Abstract
We have recently shown that the prolongation of prostaglandin E2 (PGE2) hyperalgesia in a preclinical model of chronic pain – hyperalgesic priming – is mediated by release of cAMP from IB4-positive nociceptors and its metabolism by ectonucleotidases to produce adenosine. The adenosine, in turn, acts in an autocrine mechanism at an A1 adenosine receptor whose downstream signaling mechanisms in the nociceptor are altered to produce nociceptor sensitization. We previously showed that antisense against an extracellular matrix (ECM) molecule, versican, which defines the population of nociceptors involved in hyperalgesic priming, eliminated the prolongation of PGE2 hyperalgesia. To further evaluate the mechanisms at the interface between the ECM and the nociceptor’s plasma membrane involved in hyperalgesia prolongation, we interrupted a plasma membrane molecule involved in versican signaling, integrin β1, with an antisense oligodeoxynucleotide. Integrin β1 antisense eliminated mechanical hyperalgesia induced by an adenosine A1 receptor agonist, cyclopentyladenosine (CPA), in the primed rat. We also disrupted a molecular complex of signaling molecules that contains integrin β1, lipid rafts, with methyl-β-cyclodextrin, which attenuated the prolongation without affecting the acute phase of PGE2 hyperalgesia, while having no effect on CPA hyperalgesia. Our findings help to define the plasma membrane mechanisms involved in a preclinical model of chronic pain.
Keywords: Extracellular matrix, versican, integrin β1, hyperalgesic priming, nociceptor, hyperalgesia, rat
INTRODUCTION
In hyperalgesic priming, a model for the study of the transition from acute to chronic pain, following recovery from an acute inflammatory state 3, 21, 23 or in association with peripheral neuropathy 8, 9, a neuroplastic change occurs in isolectin B4 (IB4)-positive nociceptors such that a subsequent exposure to a pronociceptive inflammatory mediator, prototypically prostaglandin E2 (PGE2) produces a marked prolongation of the mechanical hyperalgesia it induces 17. We have recently shown that the prolongation phase of PGE2 hyperalgesia is mediated by an autocrine mechanism initiated by PGE2 acting at its cell surface receptor on the nociceptor to induce release of cAMP, as well as stimulating cAMP production. In the extracellular space cAMP is metabolized to adenosine by ectonucleotidases, a process dependent on an extracellular matrix (ECM) chondroitin sulfate proteoglycan, versican. Acting at the A1 adenosine receptor on nociceptors, adenosine, which is analgesic in control (non-primed) animals, now couples to protein kinase C epsilon (PKCε to produce prolonged hyperalgesia 15. In the present study we have pursued plasma membrane mechanisms involved in the prolongation of PGE2 hyperalgesia in the setting of hyperalgesic priming. Since versican’s interaction with cells is mediated, in part, by its binding to integrin β1 on the cell surface 33-36, we first evaluated the effect of attenuation of the level of nociceptor integrin β1 on the hyperalgesia induced by the adenosine A1 receptor agonist cyclopentyladenosine (CPA) in the setting of priming. And, since aspects of integrin β1 signaling are dependent on complexes in the cholesterol rich macromolecular complexes, lipid rafts 24, 30, 31, we examined whether disruption of these rafts eliminates the prolongation of PGE2 hyperalgesia in the setting of hyperalgesic priming. Disruption of lipid rafts in the primary afferent nociceptor did reversibly attenuate the prolongation of PGE2 hyperalgesia in the setting of priming.
METHODS
Experimental animals
Experiments were performed on adult male Sprague–Dawley rats (200–220 g; Charles River, Hollister, CA, USA). Animals were housed three per cage under a 12-h light–dark cycle in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All nociceptive testing was done between 10:00 and 16:00 h. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Nociceptive threshold testing
The mechanical nociceptive withdrawal threshold on the dorsum of the rat’s hind paw was measured using an Ugo Basile Algesymeter, as previously described 12, 26, 28. Briefly, rats were placed in an acrylic cylindrical restrainer with triangular ports through which the hind legs were free to extend. Four measures of mechanical nociceptive threshold in each paw were taken at 5-min intervals, and the mean of the last three measures was defined as the nociceptive threshold. Hyperalgesia is expressed as percentage decrease in nociceptive paw-withdrawal threshold compared with the control paw-withdrawal threshold obtained before intradermal drug administration.
Hyperalgesic priming
Hyperalgesic priming, a model of chronic pain, was produced using a procedure we have used in previous studies 14, 15, 17, 21, 22; the selective protein kinase C epsilon (PKCε) activator, ψεRACK (1 μg/5 μL) 19, was injected intradermally on the dorsum of the hind paw. This induces mechanical hyperalgesia that resolves in 3–5 days, subsequent to which intradermal injection of PGE2 at the same site produces markedly prolonged, PKCε-dependent, mechanical hyperalgesia 3, 21, 22.
Drugs
The drugs used in this study were: PGE2 (direct-acting hyperalgesic inflammatory mediator), ψεRACK (Receptor for Activated C Kinase; selective activator of PKCε), N6-cyclopentyladenosine (CPA; A1 adenosine receptor agonist) (all from Sigma, St Louis, MO, USA).
The selection of the drug doses used was based on dose–response curves determined in previous studies 15, 18, 20, 21, 27, 29. The stock solution of PGE2 (1 μg/μL) was prepared in 10% ethanol, and additional dilutions made with physiological saline (0.9% NaCl), yielding a final concentration of ethanol < 1%. All drugs were administered intradermally in a volume of 5 μL using a 30-gauge hypodermic needle adapted to a Hamilton (Reno, NV, USA) syringe. For the administration of ψεRACK, to increase its permeance through the plasma membrane, a hypotonic shock was induced by initial injection of distilled water (1 μL), separated by a bubble in the same syringe 6, 7.
Paw withdrawal threshold was determined before and 30 min and 4 h after drug administration, as indicated in individual experiments. Each experiment was performed on different groups of rats.
Oligodeoxynucleotide (ODN) antisense (AS) and mismatch 25 for integrin β1 mRNA
ODN-AS and MM for integrin β1 mRNA, synthesized by Invitrogen (San Francisco, CA, USA), were prepared and administered intrathecally as previously described 1, 11, 13. The ODN-AS sequence 5′-CAA ATT CAT CTT TTC GCA GCG TCC-3′ was directed against a unique sequence of rat integrin β1 subunit. The corresponding GenBank accession number and ODN position within the cDNA sequence are U12309 and 30–53, respectively. The ODN-MM sequence 5′-CAA TTT GTT GAT TTG CCA GCG TCC-3′ corresponds to the integrin β1 AS sequence with seven bases mismatched (denoted in bold).
ODN administration
The integrin β1 ODN-AS and MM used in this study were reconstituted in nuclease-free 0.9% NaCl to a concentration of 10 μg/μL and stored at −20°C until use. For each injection, rats were anesthetized with 2.5% isoflurane. A dose of 40 μg (injection volume 20 μL) of the ODN-AS or -MM was administered using an insulin syringe with a 29-gauge needle inserted intrathecally, on the midline between the 4th and 5th lumbar vertebrae, once daily for 3 consecutive days.
Statistics
In all experiments, the dependent variable was paw-withdrawal threshold, expressed as the percentage change from pre-intervention baseline. Average mechanical threshold immediately before the experiments was 118.1 ± 1.0 g (n = 36 paws); no statistical difference in the mechanical thresholds was observed before the injection of the priming stimulus (ψεRACK) and before injection of CPA (Fig. 1) or PGE2 (Fig. 2A, first test with PGE2)/CPA (Fig. 2 B): t35=0.05780; p=0.9542, paired Student’s t-test; For Fig. 2A, before the second test with PGE2 (1 week after the first test), t23=0.2575; p=0.7991, paired Student’s t-test. Importantly, the treatment with ODN-AS or MM to integrin β1 mRNA, or the lipid raft disruptor methyl-β-cyclodextrin, did not induce changes in the mechanical nociceptive threshold (data not shown). To compare the magnitude of the hyperalgesia produced by PGE2 or CPA in different experimental conditions, two-way repeated-measures analysis of variance (ANOVA), followed by Bonferroni’s post-test, were performed (as noted in the figure legends). GraphPad prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to plot the graphics and to perform the statistical analysis; P < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
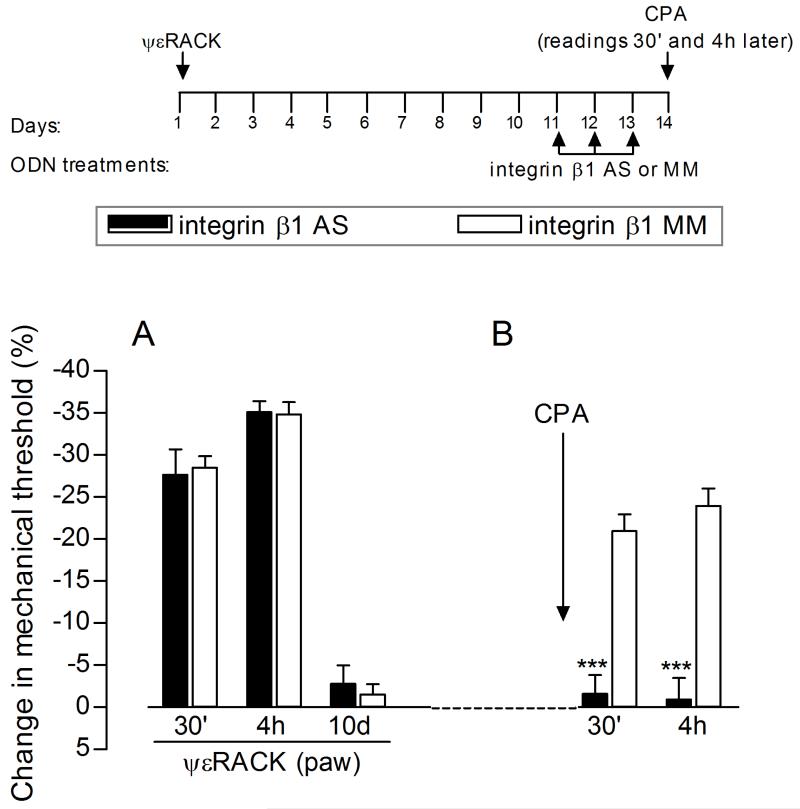
Figure 1. Role of integrin β1 in expression of hyperalgesic priming.
The schematic on the top of the figure represents the protocol used in the experiment, with the arrowheads indicating the time points when the rat hind paw mechanical threshold was evaluated. (A) The PKCε activator, ψεRACK (1 μg), was injected intradermally on the dorsum of the hind paw and, the mechanical nociceptive threshold, evaluated 30 min, 4 h and 10 days later. Significant mechanical hyperalgesia was observed at 30 min and 4 h, and by the 10th day after injection, the mechanical thresholds were not significantly different from pre-ψεRACK values; (B) Rats were then treated intrathecally, from days 11 to 13 post-ψεRACK, with ODN-AS (black bars) or -MM (white bars) for integrin β1 mRNA. On day 14, CPA (1 μg) was injected at the same site where ψεRACK had previously been administered and, the mechanical nociceptive threshold, evaluated 30 min and 4 h later. No hyperalgesia was observed in the group treated with ODN-AS, as opposed to the MM group (F1,10=70.74; ***p<0.0001 when comparing both groups; two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), suggesting that the expression of priming (i.e., induction of mechanical hyperalgesia by CPA) may be dependent on the presence of integrin β1 at the peripheral terminal of the nociceptor.
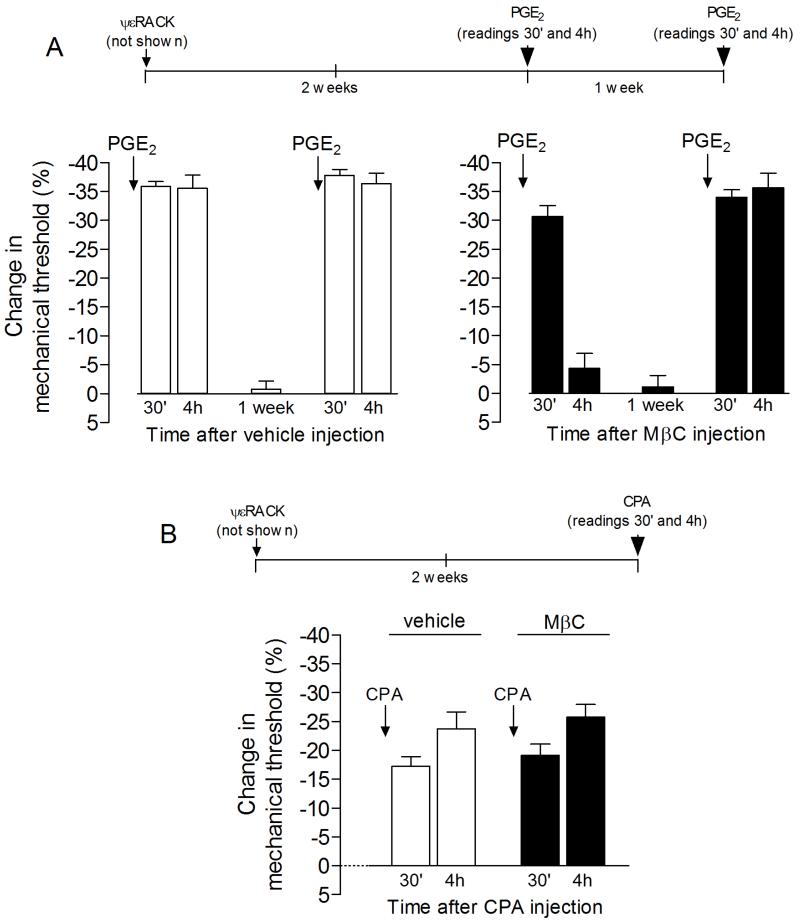
Figure 2. Role of lipid rafts in the expression of hyperalgesic priming.
The time lines on the top of the graphs show the protocols used in the respective experiments. Panel A: Rats previously (2 weeks) primed with intradermal injection of the PKCε activator ψεRACK (1 μg; not shown) on the dorsum of the right hind paw received an injection, at the same site, of vehicle (white bars) or the lipid raft disruptor methyl-β-cyclodextrin (MβC, 1 μg, black bars). 15 min later, PGE2 was injected and, the mechanical hyperalgesia, evaluated 30 min and 4 h later. We found that, while the PGE2-induced hyperalgesia was still significant at the 4th h in the vehicle-treated paws (white bars, t5=7.897, p=0.0005, when the mechanical nociceptive threshold at 4 h is compared to baseline; paired Student’s t-test), in the paws pretreated with MβC it was significantly attenuated by the 4th h after injection (black bars, second bar on the left: t5=1.136, p=0.3075, when the mechanical nociceptive threshold at 4 h is compared to baseline; paired Student’s t-test). To evaluate if the effect caused by lipid raft disruption on the expression of hyperalgesic priming was permanent, we tested again with PGE2 one week later, a period of time that allowed the recovery from the effect of MβC and return of the mechanical thresholds to baseline values (white bars: t5=0.1832; p=0.8018; black bars: t5=0.2225; p=0.8327, when comparing the mechanical thresholds before the first test with PGE2 and 1 week later; paired Student’s t-test). We observed, in both groups, that the mechanical hyperalgesia induced by PGE2 was still significant 4 h after injection, indicating that the attenuation of hyperalgesic priming by disruption of the lipid raft by MβC is reversible (white bars: t5=18.23; p<0.0001; black bars: t5=42.60; p<0.0001, when comparing the mechanical nociceptive threshold before and 4 h after PGE2 injection, respectively; paired Student’s t-test); Panel B: Rats that received intradermal injection of ψεRACK (1 μg; not shown) on the dorsum of the hind paw 2 weeks previously were treated, at the same site, with vehicle (white bars) or MβC (1 μg, black bars). 15 min later, CPA (1 μg) was injected and, the mechanical hyperalgesia, evaluated 30 min and 4 h later. Differently from what was observed in Panel A, where the disruption of lipid rafts significantly attenuated the expression of hyperalgesic priming (i.e., prolongation of PGE2-induced hyperalgesia), MβC did not affect the hyperalgesia induced by CPA in the primed paws (F1,10=0.13; p=0.4848, when comparing with the vehicle-treated group, two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group). This suggests that the hyperalgesic effect of CPA in the primed paw is not dependent on plasma membrane lipid rafts.
RESULTS
Role of integrin β1
To test the hypothesis that integrin β1 is important for the expression of hyperalgesic priming we evaluated if its knockdown would attenuate the hyperalgesia induced by the adenosine A1 receptor agonist CPA. It was not possible to use PGE2 as we have previously shown in control animals that integrin β1 antisense eliminates PGE2 hyperalgesia 10. Rats received an injection of the PKCε activator ψεRACK, which we have previously shown to induce priming 22, on the dorsum of the hind paw. Treatment with ODN against integrin β1 was started 11 days later, at which time ψεRACK-induced mechanical hyperalgesia had resolved (Fig. 1A) and, then, after 3 consecutive daily injections, testing for priming was performed by intradermal injection of the A1 adenosine receptor agonist CPA 15. Of note, we have previously shown that, although intradermal injection of CPA does not induce changes in the mechanical threshold in non-primed animals, following priming it produces prolonged PKCε-dependent mechanical hyperalgesia 15. We observed that, when rats previously primed with ψεRACK, 2 weeks before, and treated with ODN-AS or MM for integrin β1 mRNA for 3 days, received injection of CPA on the dorsum of the hind paw, hyperalgesia developed only in the group treated with MM, showing that the expression of priming (i.e., the CPA-induced hyperalgesia) is integrin β1 dependent (Fig. 1B).
Role of lipid rafts
The results from the experiments using integrin β1 antisense demonstrated that the expression of hyperalgesic priming (i.e., development of a hyperalgesic effect of the A1 adenosine receptor agonist CPA) is integrin β1 dependent, presumably by organizing second messenger signaling downstream of the activated A1 receptor, in the primed nociceptor. To test the hypothesis that signaling molecules involved in hyperalgesic priming are organized in a distinct compartment in the cell membrane, we tested the effect of the lipid raft disruptor methyl-β-cyclodextrin on the prolongation of PGE2-induced hyperalgesia and the hyperalgesia induced by CPA in primed rats. Priming was induced by injection of ψεRACK on the dorsum of the hind paw and, 2 weeks later, methyl-β-cyclodextrin or vehicle was injected at the same site; 30 min later, PGE2 (Fig. 2A) or CPA (Fig. 2B) was administered and the mechanical nociceptive threshold evaluated. We found that the lipid raft disruptor methyl-β-cyclodextrin attenuated the prolongation of the hyperalgesia induced by PGE2 while leaving the acute phase of PGE2 hyperalgesia unattenuated (Fig. 2A, panel on the right), but had no effect on the hyperalgesia induced by CPA (Fig. 2B). These results suggest that the prolongation of the PGE2-induced hyperalgesia is dependent on the organization of molecular components at a site in the plasma membrane (lipid raft), as previously shown 15, whereas the hyperalgesic effect of CPA is not, rather depending on changes in the intracellular signaling pathway triggered by A1 receptor activation in the setting of the neuroplastic change in the nociceptor responsible for hyperalgesic priming.
A summary of the proposed plasma membrane mechanism involved in the expression of hyperalgesic priming is illustrated, schematically, in Fig. 3.
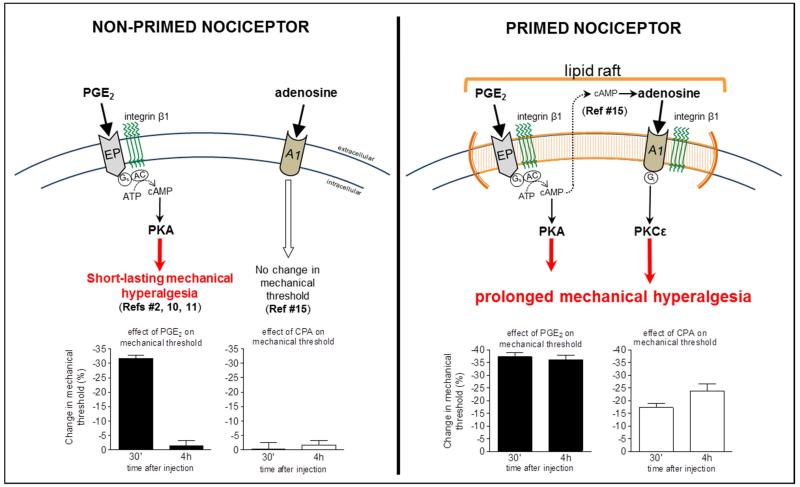
Figure 3. Schematic summary of the proposed mechanism in the plasma membrane involved in the prolongation of the PGE2-induced hyperalgesia in the primed nociceptor.
In the normal, non-primed nociceptor (LEFT SIDE), the function of EP receptors is dependent on integrin β1 10, 11. Activation of the receptor by intradermal injection PGE2 stimulates adenyl cyclase and cAMP production, which will, in turn, activate protein kinase A (PKA), and induce a short-lasting mechanical hyperalgesia, no longer present by the 4th h after injection 2. In the non-primed nociceptor, activation of the A1 receptor by adenosine does not affect the mechanical nociceptive threshold. On the other hand, in the primed nociceptor (RIGHT SIDE), in addition to the initial hyperalgesia induced by the activation of the PGE2 receptor-AC-PKA signaling pathway, a delayed component responsible for the prolongation of the PGE2 hyperalgesia, is observed. This late component is produced by the activation of an autocrine mechanism in which there is transport of cAMP to the extracellular space and further conversion to adenosine 15, followed by activation of the Gi-coupled A1 adenosine receptor (A1), which activates PKCε, prolonging the hyperalgesia produced by PGE2 in the primed nociceptor. This change in the profile of PGE2 hyperalgesia observed in hyperalgesic priming is dependent on the integrin β1 and lipid raft, the compartment in the plasma membrane where the components of the autocrine mechanism responsible for the conversion of cAMP to adenosine are located.
DISCUSSION
The role of extracellular mechanisms in pain is a relatively new area of research. We have studied the role of the extracellular matrix (ECM) in nociceptor function, in a model of the transition from acute to chronic pain, hyperalgesic priming 15, which has been shown to occur selectively in the IB4-positive subset of nociceptors. The binding of IB4 to this class of nociceptors is related to this plant lectin binding to versican, a large chondroitin sulfate proteoglycan extracellular matrix molecule, which we have shown impacts the function of the IB4-positive nociceptor 5. We have previously shown that an ODN-AS to versican mRNA, covering all its molecular isoforms, reversibly prevents hyperalgesic priming and can reverse the prolongation of PGE2 hyperalgesia in the setting of priming 15. To begin to understand how versican contributes to the marked prolongation of PGE2-induced hyperalgesia in the primed IB4-positive nociceptor, we used ODN-AS to attenuate an integrin subunit, integrin β1, in the plasma membrane, through which versican has been shown to affect cell function 33-36. We found that transient attenuation of the integrin β1 subunit in the primary afferent nociceptor reversed adenosine A1 receptor agonist (CPA)-induced hyperalgesia, possibly reflecting transient reversal of the switch that changes the coupling of the A1 adenosine receptor to signaling through PKCε to induce hyperalgesia, back to a state observed in control animals where it has no effect on pain threshold. While the details of how versican regulates nociceptor function in an integrin β1-dependent manner is not well understood, studies in non-neuronal cells indicate that versican binds to integrin β1, which we have shown to play a role in nociceptor function 1, 11, through its C-terminal G3 domain 34. Importantly, while our data indicate a dependence on integrin β1 we still do not know how A1 adenosine receptor signaling alters from Gi-protein-mediated inhibition of cAMP production and anti-hyperalgesia to coupling to the enhancement of PKCε signaling, to induce hyperalgesia.
Given that both integrin β1 and versican signaling have demonstrated dependence on lipid raft domains for their function 4, 16, 31, 32, we also evaluated the effect of disrupting lipid rafts on the hyperalgesia induced by PGE2 and CPA in the primed rat. The lipid raft disruptor methyl-β-cyclodextrin reversibly attenuated PGE2-, but not CPA-induced mechanical hyperalgesia in primed rats. The effect of lipid raft disruption could be related to disruption of signaling through the receptor at which PGE2 acts to produce hyperalgesia or the mechanisms downstream from this receptor leading to activation of the A1 adenosine receptor 15. However, given that the acute effect of PGE2 (i.e., hyperalgesia at 30 minutes) is not attenuated by disrupting lipid rafts, we favor the interpretation that the disruption of the lipid raft affects the extracellular signaling pathway between activation of the prostaglandin receptor and the production of an A1 adenosine receptor agonist in the extracellular space, which then produced mechanical hyperalgesia by acting at the A1 adenosine receptor on the primed nociceptor.
In conclusion, we have shown a role of the lipid rafts and integrin β1 function in the increased response to PGE2 observed in our model of hyperalgesic priming. The current results indirectly confirm previous observations 15 that the prolongation of PGE2-induced hyperalgesia depends on the activation of A1 adenosine receptors, and define the lipid rafts as the site where the machinery for the autocrine mechanism is located. Moreover, our experiments also suggest the signaling from A1 adenosine receptor to integrin β1 as the final step of the autocrine mechanism involved in the expression of priming.
Perspectives.
The present study contributes to a further understanding of mechanisms involved in the organization of messengers at the plasma membrane that participate in the transition from acute to chronic pain.
Highlights.
Integrin β1 transduces signals from extracellular matrix in a model of chronic pain;
Membrane lipid rafts also contribute to mechanisms of in the model of chronic pain;
Our findings help define plasma membrane mechanisms in a preclinical model of chronic pain.
Acknowledgments
This study was funded by the National Institutes of Health (NIH), NS084545.
Abbreviations
- AS
antisense
- CPA
adenosine A1 receptor agonist N6-cyclopentyladenosine
- ECM
extracellular matrix
- IB4
isolectin B4
- MM
mismatch
- ODN
oligodeoxynucleotide
- PGE2
prostaglandin E2
- PKCε
protein kinase C epsilon
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
REFERENCES
- 1.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–52. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–6. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–5. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi J, Wang R, Zhang Y, Han X, Ampah KK, Liu W, Zeng X. Identification of nucleolin as a lipid-raft-dependent beta1-integrin-interacting protein in A375 cell migration. Mol Cells. 2013;36:507–17. doi: 10.1007/s10059-013-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogen O, Dreger M, Gillen C, Schroder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J. 2005;272:1090–102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- 6.Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–4. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- 7.Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–8. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–9. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–42. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33:11002–11. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari LF, Bogen O, Levine JD. Second Messengers Mediating the Expression of Neuroplasticity in a Model of Chronic Pain in the Rat. J Pain. 2014 doi: 10.1016/j.jpain.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari LF, Levine E, Levine JD. Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci. 2013;37:1705–13. doi: 10.1111/ejn.12145. [DOI] [PubMed] [Google Scholar]
- 16.Grove LM, Southern BD, Jin TH, White KE, Paruchuri S, Harel E, Wei Y, Rahaman SO, Gladson CL, Ding Q, Craik CS, Chapman HA, Olman MA. Urokinase-type plasminogen activator receptor (uPAR) ligation induces a raft-localized integrin signaling switch that mediates the hypermotile phenotype of fibrotic fibroblasts. J Biol Chem. 2014;289:12791–804. doi: 10.1074/jbc.M113.498576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–5. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN, Yatani A, Robbins J, Dorn GW., 2nd Cardiotrophic effects of protein kinase C epsilon: analysis by in vivo modulation of PKCepsilon translocation. Circ Res. 2000;86:1173–9. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- 20.Ouseph AK, Khasar SG, Levine JD. Multiple second messenger systems act sequentially to mediate rolipram-induced prolongation of prostaglandin E2-induced mechanical hyperalgesia in the rat. Neuroscience. 1995;64:769–76. doi: 10.1016/0306-4522(94)00397-n. [DOI] [PubMed] [Google Scholar]
- 21.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–90. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–26. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 23.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel AP, Kimble-Hill A, Garg S, Jordan R, Naumann CA. Native ligands change integrin sequestering but not oligomerization in raft-mimicking lipid mixtures. Biophys J. 2011;101:1642–50. doi: 10.1016/j.bpj.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson E, Persson J, Fitzsimmons B, Yaksh TL. Intrathecal neurosteroids and a neurosteroid antagonist: effects on inflammation-evoked thermal hyperalgesia and tactile allodynia. Neurosci Lett. 2013;548:27–32. doi: 10.1016/j.neulet.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain res. 1989;487:148–51. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- 27.Taiwo YO, Heller PH, Levine JD. Characterization of distinct phospholipases mediating bradykinin and noradrenaline hyperalgesia. Neuroscience. 1990;39:523–31. doi: 10.1016/0306-4522(90)90288-f. [DOI] [PubMed] [Google Scholar]
- 28.Taiwo YO, Levine JD. Contribution of guanine nucleotide regulatory proteins to prostaglandin hyperalgesia in the rat. Brain res. 1989;492:400–3. doi: 10.1016/0006-8993(89)90929-3. [DOI] [PubMed] [Google Scholar]
- 29.Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–62. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- 30.Vassilieva EV, Gerner-Smidt K, Ivanov AI, Nusrat A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G965–76. doi: 10.1152/ajpgi.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Yoo Y, Fan H, Kim E, Guan KL, Guan JL. Regulation of Integrin beta 1 recycling to lipid rafts by Rab1a to promote cell migration. J Biol Chem. 2010;285:29398–405. doi: 10.1074/jbc.M110.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Bi J, Ampah KK, Zhang C, Li Z, Jiao Y, Wang X, Ba X, Zeng X. Lipid raft regulates the initial spreading of melanoma A375 cells by modulating beta1 integrin clustering. Int J Biochem Cell Biol. 2013;45:1679–89. doi: 10.1016/j.biocel.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Chen L, Cao L, Sheng W, Yang BB. Overexpression of the C-terminal PG-M/versican domain impairs growth of tumor cells by intervening in the interaction between epidermal growth factor receptor and beta1-integrin. J Cell Sci. 2004;117:2227–37. doi: 10.1242/jcs.01057. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Wu J, Lee DY, Yee A, Cao L, Zhang Y, Kiani C, Yang BB. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005;24:3–13. doi: 10.1016/j.matbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]