost Glucose Control) for use during the 6 initial weeks of the program and as needed during the first year. Participants paid their copayments and insurance deductibles for RYGB and Why WAIT interventions. Surgical costs were covered by an investigator-initiated award from Covidien for participants with BMI less than 35 because insurance does not cover these procedures.
Follow-up and Outcome Assessments
Metabolic assessments were performed at baseline and repeated at 10% of initial body weight loss to obtain assessments at a comparable level of weight lost in both cohorts. If 10% weight loss did not occur, metabolic assessments were performed at 3 months. Final assessments were repeated at 12 months, providing a time-based comparison. Metabolic assessments included medications and dosing, weight (model 0501 electronic scale; ACME), height (wall-mounted stadiometer), waist circumference (Gulak tape measure according to the National Heart, Lung, and Blood Institute Clinical Guidelines8), and seated blood pressure using an automated device (BP742, Omron Healthcare). Body composition and basal metabolic rate were assessed by bioelectrical impedance (TBF-215; Tanita Corporation). A 6-minute walk test was performed.9 Patient-reported outcomes were systematically assessed using surveys including the 36-item Short-Form (SF-36), version 210; Barriers to Being Physically Active11; EuroQol 5 Dimensions (EQ-5D) (EuroQol Group)12; Problem Areas In Diabetes (PAID)13,14; and Impact of Weight on Quality of Life–Lite (IWQOL) (which assesses weight-related physical function, self-esteem, sexual life, public distress, and work-related stress).15 The United Kingdom Prospective Diabetes Study (UKPDS) Risk Engine was used to calculate cardiovascular risk.16
Laboratory Tests
Clinical laboratory evaluations were performed by Quest Diagnostics. Quest Laboratories is certified by both the Clinical Laboratory Improvement Amendment and the College of American Pathologists.
Statistical Analysis
The primary outcome was attaining glycemic control (fasting plasma glucose levels below 126 mg/dL [to convert to millimoles per liter, multiply by 0.0555] and HbA1c below 6.5%) at 1 year of follow-up, regardless of whether patients were using pharmaceutical interventions.
We estimated the sample size assuming that RYGB would result in resolution of hyperglycemia in 80% of the patients and medical management in 20%. Twenty participants per group provided 97% power to detect a significant difference between groups, with α = .05. Dichotomous and continuous variables were analyzed using logistic regression and a general linear mixed model, respectively, to test the null hypotheses of equal resolution of hyperglycemia and other major outcomes at 1 year while controlling for covariates. Each measure’s outcome analysis during the 1-year study was adjusted for baseline, unless noted otherwise. The primary analysis was intention-to-treat and involved all randomly assigned patients who received at least 1 postrandomization assessment (modified per-protocol analysis). Sensitivity analysis included all randomized participants (Supplement [eTable 3]). Baseline characteristics are presented as mean (SD) and outcome data are mean (95% CI) or median (interquartile range [IQR]). No interim analyses for superiority or futility were performed. All participants completed the visits before data analysis.
Results
Participants
During recruitment (March 12, 2010, to September 7, 2011), 822 potential participants underwent telephone screening, and 148 subsequently attended an orientation session (Figure 1). Additional information on recruitment approaches and reported reasons for not pursuing trial involvement are provided in the Supplement (eTable 4 and eTable 5). Of those individuals, 93 underwent full medical screening. The most common reasons for screening failure were preference for an alternative surgical procedure, out-of-range HbA1c, poor surgical candidacy, inability to participate in an unsupervised exercise program, and renal dysfunction. Forty-three participants were randomized to surgical (RYGB, 22) or medical (Why WAIT, 21) interventions. Before any intervention, 3 participants withdrew consent, 1 received a diagnosis of breast cancer, and 1 received a diagnosis of severe depression; these individuals were not included further in summary data (primary end-point analysis including all randomized participants is provided in the Supplement [eTable 3]). Nineteen patients were included in each group for the final analysis. Baseline demographics of the patients undergoing intervention are provided in Table 1 and include 6 participants (32%) with BMI under 35 in the surgical group and 7 (37%) in the nonsurgical group. Established microvascular complications were mild and infrequent.
Table 1.
Baseline Characteristics by Study Group
| Characteristic | Roux-en-Y Gastric Bypass |
Why WAIT |
|---|---|---|
| Age, mean (SD), y | 50.7 (7.6) | 52.6 (4.3) |
| Sex, No. (%) | ||
| Male | 6 (32) | 9 (47) |
| Female | 13 (68) | 10 (53) |
| Race/ethnicity, No. (%) | ||
| White | 14 (74) | 10 (53) |
| African American | 3 (16) | 8 (42) |
| Asian | 1 (5) | 0 |
| Hispanica | 1 (5) | 1 (5) |
| BMI, mean (SD) | 36.0 (3.5) | 36.5 (3.4) |
| BMI <35, No. (%) | 6 (32) | 7 (37) |
| Weight, mean (SD), kg | 104.6 (15.5) | 102.7 (17.0) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 132.8 (10.5) | 126.3 (14.7) |
| Diastolic | 81.7 (7.4) | 76.6 (8.8) |
| Diabetes mellitus | ||
| Years since diagnosis, mean (SD) | 10.6 (6.6) | 10.2 (6.1) |
| Complications, No. (%) | ||
| Retinopathy | 1 (5) | 6 (32) |
| Neuropathy | 3 (16) | 5 (26) |
| Nephropathy | 0 | 1 (5) |
| Medications, No. (%) | ||
| Insulin | 15 (79) | 8 (42) |
| Metformin hydrochloride | 17 (89) | 15 (79) |
| GLP-1 agonist | 5 (26) | 2 (11) |
| Pramlintide acetate | 0 | 1 (5) |
| Other glycemic medication | 7 (37) | 12 (63) |
| Statin | 15 (79) | 16 (84) |
| Other lipid-lowering medication | 3 (16) | 2 (11) |
| ACE inhibitor/ARB | 17 (89) | 14 (74) |
| Other antihypertensive medication | 14 (74) | 12 (63) |
| Laboratory values, mean (SD)b | ||
| HbA1c, % | 8.24 (1.42) | 8.83 (1.01) |
| Glucose, mg/dL | 132.3 (49.7) | 162.2 (53.8) |
| Total cholesterol, mg/dL | 154.2 (34.0) | 162.5 (38.6) |
| Triglycerides, mg/dL | 119.7 (65.7) | 156.3 (75.7) |
| HDL-C, mg/dL | 43.6 (9.7) | 39.1 (9.9) |
| LDL-C, mg/dLc | 88.1 (27.7) | 98.9 (29.3) |
| UKPDS risk scores, mean (SD) | ||
| CHD | 9.8 (9.6) | 10.9 (6.9) |
| Fatal CHD | 6.5 (7.7) | 6.9 (4.9) |
| Stroke | 4.0 (4.1) | 4.0 (2.3) |
| Fatal stroke | 0.6 (0.6) | 0.5 (0.3) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; GLP-1, glucagonlike peptide 1; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UKPDS, United Kingdom Prospective Diabetes Study; WAIT, Weight Achievement and Intensive Treatment.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; HbA1c to a proportion of total Hb, 0.01; HDL-C, LDL-C, and total cholesterol to millimoles per liter, 0.0259; and triglycerides to millimoles per liter, 0.0113.
Hispanic participants may be any race.
Laboratory assessments were made after patients fasted overnight.
Direct measurement was performed.
Primary End Point
Eleven participants (58%) in the RYGB group reached the target HbA1c level of less than 6.5% and the fasting plasma glucose level below 126 mg/dL at 12 months, compared with 3 (16%) in the medical therapy group (P = .03). The odds of resolution of hyperglycemia, as defined above, were 6.9 times greater in the surgical group at 1 year. All patients in the surgical group who achieved target glycemia were no longer receiving diabetes medications at 1 year.
Weight and Glycemia
Early assessment was performed when participants lost 10% of their body weight or at 3 months if a 10% loss was not achieved by then. All RYGB participants achieved 10% weight loss before 3 months, at a median of 39 days (range, 23–85 days). In comparison, 37% (7 of 19) of participants in the Why WAIT group achieved this 10% weight loss goal. Of participants who did not lose 10% of their body weight by 3 months, mean weight loss was 5.4% (range, +0.3% to −9.2%) at 3 months, with mean group weight lost 7.7% (0.8%) at 3 months. Thus, both groups were successful in weight loss, but there were greater reductions in weight following RYGB than Why WAIT, and differences emerged over time (Figure 2). Reductions in waist circumference, and fat and lean mass by bioelectrical impedance were also greater following RYGB compared with Why WAIT (Table 2).
Figure 2. Changes in Cardiometabolic Outcomes Following Bariatric Surgery and Medical Management.
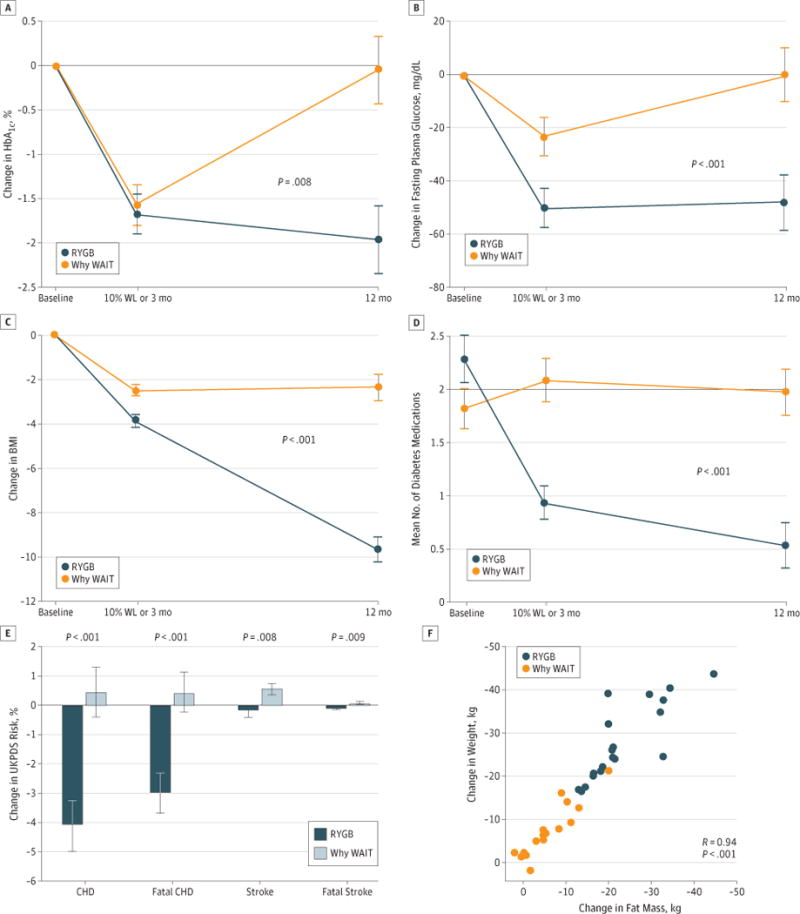
Changes in hemoglobin A1c (HbA1c) (A), fasting plasma glucose (B), and body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) (C) graphed by treatment group and time as baseline-adjusted mean, with SE indicated with limit lines. P values indicate the significant difference between groups in linear mixed model adjusted for baseline. Mean number of diabetes medications (D). Change from baseline for United Kingdom Prospective Diabetes Study (UKPDS) Risk Scores for coronary heart disease (CHD), fatal CHD, stroke, and fatal stroke. Variance indicated with the limit lines is SE (E). The relationship between total weight lost (WL) and change in fat by bioelectrical impedance (F). RYGB indicates Roux-en-Ygastric bypass; WAIT, Weight Achievement and Intensive Treatment.
Table 2.
Metabolic Changes Following RYGB and Diabetes and Weight Medical Management
| End Point | Baseline, Mean (SD) | Baseline-Adjusted Mean Change (95% CI) | P Valuea | ||||
|---|---|---|---|---|---|---|---|
| 10% Weight Lost or 3 mo | 12 mo | ||||||
| RYGB | Why WAIT | RYGB | Why WAIT | RYGB | Why WAIT | ||
| Waist, cm | 117.8 (14.9) | 114.1 (12.2) | −9.8 (−12.9 to −6.6) | −5.3 (−8.4 to −2.1) | −26.9 (−30.4 to −23.5) | −6.6 (−10.3 to −2.9) | <.001b |
| Mass, kg | |||||||
| Lean | 59.2 (14.1) | 60.1 (10.8) | −1.8 (−3.0 to −0.6) | −1.3 (−2.5 to −0.1) | −5.1 (−7.0 to −3.3) | −1.4 (−3.4 to 0.6) | .04b |
| Fat | 45.5 (9.4) | 42.6 (9.8) | −9.4 (−11.4 to −7.5) | −6.1 (−8.0 to −4.1) | −22.7 (−25.6 to −19.8) | −6.2 (−9.3 to −3.0) | <.001b |
| BP, mm Hg | |||||||
| Systolic | 132.8 (10.5) | 126.3 (14.7) | −7.3 (−13.1 to −1.4) | −6.3 (−12.1 to −0.4) | −12.3 (−18.5 to −6.2) | −1.0 (−7.2 to 5.3) | .02c |
| Diastolic | 81.7 (7.4) | 76.6 (8.8) | 0.9 (−2.3 to 4.1) | −4.3 (−7.5 to −1.1) | −5.1 (−8.3 to −1.9) | −2.1 (−5.3 to 1.2) | <.001c |
| Laboratory values | |||||||
| Total cholesterol, mg/dL | 154.2 (34.0) | 162.5 (38.6) | −18.6 (−32.3 to −4.9) | −3.7 (−17.4 to 10.0) | −3.2 (−17.7 to 11.3) | 8.3 (−7.0 to 23.5) | .11 |
| Triglycerides, mg/dL | 120 (66) | 156 (76) | −32 (−43 to −21) | −31 (−42. to −19) | −47 (−63 to −30) | −5 (−23 to 12) | .02b |
| HDL-C, mg/dL | 43.6 (9.7) | 39.1 (9.9) | −5.8 (−8.5 to −3.2) | 0.1 (−2.5 to 2.8) | 10.2 (6.7 to 13.6) | 0.4 (−3.3 to 4.0) | <.001c |
| LDL-C, mg/dLd | 88.1 (27.7) | 98.9 (29.3) | −7.4 (−19.0 to 4.1) | −4.4 (−16.1 to 7.3) | −5.4 (−17.8 to 6.9) | 8.6 (−4.5 to 21.6) | .22 |
| ALT, IU/L | 32.2 (16.3) | 27.7 (12.3) | −7.4 (−10.7 to −4.0) | −3.8 (−7.2 to −0.5) | −10.6 (−15.5 to −5.6) | −4.7 (−10.1 to 0.6) | .06 |
| AST, IU/L | 30.6 (21.6) | 23.3 (13.4) | −1.4 (−5.5 to 2.6) | −0.8 (−4.8 to 3.2) | −5.8 (−10.0 to −1.6) | −3.3 (−7.6 to 1.1) | .49 |
| Creatinine, mg/dL | 0.71 (0.14) | 0.86 (0.21) | −0.06 (−0.10 to −0.02) | 0.02 (−0.02 to 0.06) | −0.07 (−0.11 to −0.03) | 0 (−0.05 to 0.04) | .01 |
| Urinary albumin to creatinine ratioe | 3 (0 to 7)f | 3 (0 to 10)f | ND | ND | −1.0 (−5.0 to 5.0) | 0 (−4.5 to 2.0) | .96f |
| Vitamin D3, ng/mL | 22.2 (9.0) | 21.0 (8.8) | 7.0 (4.3 to 9.7) | 0.3 (−3.1 to 3.7) | 4.6 (0.5 to 8.6) | 1.0 (−3.3 to 5.3) | .01 |
| Hematocrit, % | 36.8 (3.3) | 40.2 (4.3) | −4.4 (−5.4 to −3.5) | 0.0 (−1.0 to 1.0) | −2.3 (−3.6 to −1.1) | 0.2-(−1.2-to1.5) | <.001 |
| WBCs, ×103/μL | 6.8 (2.1) | 6.5 (1.8) | −1.2 (−1.7 to −0.7) | −0.4 (−0.9 to 0.1) | −0.9 (−1.4 to −0.4) | 0.1 (−0.4 to 0.6) | .01 |
| 6-Min walk, m | 458.7 (65.5) | 466.7 (55.9) | −6.4 (−29.7 to 16.8) | 33.2 (10.0 to 56.5) | 17.8 (−8.3 to 44.0) | 27.7 (0.4 to 55.1) | .11 |
| Heart rate recovery (1 min), beats/min | 92.2 (15.2) | 87.5 (12.0) | −4.6 (−9.2 to 0) | −1.1 (−5.8 to 3.5) | −10.7 (−15.9 to −5.5) | 1.0 (−4.5 to 6.5) | .01 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; ND, not determined; RYGB, Roux-en-Y gastric bypass; WAIT, Weight Achievement and Intensive Treatment; WBCs, white blood cells.
SI conversion factors: To convert ALT and AST to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, 88.4; glucose to millimoles per liter, 0.0555; HDL-C, LDL-C, and total cholesterol to millimoles per liter, 0.0259; hematocrit to proportion of 1.0, 0.01; triglycerides to millimoles per liter, 0.0113; and WBCs to ×109/L, 1.0.
P values represent treatment effects from linear mixed model corrected for baseline, unless otherwise noted.
P value for time × treatment interaction was also significant at P < .05.
P value represents time × treatment interaction; treatment effect was not significant.
Direct measurement was performed.
Urinary albumin reported in micrograms of protein per milligrams of creatinine, obtained in a spot morning void.
Median and IQR are provided and were analyzed using the Kruskal-Wallis test for nonparametric data.
At the early assessment, HbA1c reduction did not differ significantly between groups, and both groups achieved significant reductions from baseline (Figure 2), although there was a shorter time interval to the early assessment in the surgical compared with the medical group. At 1 year, the change from baseline for HbA1c was significantly greater after RYGB than Why WAIT, and a significant reduction from baseline was sustained only in the surgical group. The pattern for fasting glucose levels was similar (Figure 2).
Blood Pressure and Lipid Levels
Systolic and diastolic blood pressure and triglycerides were lower at 1 year and high-density lipoprotein cholesterol was increased only in the RYGB group. The difference between the groups was significant (Table 3) and was observed despite greater reductions in antihypertensive and lipid-lowering medication use following RYGB (Supplement [eFigure A and B]).
Table 3.
Glycemic Changes Following RYGB and Diabetes and Weight Medical Management
| Primary End Point | 12 mo, No. (%) | P Valuea | |
|---|---|---|---|
| RYGB (n = 19) |
Why WAIT (n = 19) |
||
| HbA1c <6.5% and FPG <126 mg/dL | 11 (58) | 3 (16) | .03 |
| Meeting ADA treatment goals | |||
| HbA1c <7.0% | 15 (79) | 4 (21) | .002 |
| LDL-C <100 mg/dLb | 15 (79) | 9 (47) | .05 |
| Systolic blood pressure <130 mm Hg | 16 (84) | 11 (58) | .04 |
| Meeting all 3 goals | 11 (58) | 1 (5) | .007 |
| Normoglycemia | |||
| HbA1c <6.0% | 6 (32) | 0 | .05c |
| FPG <100 mg/dL | 14 (74) | 3 (16) | .003 |
| Meeting both criteria | 6 (32) | 0 | .02d |
Abbreviations: ADA, American Diabetes Association; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; RYGB, Roux-en-Y gastric bypass; WAIT, Weight Achievement and Intensive Treatment.
SI conversion factors: To convert HbA1c to a proportion of total Hb, multiply by 0.01; LDL-C to millimoles per liter, 0.0259.
P values are logistic regression between groups corrected for baseline, unless noted.
Direct measurement was performed.
P value is exact logistic regression corrected for baseline.
P value cannot be adjusted for both baseline HbA1c and fasting glucose because there were no patients with HbA1c less than 6.0% in the Why WAIT program; thus, this reported value is unadjusted for baseline.
Cardiometabolic Risk
At randomization, participants were free from active cardiovascular or other diseases prohibiting them from exercising safely, including unsupervised exercise. However, fitness assessed by the 6-minute walk test improved in those randomized to the structured Why WAIT program, but heart rate recovery from exercise was better following surgery. Nonsignificant improvement in fitness tended to occur in the RYGB group by 1 year such that the difference between the groups was not significant.
Cardiometabolic risk scores for coronary heart disease, fatal coronary heart disease, stroke, and fatal stroke, estimated using the UKPDS Risk Engine, were all reduced more at 1 year following RYGB than Why WAIT (Figure 2). In addition, creatinine, white blood cell count, and hematocrit were lower, but the vitamin D level was greater following RYGB than Why WAIT.
Patient-Reported Outcomes
At baseline participants exhibited moderately low SF-36 total, physical health, and mental health scores, and high IWQOL and PAID health status scores, consistent with moderate distress across all axes (Figure 3 and Supplement [eTable 6]). At early assessment, Why WAIT participants reported greater improvements compared with RYGB participants in quality of life, assessed by SF-36 total, physical health, and mental health scores. Differences between the groups did not persist at 1 year. PAID captured reductions in emotional distress, eating behaviors, and difficulty with diabetes self-management after both interventions and were similar in magnitude between the groups. The number of barriers to being active was reduced, and the magnitude of improvement was similar between the groups. The visual analog scale score of the EQ-5D also improved similarly between groups, with no significant change within or between groups for the EQ-5D index score (data not shown). The IWQOL score also improved significantly following RYGB and Why WAIT, and the magnitude of improvement was significantly greater in the RYGB group at 1 year. In the groups combined, improvement in IWQOL scores correlated with greater weight loss (r = 0.70; P < .001).
Figure 3. Patient-Reported Outcomes and Change in Body Mass Index (BMI) and Impact of Weight on Quality of Life–Lite (IWQOL).
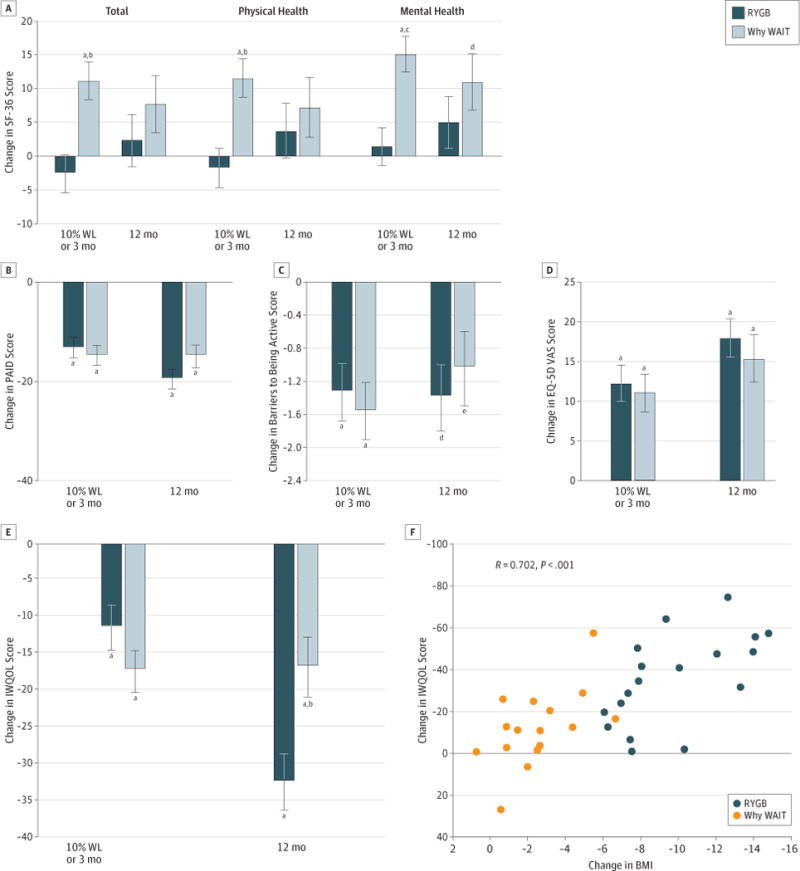
A, Short-Form 36 (SF-36). B, Problem Areas in Diabetes (PAID). C, Barriers to Being Active. D, EuroQol 5 Dimensions (EQ-5D) visual analog scale (VAS). E, IWQOL. F, Relationship between change in BMI and change in IWQOL scores. Data are graphed by treatment group and time as baseline-adjusted mean change from baseline and SE, indicated with limit lines. Baseline mean (SD) of all patient-reported outcomes are provided in the Supplement (eTable 6). RYGB indicates Roux-en-Y gastric bypass; WAIT, Weight Achievement and Intensive Treatment; and WL, weight loss.
aP < .001 (within-group comparison).
bP < .01 (between-group comparison).
cP < .001 (between-group comparison).
dP < .01 (within-group comparison).
eP < .05 (between-group comparison).
Adverse Events
No participant experienced severe hypoglycemia (requiring assistance). Surgical arm postintervention serious adverse events included ischemic heart disease with coronary artery bypass surgery, new breast cancer diagnosis, nephrolithiasis, exacerbated depression with suicide attempt, and hip arthroplasty. Notably, hip pain preceded enrollment and did not improve following weight loss; thus, hip arthroplasty following RYGB was not the result of improved surgical candidacy. Three different participants in the nonsurgical arm had presyncope serious adverse events.
Discussion
Risks and benefits of bariatric surgery compared with nonsurgical medical management for obese patients with type 2 diabetes, particularly for those with lesser-magnitude obesity, are of increasing interest. The present study and others17,18 confirm that a randomized trial of bariatric surgery compared with medical and lifestyle intervention for diabetes is feasible in the US population consistent with reported trials in other countries18–20 and with studies comparing surgery with medical approaches for coronary disease management.21 Patients often have a strong preference for the type of surgery, and if larger trials to directly address mortality or cardiovascular outcomes are conducted, pragmatic or innovative designs to accommodate patients’ surgical preference may be needed.
We found that obese patients with type 2 diabetes are more likely to achieve the target HbA1c level of less than 6.5% and fasting plasma glucose less than 126 mg/dL 1 year after randomization to RYGB compared with intensive medical diabetes and weight management. Other glycemic thresholds often used to quantify achieving diabetes goals were also higher following RYGB. Notably, all patients in the surgical group achieved glycemic control without using diabetes medications. Likewise, the surgical group experienced improved blood pressure and lipid levels with reduction or elimination of concomitant medications in many patients (Supplement). Our study also adds to the relatively sparse data available on patients with lower-magnitude obesity.22 To our knowledge, our trial was the first to use a pragmatic, clinically available intensive diabetes weight management program designed specifically for application in real-world clinical practice6 modeled off clinical trial practices with demonstrated effectiveness, such as the Diabetes Prevention Program3 and Look AHEAD studies.23 Initially favorable glycemic and weight reduction occurred with medical and lifestyle intervention. Although weight loss was maintained, dysglycemia recidivism rates were high during the study year. In general, participants and providers appeared hesitant to add glycemic management pharmacotherapies after the initial success lowering the HbA1c concentration with fewer medications or lower dosages. At follow-up visits, participants reported their willingness to increase adherence to dietary and exercise programs. In contrast, although the shorter time to early assessment after RYGB compared with Why WAIT could confound the change in HbA1c at this time, weight and glycemic improvements after RYGB occurred quickly and were maintained throughout the 1-year follow-up period.
Although the study was not powered to assess the effects of interventions on additional metabolic measures, we observed improvements in multiple cardiovascular risk factors including substantial differences in improvement in UKPDS-calculated cardiovascular risk scores. These findings concur with cardiovascular outcomes reductions found in multiple nonrandomized, observational, controlled trials24–28 and may portend improved major cardiovascular event rates for surgical patients.
Both RYGB and Why WAIT interventions improved self-reported total, physical, and mental health status (SF-36); problems associated with diabetes management (PAID); barriers to being active; and adverse effects of weight on life quality (IWQOL). Early deterioration in the SF-36 total and physical health scores reported in surgical patients could be the result of the short postoperative time interval to the 10% weight lost outcome for this assessment. At 1 year, improvements were comparable between the groups. Similar-magnitude improvements in patient-reported diabetes burden were achieved in different ways: with resolution of hyperglycemia following RYGB and with education, lifestyle, and medication changes in the Why WAIT program. Barriers to being active were similarly improved in both groups. Weight-specific quality-of-life improvements were proportional to weight lost. Greater differences between the groups appeared at 1 year compared with the earlier assessment.
This study had limitations. Duration of diabetes and insulin use, as proxies for β-cell function, were not inclusion or exclusion criteria. Thus, our study population had a wide range across these variables. There were relatively few patients with diabetes-related coexisting established microvascular or cardiovascular disease, limiting the applicability of the findings to patients with more extensive diabetes-related complications. It is possible that participants willing to be randomized to surgery are not representative of motivated patients willing only to participate in an intensive medical-management program—thus affecting the amount of weight lost in this group. Despite the randomization process, participants in the medical arm had numerically higher baseline HbA1c concentrations and fasting glucose levels and thus, despite statistical corrections for baseline dysglycemia, could be less likely to achieve a dichotomous end point. We did not study emerging surgical approaches, such as the now frequently used gastric sleeve.29 The small number of participants available at the 1 year follow-up disallows assessment of infrequent or long-term adverse events, cardiovascular or mortality outcomes, metabolic response durability over time, or cost-effectiveness. These factors are especially relevant considerations for public health policy changes recommending surgical intervention for diabetes management.30 Serious adverse events were numerically more frequent in surgical patients, and possible debilitating surgical events18 can substantially offset any favorable metabolic improvements. Individual and societal risk tolerance may differ. The American Recovery and Reinvestment Act feasibility funding for a randomized trial comparing bariatric and metabolic surgeries with medical approach did not permit extended follow-up. At this time, the potential effect of long-term nutritional deficiencies and lack of data on cardiovascular and mortality outcomes must temper any enthusiasm for an endorsement of surgical procedures for diabetes management.
Although resolution of hyperglycemia may not last indefinitely following surgery,31 the UKPDS32 and Steno-2 Study33 in patients with type 2 diabetes and the Diabetes Control and Complications Trial34 in patients with type 1 diabetes all suggest the health benefits of previous glycemic control may take years to emerge. Despite a lack of significant differences in glycemic control during the extended observational follow-up period, patients previously randomized to intensive control demonstrated a significantly lower risk of diabetes complications. The continuing benefit of early improved metabolic control has been termed metabolic memory or legacy effect. These data suggest that optimal maintenance of metabolic control may minimize the long-term risk of diabetic complications, although this hypothesis remains controversial and may not be true for patients with longer-duration diabetes.35–37 Low operative morbidity permits consideration of bariatric and metabolic surgeries specifically for diabetes management, although few data are available for patients with a lower amount of excess weight22 and currently available studies suggest that improved mortality may be limited to the patients with the highest level of obesity.24 Our trial and other small studies17,19,20 suggest health benefits for patients with type 2 diabetes and lower-degree obesity who accept surgical risk. However, the short- and long-term risk and benefits need serious evaluation.
Prospective and case-control, but not randomized, studies suggest significant benefits associated with bariatric surgery in diabetes treatment and prevention, reduced incidence of cancer in women,38 and reduced cardiovascular26,39 and all-cause mortality24,25,39–41 for obese patients. Although upstream bias in patient selection is possible in these nonrandomized trials, the potential magnitude of these benefits, if they are confirmed, is substantial. Without a unified long-term outcome trial to compare bariatric surgery with intensive medical weight management, our study and other small randomized clinical trials provide data to support the observational studies and suggest a role for surgical approach to diabetes management.
Conclusions
After a 1-year follow-up period in a clinical setting, better weight loss and glycemia control, as well as improvement in other cardiovascular risk markers, occurred in the present study following RYGB compared with an intensive diabetes and weight management program. Metabolic improvements have the potential to reduce cardiovascular morbidity and mortality, as seen in nonrandomized studies. Thus, our short-duration study suggests that RYGB may be useful in managing type 2 diabetes, including for patients with lower levels of obesity (BMI 30–42). Individual risks and benefits should be carefully considered. Improvements in patient-reported outcomes were similar at 1 year despite the different therapeutic approaches.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RC1-DK086918, R56-DK095451, and P30-DK03836; the Marietta Blau grant ICM-2010-02797 from the Österreichischer Austausdienst; and the Herbert Graetz Fund. Covidien provided funds for the surgical costs of participants with BMI less than 35 who were randomized to undergo surgery; Lifescan, a Division of Johnson & Johnson, provided home glucose monitoring supplies; Nestle Nutrition Inc provided Boost; and Mercodia provided assay materials.
Additional Contributions: We acknowledge the support of the Joslin Clinical Research Center and thank its philanthropic donors.
Footnotes
Author Contributions: Drs Halperin and Ding contributed equally to the work. Drs Ding and Simonson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Halperin, Simonson, Hamdy, Abrahamson, Lautz, Goldfine.
Acquisition, analysis, or interpretation of data: Halperin, Ding, Simonson, Panosian, Goebel-Fabbri, Wewalka, Clancy, Foster, Vernon, Goldfine.
Drafting of the manuscript: Halperin, Ding, Simonson, Panosian, Foster, Vernon, Goldfine.
Critical revision of the manuscript for important intellectual content: Halperin, Ding, Simonson, Panosian, Goebel-Fabbri, Wewalka, Hamdy, Abrahamson, Clancy, Lautz, Goldfine.
Statistical analysis: Halperin, Simonson.
Obtained funding: Halperin, Simonson, Lautz, Vernon, Goldfine.
Administrative, technical, or material support: Halperin, Ding, Simonson, Panosian, Goebel-Fabbri, Wewalka, Hamdy, Abrahamson, Clancy, Foster, Vernon, Goldfine.
Study supervision: Halperin, Simonson, Wewalka, Hamdy, Abrahamson, Clancy, Lautz, Vernon, Goldfine.
Conflict of Interest Disclosures: Dr Hamdy serves as a consultant for Abbott Nutrition and Merck Pharmaceuticals and receives research support from Neurometrix and Metagenics. Dr Abrahamson is a member of the advisory boards of Novo Nordisk, Halozyme, Jannsen Pharmaceuticals, and WebMD Health Services. Dr Goldfine receives supplies for investigator-initiated studies from Caraco Pharmaceuticals; Amneal Pharmaceuticals; Novo Nordisk; Lifescan, a Division of Johnson & Johnson; Nestle Nutrition; and Mercodia; and grant support from Daiichi Sanky; and has served as a consultant for Novo Nordisk. No other disclosures were reported.
Supplemental content at jamasurgery.com
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01073020
References
- 1.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Highest priority challenge topics [page 20] https://grants.nih.gov/archive/grants/funding/challenge_award/High_Priority_Topics.pdf. Accessed April 23, 2014.
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diab Rep. 2008;8(5):413–420. doi: 10.1007/s11892-008-0071-5. [DOI] [PubMed] [Google Scholar]
- 7.Giusti J, Rizzotto JA. Interpreting the Joslin Diabetes Center and Joslin Clinic clinical nutrition guideline for overweight and obese adults with type 2 diabetes. Curr Diab Rep. 2006;6(5):405–408. doi: 10.1007/s11892-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute. Guidelines on overweight and obesity: electronic textbook. https://www.nhlbi.nih.gov/guidelines/obesity/e_txtbk/txgd/4142.htm. Accessed April 23, 2014.
- 9.Beriault K, Carpentier AC, Gagnon C, et al. Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009;30(10):725–727. doi: 10.1055/s-0029-1231043. [DOI] [PubMed] [Google Scholar]
- 10.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Barriers to Being Physically Active Quiz. National Center for Chronic Disease Prevention and Health Promotion; http://www.cdc.gov/diabetes/ndep/pdfs/8-road-to-health-barriers-quiz-508.pdf. Accessed May 2, 2014. [Google Scholar]
- 12.UK Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) Diabetes Care. 1999;22(7):1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 13.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 14.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 15.Kolotkin RL, Crosby RD. Psychometric evaluation of the Impact of Weight on Quality of Life–Lite questionnaire (IWQOL-Lite) in a community sample. Qual Life Res. 2002;11(2):157–171. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 16.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 17.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 20.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 21.Frye RL, August P, Brooks MM, et al. BARI 2D Study Group A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309(21):2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 23.Wing RR, Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 25.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 26.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 27.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98(24):1763–1777. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 28.Johnson BL, Blackhurst DW, Latham BB, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545–556. doi: 10.1016/j.jamcollsurg.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 30.Rubino F, Kaplan LM, Schauer PR, Cummings DE, Diabetes Surgery Summit Delegates The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251(3):399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 31.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23(1):93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 33.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 34.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 37.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 38.Sjöström L, Gummesson A, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 39.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;199(4):543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–423. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDonald KG, Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non–insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1(3):213–220. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


