Abstract
Previous research has shown that type 2 diabetes mellitus (T2DM) is associated with white matter microstructural changes, cognitive impairment, and decreased resting-state functional connectivity and spontaneous brain activity. This study used magnetization transfer imaging to examine, for the first time, the integrity of macromolecular protein pools in fronto-striato-thalamic circuits and its clinical and cognitive correlates in patients with T2DM. T2DM patients without mood disorders (n = 20, aged 65.05 ± 11.95 years) and healthy control subjects (HCs; n = 26, aged 62.92 ± 12.71 years) were recruited. Nodes of fronto-striato-thalamic circuits—head of the caudate nucleus (hCaud), putamen, globus pallidus, thalamus—and four cortical regions—rostral and dorsal anterior cingulate cortex, dorsolateral prefrontal cortex, and lateral orbitofrontal cortex—were examined. Compared with HCs, patients with T2DM had significantly lower magnetization transfer ratio (MTR) in bilateral anterior cingulate and hCaud. Reduced MTRs in the above regions showed correlations with T2DM-related clinical measures, including hemoglobin A1c level and vascular risk factors, and neuropsychological task performance in the domains of learning and memory, executive function, and attention and information processing. The impaired biophysical integrity of brain macromolecular protein pools and their local microenvironments in T2DM patients may provide insights into the neurological pathophysiology underlying diabetes-associated clinical and cognitive deficits.
Introduction
Type 2 diabetes mellitus (T2DM) is associated with metabolic and macro- and microvascular complications in multiple organ systems, including the brain (1). T2DM is also associated with behavioral aberrations, including mood disorders, cognitive impairment, and increased risk of dementia in the elderly (2–5). This may be due in part to neuroanatomical alterations as revealed by structural MRI, including atrophy in prefrontal cortex and the anterior cingulate (6–8). Findings from diffusion tensor imaging studies indicate reduced fractional anisotropy in the frontal white matter (9), cingulum bundle, uncinate fasciculus (10), and anterior limb of internal capsule (ALIC) (11) in patients with T2DM relative to nondiabetic control subjects. Moreover, recent resting-state functional MRI studies demonstrated disrupted functional connectivity within the default mode network and decreased spontaneous brain activity in the occipital lobe and postcentral gyrus in T2DM patients compared with control subjects (10,12,13). However, it is not clear whether macromolecular protein pools in these regions and circuits are also impaired in T2DM. Investigating the integrity of macromolecular protein pools in gray and white matter may provide complementary information and would aid in our understanding of the mechanisms underlying T2DM-related brain alterations and the neurobiology of diabetes.
Magnetization transfer (MT) imaging exploits magnetization exchange between protons bound to macromolecules and free protons in tissue water. In MT imaging, an off-resonance prepulse is applied to selectively saturate bound protons, and magnetization is then transferred from saturated bound protons to free protons through chemical exchange and direct dipolar coupling. This transfer of magnetization leads to decreased magnetic resonance signal from free protons. The contrast between MT images with and without the saturation prepulse is defined as magnetization transfer ratio (MTR) (14), which reflects the biophysical integrity of macromolecular protein pools and their local microenvironment. Postmortem MT and histopathology studies of multiple sclerosis revealed that in white matter, lower MTR is associated with axonal loss and myelin compromise (15,16). The origins of MTR changes in gray matter are more complex and heterogeneous and may reflect multiple neurobiological aberrations (17–20). In gray matter, cell membrane proteins and phospholipids contribute to macromolecular density (19). Therefore, damage to cell membranes, reduction in dendritic density and neuronal size and number may independently or collectively lead to decreased MTR (19). Wallerian degeneration, secondary to proximal and/or distal axonal damage, has also been implicated as a mechanism contributing to lower MTR (18,20). Although lacking of specificity in its origins, MTR imaging provides an innovative way to probe the integrity of macromolecular protein pools in the brain.
Despite the unique features of MT imaging, there are very few MT studies on T2DM in the literature. The only published MT study was on T2DM and major depression in combination, which focused on subcortical regions and found compromised macromolecular protein pools in the head of the caudate nucleus (hCaud) in T2DM patients (4). However, it is unknown whether there are accompanied changes in macromolecular protein pools in the connected cortical regions.
Fronto-striato-thalamic circuits have been implicated in the pathophysiology of several mood and associated cognitive disorders (21,22). T2DM is consistently associated with disturbances in mood and cognition (4,23). Particularly, individuals with T2DM are at a greater risk for depression (24,25). However, neurosubstrates underlying the increased risk of mood disturbance and/or cognitive impairments in T2DM are unclear. In this study, we specifically evaluated the biophysical integrity of macromolecular protein pools in fronto-striato-thalamic circuits in patients with T2DM without comorbid mood or cognitive disturbances. Such a study design allows us to elucidate T2DM-specific influence on brain circuits implicated in mood and cognitive functions, which may potentially inform mechanisms underlying increased vulnerability to mood and cognitive disorders in T2DM.
More specifically, this study investigated brain regions/structures associated with the dorsolateral prefrontal, lateral orbitofrontal, and anterior cingulate frontal-subcortical circuits—circuits that are neurobiologically relevant in human behavior, including cognitive control, decision making, action planning and execution, learning and working memory, attention, and emotional processing (21). Additionally, we were interested in examining the relationships between altered macromolecular protein pools and T2DM-related clinical measures as well as neuropsychological metrics in different cognitive domains. We hypothesized that MTR would be lower at nodes of fronto-striato-thalamic circuits in T2DM patients compared with healthy control subjects (HCs). We additionally hypothesized that MTR in these node regions in patients with T2DM would be negatively correlated with clinical measures reflecting vascular comorbidities and blood glucose regulation and positively correlated with neuropsychological performance.
Research Design and Methods
Subjects
The subject population consisted of 20 T2DM patients without mood disorders (aged 65.05 ± 11.95 years) and 26 nondiabetic HCs (aged 62.92 ± 12.71 years). Subjects were selected from a larger sample of a research program on diabetes and depression at the University of Illinois at Chicago. All participants were aged 30 years and older and recruited from the greater Chicago area through flyers, local advertisements, and relevant outpatient clinics. The study was approved by the University of Illinois at Chicago Institutional Review Board, and written informed consent was obtained from all participants.
The diagnosis of T2DM in patients was made by their primary care physicians and was confirmed using American Diabetes Association guidelines (26) (an elevated nonfasting hemoglobin A1c [HbA1c] level [>6.5% (48 mmol/mol)] or the use of antidiabetic medications [oral hypoglycemic and/or insulin] when enrolled for this study). T2DM patients reported using oral hypoglycemic medications and/or insulin for glycemic control (six patients with one oral hypoglycemic medication or insulin; seven with two or more hypoglycemic medications; two with both medications and insulin; five without any hypoglycemic medication or insulin). With respect to diabetic vascular complications (27), 5 patients had diabetic microvascular complications (e.g., diabetic nephropathy, neuropathy, and retinopathy), 1 had diabetic macrovascular complications (e.g., coronary artery disease, peripheral arterial disease, and myocardial infarction), 2 had both, and 12 were without any vascular complications to their diabetes. HCs were free of diabetes and had HbA1c levels within normal limits. All participants received the Mini-Mental State Examination (MMSE) (28), the Structured Clinical Interview for DSM-IV (29), and the 17-item Hamilton Depression Rating Scale (HAM-D) (30), administered by a trained research assistant and a board-certified (A.K.) or board-eligible (O.A.) psychiatrist. Both control and patient subjects denied a history of depressed mood, obtained scores of 8 or lower on the HAM-D, were free of unstable medical conditions, and were of comparable age and sex.
Exclusion criteria included any current or past history of neurological and psychiatric disorders (e.g., dementia, stroke, seizure, transient ischemic attack, or depression), learning disability or attention deficit hyperactivity disorder, psychotropic medication, current or past history of substance abuse or dependence, history of head injury or loss of consciousness, a MMSE score less than 24, or any contraindication to MRI scan such as metal in the body, surgically implanted devices containing metal, claustrophobia, and pregnancy.
All participants were assessed for medical comorbidity using the Cumulative Illness Rating Scale (CIRS) (31) and for vascular comorbidities using the Framingham Stroke Risk Profile (FSRP) score (32). We also designed a modified FSRP (mFSRP) score in which the contribution of T2DM was removed to represent vascular complications only. All participants received a nonfasting blood draw to document HbA1c levels, HDL cholesterol, and LDL cholesterol and to verify diabetes status. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and BMI were documented on each participant. All participants were also assessed for IQ using the Wechsler Test of Adult Reading (WTAR) (33). Demographic and T2DM-related clinical measures are summarized in Table 1. Obesity, hypertension, vascular diseases, and/or elevated bad cholesterol levels are often seen in patients with T2DM, and each of these comorbidities has been reported to exert their own effects on the human brain. It must be noted that in this study, these comorbidities (including the following variables: BMI, SBP, DBP, mFSRP, and LDL cholesterol) were of comparable levels between two groups (P > 0.20). So the possible effects of these comorbidities on the group difference in MTR have been minimized, and the findings from this study are primarily attributable to T2DM.
Table 1.
Demographic and clinical measures across subject groups
| Measures | HC
(N = 26) |
T2DM
(N = 20) |
Statistics |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | df | P | |
| Age (years) | 62.92 | 12.71 | 65.05 | 11.95 | 0.333 | 1, 44 | 0.567 |
| Sex (n) | 11 M, 15 F | 12 M, 8 F | χ2 = 1.415 | 1 | 0.234 | ||
| Race (n) | 10 B, 2 H, 1 A, 11 W, 2 O | 11 B, 1 H, 0 A, 8 W, 0 O | χ2 = 3.125 | 4 | 0.537 | ||
| Handedness (n) | 25 R, 1 L, 0 M | 16 R, 3 L, 1 M | χ2 = 3.248 | 2 | 0.197 | ||
| Education (years) | 14.54 | 1.86 | 14.80 | 2.28 | 0.184 | 1, 44 | 0.670 |
| WTAR | 102.52 | 11.09 | 99.95 | 16.54 | 0.387 | 1, 43a | 0.537 |
| MMSE | 28.92 | 0.98 | 28.35 | 1.18 | 3.241 | 1, 44 | 0.079 |
| HAM-D | 0.96 | 1.311 | 1.65 | 1.843 | 2.193 | 1, 44 | 0.146 |
| BMI (kg/m2) | 30.18 | 17.54 | 31.43 | 5.91 | 0.092 | 1, 44 | 0.763 |
| SBP (mmHg) | 136.38 | 14.05 | 139.30 | 17.87 | 0.384 | 1, 44 | 0.539 |
| DBP (mmHg) | 79.85 | 10.06 | 83.70 | 10.30 | 1.627 | 1, 44 | 0.209 |
| T2DM duration (months) | — | — | 115.15 | 85.19 | — | — | — |
| HDL cholesterol | 72.88 | 22.21 | 48.15 | 13.27 | 19.406 | 1, 44 | <0.001 |
| LDL cholesterol | 93.27 | 23.99 | 88.00 | 22.56 | 0.574 | 1, 44 | 0.453 |
| CIRS | 3.69 | 2.83 | 7.15 | 3.05 | 15.812 | 1, 44 | <0.001 |
| FSRP | 8.81 | 4.61 | 13.00 | 4.90 | 8.848 | 1, 44 | 0.005 |
| mFSRP (T2DM removed) | 8.81 | 4.61 | 10.60 | 5.14 | 1.544 | 1, 44 | 0.221 |
| HbA1c (%) (mmol/mol) | 5.70 (39) | 0.36 (3.9) | 7.37 (57) | 1.67 (18.3) | 24.503 | 1, 44 | <0.001 |
A, Asian; B, black; F, female; H, Hispanic; L, left; M (handedness), mixed; M (sex), male; O, other; R, right; W, white.
aOne control subject’s WTAR was not recorded. Measures with significant group differences are in boldface text.
Neuropsychological Tests
A neuropsychological battery was conducted on each participant across three domains: learning and memory (California Verbal Learning Test [Second Edition] immediate total recall and long-delay free recall [34]; Wechsler Memory Scale [Third Edition] Logical Memory I and II and Visual Reproduction I and II [35]); attention and information processing (Stroop Color and Word tests [36]; Trail Making Test A; and Wechsler Adult Intelligence Scale [Third Edition] Digit Symbol-Coding [37]); and executive function (Delis-Kaplan Executive Function System Category Switching [38]; Trail Making Test B [39]; Stroop Interference Score [36]; Wechsler Adult Intelligence Scale [Third Edition] Digit Span Backward [37]; and Self-Ordered Pointing Task Total Errors [40]). Raw scores from the neuropsychological battery were standardized using the HC sample mean and SD. Relevant scores were reversed so that high scores consistently reflected better performance. Composite Z scores were calculated for each domain, and Cronbach α suggested that each variable measured a unidimensional latent construct (learning and memory, α = 0.88; attention and information processing, α = 0.85; executive function, α = 0.76).
MT Image Data Acquisition
MRI was performed on a Philips Achieva 3.0T scanner (Philips Medical Systems, Best, the Netherlands) with a body coil for transmission and a Philips 8-channel SENSE Head coil for reception. Subjects were equipped with soft ear plugs, positioned comfortably in the head coil using custom-made foam pads to minimize head motion, and instructed to remain still. MT images were acquired using a three-dimensional (3D) spoiled gradient echo sequence with multishot echo-planar imaging (EPI) readout and the following parameters: repetition time (TR)/echo time (TE) = 64/15 ms, flip angle = 9°, field of view (FOV) = 24 cm, 67 axial slices, slice thickness/gap = 2.2 mm/no gap, EPI factor = 7, and reconstructed voxel size = 0.83 × 0.83 × 2.2 mm3, with a nonselective five-lobed Sinc-Gauss off-resonance MT prepulse (B1/Δf/duration = 10.5 μT/1.5 kHz/24.5 ms) optimized for maximum white matter/gray matter contrast (41). Image slices were parallel to the anterior commissure–posterior commissure line. Parallel imaging was used with a reduction factor of 2. Before the MT scan, high-resolution 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) images were acquired with the following sequence parameters: TR/TE = 8.4/3.9 ms, flip angle = 8°, FOV = 24 cm, 134 axial slices/no gap, and reconstructed voxel size = 0.83 × 0.83 × 1.1 mm3. In addition, T2-weighted fluid-attenuated inversion recovery (FLAIR) images were also acquired using turbo spin echo sequence with the following sequence parameters: TR/inversion time (TI)/TE = 11,000/2,800/68 ms, FOV = 24 cm, 67 axial slices without gap, and reconstructed voxel size = 0.83 × 0.83 × 2.2 mm3, for delineation of hyperintense areas in the brain.
Image Processing
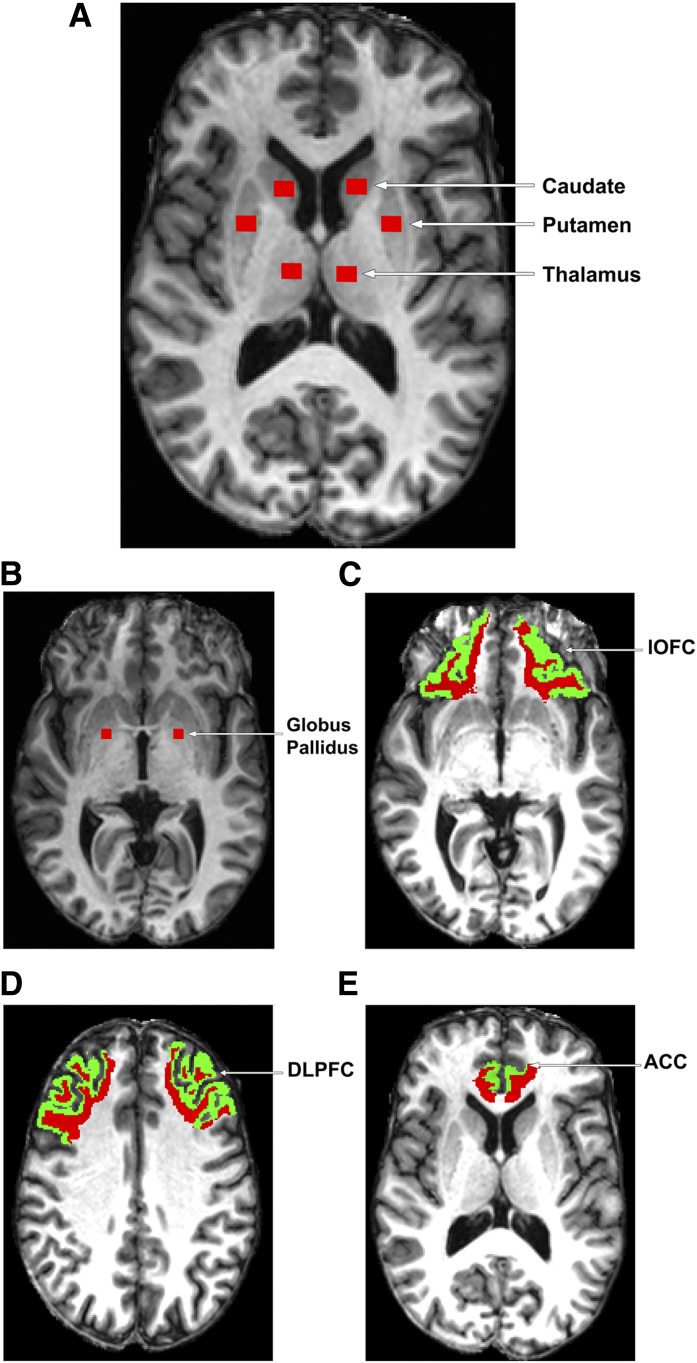
For each participant, T1-weighted MPRAGE image, T2-weighted FLAIR image, and MT images (with and without the off-resonance MT prepulse: Ms and M0) were coregistered. MTR values were calculated on a voxel-by-voxel basis using coregistered M0 and Ms with the formula MTR = (M0 − Ms)/M0. Regions of interest (ROIs) were placed on the coregistered high-resolution T1-weighted image at the nodes of fronto-striato-thalamic circuits (21), including four subcortical regions (i.e., hCaud, putamen, globus pallidus, and thalamus) and four cortical regions (i.e., rostral anterior cingulate cortex [rACC], dorsal anterior cingulate cortex [dACC], dorsolateral prefrontal cortex [DLPFC], and lateral orbitofrontal cortex [lOFC]) in both hemispheres (see Fig. 1). Care was taken to ensure consistent placement of the subcortical ROIs for the MTR analysis. The slice displaying the most anterior margin of the genu of the corpus callosum (Montreal Neurological Institute (MNI) [x, y, z] coordinates, [1, 32, 6] mm) was chosen as the reference slice for placing the subcortical ROIs hCaud, putamen, and thalamus, because this landmark could be easily and consistently identified across subjects and these ROIs are visible at this slice level (4) (see Fig. 1A). In the case that putamen was not completely visualized on this slice, the next inferior slice was used as the reference slice (4). For the ROI of globus pallidus, the slice clearly displaying the anterior commissure (MNI coordinates [0, 2, −4] mm) was chosen as the reference slice (see Fig. 1B). During the placement of the subcortical ROIs, the coregistered FLAIR image was closely examined to ensure the ROIs were not placed in hyperintense areas. Moreover, we used constant volumes of ROIs in all of the defined subcortical regions, i.e., the volume was fixed to 73.3 mm3 for hCaud, putamen, and thalamus and was fixed to relatively smaller 55 mm3 for globus pallidus. These two volumes were selected so that the MTR calculation in each subcortical ROI could be devoid of any partial volume effects from adjoining brain regions and/or cerebrospinal fluid on all the involved subjects. For the four cortical ROIs (i.e., rACC, dACC, DLPFC, and lOFC), we used the FreeSurfer package (https://surfer.nmr.mgh.harvard.edu/) to parcellate these structures and evaluated MTR within cortical white matter to minimize partial volume effects from the cerebrospinal fluid. To better illustrate the anatomical location of each cortical region, both gray (green) and white (red) matter areas are displayed in Fig. 1C–E (the region of dACC is not shown for simplicity). Generation of ROI masks and calculation of MTR were performed using in-house developed programs.
Figure 1.
ROIs for MTR analysis.
Statistical Analysis
Clinical and demographic measures were analyzed using univariate ANOVA for continuous variables and χ2 tests for categorical variables. Group differences in MTR in bilateral regions were assessed using a mixed-model analysis with diagnostic group as the between-group factor and hemisphere as a within-subject factor. Group differences in MTR in individual regions on each hemisphere were further analyzed using univariate ANCOVA controlling for age. Correlations between MTR and FSRP, mFSRP, HbA1c level, or other related clinical variables were analyzed using partial Pearson product-moment correlations controlling for age. In the correlation analysis of MTR and HbA1c, a natural log transformation was first performed on HbA1c (i.e., log-transformed HbA1c) to reduce the skewness of this variable when the sample was combined from both HC and T2DM groups. Correlations between MTR and neuropsychological task performance were also analyzed using partial Pearson product-moment correlations controlling for age and MMSE as initial covariates. Multiple comparison correction was performed using false discovery rate (FDR) approach (42–44) with the maximum acceptable FDR set at 0.15. All statistical analyses were carried out using SPSS version 18 (SPSS Inc., Chicago, IL).
Results
Demographic and Clinical Measures
Table 1 summarizes the demographic and clinical characteristics of the T2DM and HC groups. There were no significant group differences in age, sex, race, handedness, years of education, IQ (WTAR), MMSE, HAM-D (both groups were free of depression, with mean scales <2), BMI, SBP, DBP, LDL cholesterol, and mFSRP. As expected, there were significant group differences in the diabetes-related clinical measures, HDL cholesterol (F = 19.406, df = 1, 44, P < 0.001), CIRS (F = 15.812, df = 1, 44, P < 0.001), FSRP (F = 8.848, df = 1, 44, P = 0.005), and HbA1c (F = 24.503, df = 1, 44, P < 0.001).
Neuropsychological Tests
There was no significant group difference in neuropsychological task performance in the three domains: learning and memory, executive function, and attention and information processing (P > 0.09) (see Table 2) (the neuropsychological data of one control subject was not recorded, one diabetic subject was color blind and did not perform the Stroop tasks, and another diabetic subject’s Trail Making Test A task was not scored).
Table 2.
Neuropsychological task performance (in composite Z score) in three domains across subject groups
| Domain | HC
(N = 26) |
T2DM
(N = 20) |
Statistics |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | df | P | |
| Learning and memory | Refa | 0.785 | −0.280 | 0.779 | 1.217 | 1, 43 | 0.276 |
| Executive function | Refa | 0.715 | −0.372 | 0.673 | 2.972 | 1, 42 | 0.092 |
| Attention and information processing | Refa | 0.826 | −0.278 | 0.861 | 1.348 | 1, 41 | 0.252 |
aRaw score of each specific cognitive task on each subject was standardized (called Z score) using the mean and SD of the HC group.
Group Differences in MTR
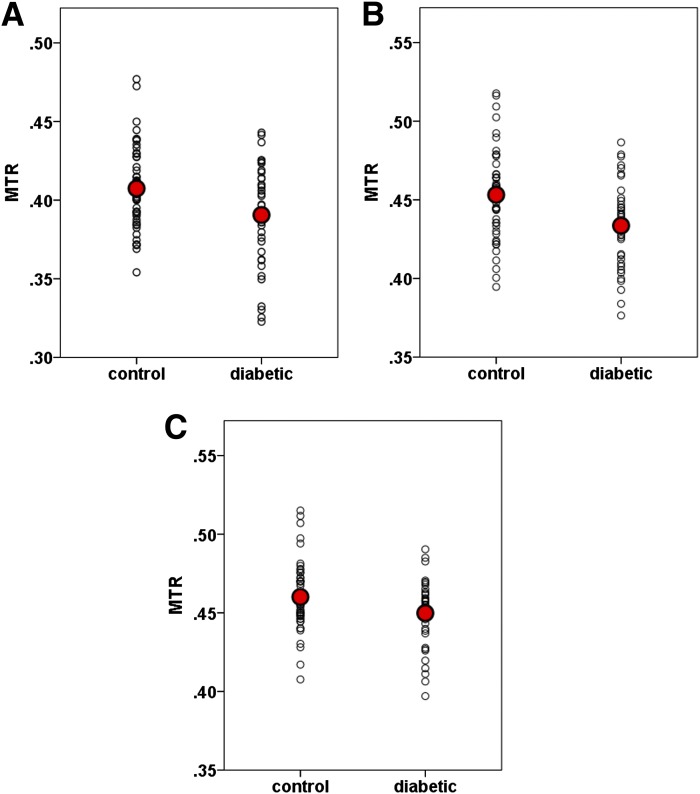
The mixed-model analysis (with diagnostic group as the between-group factor and hemisphere as a within-subject factor) showed that among the ROIs examined, MTR was significantly lower in bilateral dACC (F = 6.082, df = 1, 44, P = 0.018) and bilateral hCaud (F = 5.085, df = 1, 44, P = 0.029), and there was a trend of lower MTR in bilateral rACC (F = 2.767, df = 1, 44, P = 0.103) in T2DM patients compared with nondiabetic control subjects (see Fig. 2). The above significant results remained significant after the FDR multiple comparison correction. There were no significant hemispheric differences in MTR in the above regions, i.e., dACC (F = 1.040, df = 1, 45, P = 0.313), hCaud (F = 0.548, df = 1, 45, P = 0.463), and rACC (F = 1.451, df = 1, 45, P = 0.235). ANCOVA analysis further revealed that the dACC MTRs in both left and right hemispheres were significantly lower in T2DM patients than in nondiabetic control subjects (left: F = 5.787, df = 1, 43, P = 0.021 and right: F = 4.847, df = 1, 43, P = 0.033), and right hCaud MTR was significantly lower in T2DM patients (F = 5.416, df = 1, 43, P = 0.025), while no significant difference was found in left hCaud MTR (F = 1.974, df = 1, 43, P = 0.167) despite a lower mean value in T2DM patients than control subjects. In the following sections, we limited our further statistical analysis to the regions showing group differences in MTR, i.e., bilateral dACC, bilateral rACC, and right hCaud.
Figure 2.
Scatterplots of MTRs in (A) hCaud, (B) dACC, and (C) rACC. A: F = 5.085, df = 1, 44, P = 0.029. B: F = 6.082, df = 1, 44, P = 0.018. C: F = 2.767, df = 1, 44, P = 0.103.
Correlation Between MTR and T2DM-Related Clinical Measures
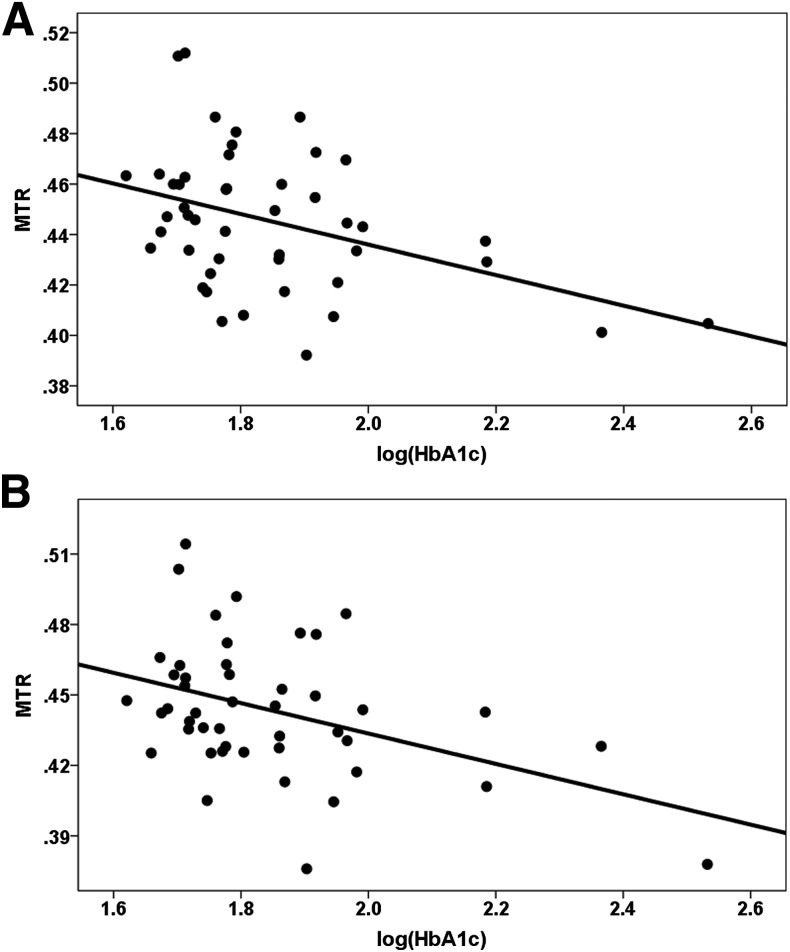
When groups were combined, MTR was negatively correlated with the log-transformed HbA1c level in bilateral dACC (left: r = −0.401, df = 43, P = 0.006 and right: r = −0.412, df = 43, P = 0.005) (see Fig. 3A and B), bilateral rACC (left: r = −0.350, df = 43, P = 0.018 and right: r = −0.329, df = 43, P = 0.027), and right hCaud (r = −0.323, df = 43, P = 0.031). These results remained significant after the FDR multiple comparison correction.
Figure 3.
Representative scatterplots of correlations between MTRs and log-transformed HbA1c level in (A) left dACC and (B) right dACC. A: r = −0.401, df = 43, P = 0.006. B: r = −0.412, df = 43, P = 0.005.
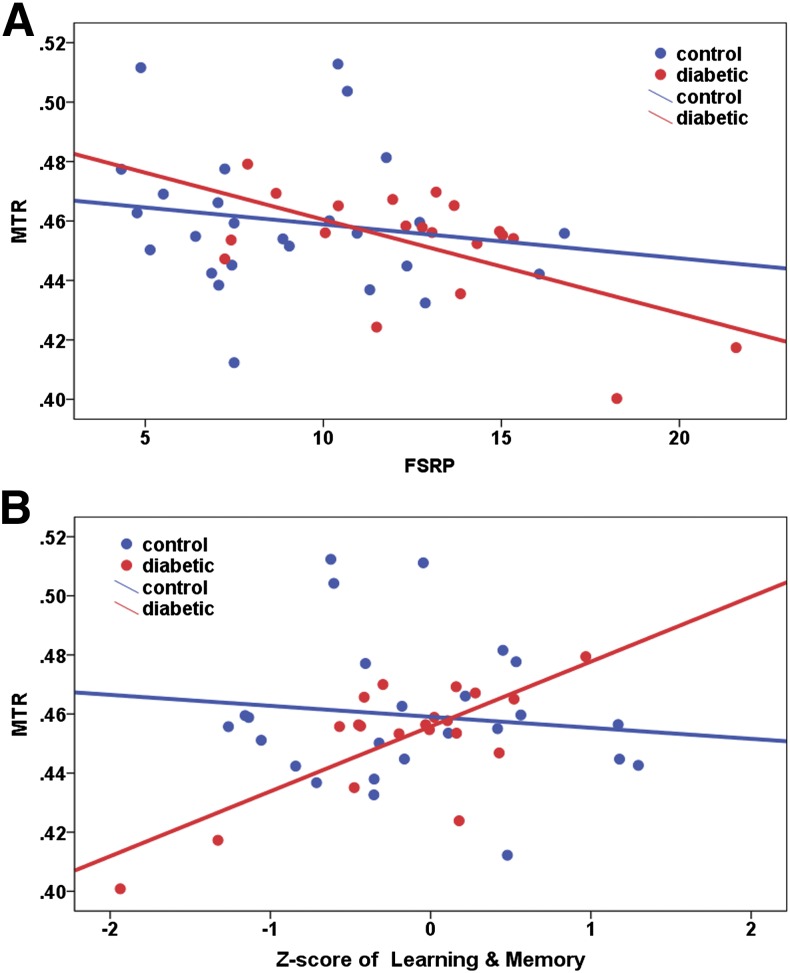
MTRs in bilateral rACC were negatively correlated with the FSRP score across the entire sample combining both groups (left: r = −0.344, df = 43, P = 0.021 and right: r = −0.371, df = 43, P = 0.012). If examining MTR in each group, significant correlation between right rACC MTR and FSRP was found in the T2DM group but not in the HC group (T2DM: r = −0.589, df = 17, P = 0.008 and HC: r = −0.158, df = 23, P = 0.450) (see Fig. 4A). The above significant results remained significant after the FDR multiple comparison correction. We repeated the above correlation analysis using the mFSRP score, and the results remained significant in bilateral rACC across the entire sample (left: r = −0.304, df = 43, P = 0.043 and right: r = −0.357, df = 43, P = 0.016) and in right rACC in the T2DM group (T2DM: r = −0.599, df = 17, P = 0.007 and HC: r = −0.158, df = 23, P = 0.450).
Figure 4.
Representative scatterplots of correlations between MTRs in right rACC and (A) vascular risk factor assessed by FSRP score and (B) neuropsychological task performance (in Z score) in learning and memory. A: T2DM: r = −0.589, df = 17, P = 0.008 and HC: r = −0.158, df = 23, P = 0.450. B: T2DM: r = 0.811, df = 16, P < 0.001 and HC: r = −0.100, df = 21, P = 0.651.
There was no significant correlation between MTR in the examined regions and the duration of T2DM (P > 0.07), SBP (P > 0.08), DBP (P > 0.13), BMI (P > 0.39), HDL cholesterol (P > 0.19), and LDL cholesterol (P > 0.12) in T2DM patients.
Correlation Between MTR and Neuropsychological Task Performance
The learning and memory Z score was positively correlated with bilateral rACC MTR in the T2DM group (left: r = 0.595, df = 16, P = 0.009 and right: r = 0.811, df = 16, P < 0.001) but not in the HC group (left: r = −0.166, df = 21, P = 0.449 and right: r = −0.100, df = 21, P = 0.651). The significant correlation in right rACC remained significant after the FDR multiple comparison correction (see Fig. 4B). Furthermore, this correlation remained significant after adding the HbA1c level (r = 0.722, df = 15, P = 0.001) or mFSRP score (r = 0.648, df = 15, P = 0.005) as an additional covariate besides age and MMSE. In addition, the right rACC MTR was also positively correlated with the executive function Z score in the T2DM group (r = 0.540, df = 15, P = 0.025) but not in the HC group (r = −0.097, df = 21, P = 0.659). Finally, the attention and information processing Z score was positively correlated with right hCaud MTR in the T2DM group only (T2DM: r = 0.549, df = 14, P = 0.028 and HC: r = −0.209, df = 21, P = 0.339). While the significant correlations between right rACC MTR and the Z score of executive function and between hCaud MTR and the attention and information processing Z score remained significant after adding HbA1c or mFSRP as an additional covariate besides age and MMSE, these two correlations did not survive the FDR multiple comparison correction.
Discussion
The primary finding of the current study is that biophysical integrity of macromolecular protein pools are compromised at node regions of frontal-subcortical circuits, i.e., bilateral anterior cingulate and head of the right caudate nucleus, in T2DM patients compared with nondiabetic control subjects. Reduced MTR in these regions correlated with T2DM-related clinical measures (including HbA1c level and increased vascular risk factors) and neuropsychological task performance in the domains of learning and memory, executive function, and attention and information processing.
Our findings of reduced MTRs in bilateral anterior cingulate and the head of the right caudate nucleus are consistent with recent reports of reduced fractional anisotropy and increased radial diffusivity (suggestive of possible demyelination) in the frontal white matter and the associations between disease duration and increased radial diffusivity in the cingulate white matter gyrus and the right caudate nucleus in T2DM patients (9). Also in line with our findings, significantly lower concentrations of glutamate and glutamine in the caudate nucleus and higher concentrations of myo-inositol in the frontal white matter were observed in patients with T2DM relative to HCs (2). Further, a trend toward significant white matter impairment in the ALIC was observed in T2DM, which was associated with elevated HbA1c in a recent diffusion tensor imaging tractography study (11). The ALIC contains the anterior thalamic radiation, prefrontal corticopontine tracts, and thalamo-striate and striato-striate tracts, providing reciprocal connections between the frontal lobe, striatum, and thalamus (11,45,46). Our study extended the finding of altered white matter integrity in ALIC and demonstrated that the biophysical integrity of macromolecular protein pools in brain regions connected through these white matter tracts is also impaired in T2DM.
hCaud and the anterior cingulate are important nodes of cortico-striatal-pallidal-thalamic circuits that are involved in cognitive functions such as learning and memory, attention and information processing, and executive function (21). Specifically, the caudate nucleus is extensively connected with cortical and subcortical structures in well-characterized circuits (4,21) that subserve the complex regulation of motor functions, cognition, and mood. Among five primary cortical-subcortical circuits (prefrontal, striatal, pallidal, thalamic, and prefrontal circuits), three circuits (i.e., the oculomotor, dorsolateral prefrontal, and lateral orbitofrontal circuits) have direct connections from the prefrontal regions to the caudate nucleus (4,21). In agreement with these circuitry functions, our results demonstrated significant association between cognition and the integrity of macromolecular protein pools in the node regions of frontal-subcortical circuits. The positive correlations between MTRs and neuropsychological task performance in distinct domains are consistent with and further expand the reported association between caudate MTR and global cognition (23). Changes in the caudate and prefrontal regions have been associated with a broad spectrum of behavioral aberrations (4,11,21). Our results demonstrated that the biophysical integrity of the cortico-striatal-pallidal-thalamic circuits was compromised in T2DM patients in the absence of significant cognitive impairments or mood disturbances. T2DM-related changes in these circuits may contribute, in part, to increased susceptibility of cognitive deficits and/or mood disorders in patients with T2DM and potentially inform the underlying substrates linking T2DM and cognitive/mood disorders.
Further, MTRs in bilateral dACC, rACC, and right hCaud were significantly correlated with the HbA1c level, and reduced MTRs in bilateral rACC were associated with increased vascular risk factors. While correlations do not prove causality, these correlations may suggest that both hyperglycemia and vascular diseases may be contributing to the abnormalities observed in our clinical sample. In contrast, there was no significant association between reduced MTRs and duration of T2DM. The HbA1c is known to reflect an average level of blood glucose over the previous 3 months. A possible explanation is that reduced MTRs may, in part, reflect the intermediate or dynamic impact of T2DM on macromolecular protein pools of these regions.
Several limitations of the current study should be considered. The study is limited by its relatively small sample size (20 patients with T2DM and 26 HCs), which may have limited the power to detect subtle changes in some regions of the fronto-striato-thalamic circuits in T2DM patients. Furthermore, the relatively small sample size restricted our ability to examine specific task-level cognitive processes. Thus a composite Z score approach was used to protect against multiple comparisons. In addition, a cross-sectional instead of longitudinal design was used in this study. The cross-sectional design is inherently more vulnerable to intersubject variance and cohort effects. Therefore, future studies are needed to increase the sample size and to investigate the relationship between biophysical integrity of macromolecular protein pools and specific cognitive functions.
In conclusion, our study found compromised macromolecular protein pools in bilateral anterior cingulate and the head of the right caudate nucleus in T2DM patients compared with control subjects. Moreover, the compromised biophysical properties of macromolecular protein pools represented by lower MTR were correlated with T2DM-related clinical measures and neuropsychological task performance in distinct domains. This study is the first to report impaired macromolecular protein pools in frontal-subcortical circuits and its clinical and cognitive correlates in patients with T2DM. These findings contribute to the growing literature in brain alterations in T2DM and have important implications for the underlying neurobiology of diabetes.
Article Information
Acknowledgments. The authors thank Dr. Peter van Zijl, Joseph S. Gillen, Terri Brawner, and Seth A. Smith (Johns Hopkins University) for the MT sequence, which was developed by the support of National Center for Research Resources grant P41 RR015241.
Funding. This work was supported by National Institutes of Health grants R01-MH63764 and R01-MH73989.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.Y. performed the investigations, designed the imaging protocol, acquired and analyzed the data, and wrote the manuscript. O.A. reviewed and edited the manuscript. M.W. analyzed the data and wrote the manuscript. M.L. reviewed and edited the manuscript. A.K. designed the study and wrote the manuscript. S.Y. and A.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Annual Meeting of the International Society for Magnetic Resonance in Medicine, Milan, Italy, 12–16 May 2014.
References
- 1.Harati Y. Diabetes and the nervous system. Endocrinol Metab Clin North Am 1996;25:325–359 [DOI] [PubMed]
- 2.Ajilore O, Haroon E, Kumaran S, et al. Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology 2007;32:1224–1231 [DOI] [PubMed] [Google Scholar]
- 3.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Gupta R, Thomas A, Ajilore O, Hellemann G. Focal subcortical biophysical abnormalities in patients diagnosed with type 2 diabetes and depression. Arch Gen Psychiatry 2009;66:324–330 [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575 [DOI] [PubMed] [Google Scholar]
- 6.Ajilore O, Narr K, Rosenthal J, et al. Regional cortical gray matter thickness differences associated with type 2 diabetes and major depression. Psychiatry Res 2010;184:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group . Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci 2010;299:126–130 [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Haroon E, Darwin C, et al. Gray matter prefrontal changes in type 2 diabetes detected using MRI. J Magn Reson Imaging 2008;27:14–19 [DOI] [PubMed] [Google Scholar]
- 9.Hsu JL, Chen YL, Leu JG, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage 2012;59:1098–1105 [DOI] [PubMed] [Google Scholar]
- 10.Hoogenboom WS, Marder TJ, Flores VL, et al. Cerebral white matter integrity and resting-state functional connectivity in middle-aged patients with type 2 diabetes. Diabetes 2014;63:728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang A, Ajilore O, Zhan L, et al. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology 2013;38:1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Jiao Y, Chen YC, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 2014;63:749–760 [DOI] [PubMed] [Google Scholar]
- 13.Musen G, Jacobson AM, Bolo NR, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 2012;61:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10:135–144 [DOI] [PubMed] [Google Scholar]
- 15.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 1999;46:747–754 [DOI] [PubMed] [Google Scholar]
- 16.Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 2004;56:407–415 [DOI] [PubMed] [Google Scholar]
- 17.Steens SC, Bosma GP, Steup-Beekman GM, le Cessie S, Huizinga TW, van Buchem MA. Association between microscopic brain damage as indicated by magnetization transfer imaging and anticardiolipin antibodies in neuropsychiatric lupus. Arthritis Res Ther 2006;8:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audoin B, Davies G, Rashid W, Fisniku L, Thompson AJ, Miller DH. Voxel-based analysis of grey matter magnetization transfer ratio maps in early relapsing remitting multiple sclerosis. Mult Scler 2007;13:483–489 [DOI] [PubMed] [Google Scholar]
- 19.Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry 2003;60:779–788 [DOI] [PubMed] [Google Scholar]
- 20.Khaleeli Z, Altmann DR, Cercignani M, Ciccarelli O, Miller DH, Thompson AJ. Magnetization transfer ratio in gray matter: a potential surrogate marker for progression in early primary progressive multiple sclerosis. Arch Neurol 2008;65:1454–1459 [DOI] [PubMed] [Google Scholar]
- 21.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381 [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Yang S, Ajilore O, Wu M, Charlton R, Lamar M. Subcortical biophysical abnormalities in patients with mood disorders. Mol Psychiatry 2014;19:710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elderkin-Thompson V, Hellemann G, Gupta RK, Kumar A. Biophysical correlates of cognition among depressed and nondepressed type 2 diabetic patients. Diabetes Care 2009;32:48–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouwen A, Winkley K, Twisk J, et al. European Depression in Diabetes (EDID) Research Consortium . Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 2010;53:2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–630 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association . Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77–82 [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis I DSM-IV Disorders. New York, Biometrics Research, 1994 [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278–296 [DOI] [PubMed] [Google Scholar]
- 31.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626 [DOI] [PubMed] [Google Scholar]
- 32.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 1994;25:40–43 [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Test of Adult Reading: WTAR. San Antonio, TX, Psychological Corporation, 2001 [Google Scholar]
- 34.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test 2nd Edition: Adult Version Manual; San Antonio, TX, Psychological Corporation, 2000 [Google Scholar]
- 35.Wechsler D. Wechsler Memory Scale (WMS-III). San Antonio, TX, Psychological Corporation, 1997 [Google Scholar]
- 36.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL, Stoelting, 1978 [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale–Third Edition (WAIS-III): Administration and Scoring Manual. San Antonio, TX, Psychological Corporation, 1997 [Google Scholar]
- 38.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX, Psychological Corporation, 2001 [Google Scholar]
- 39.Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC, War Department, Adjutant General’s Office, 1944 [Google Scholar]
- 40.Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci U S A 2002;99:5649–5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith SA, Farrell JA, Jones CK, Reich DS, Calabresi PA, van Zijl PC. Pulsed magnetization transfer imaging with body coil transmission at 3 Tesla: feasibility and application. Magn Reson Med 2006;56:866–875 [DOI] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 43.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;25:60–83 [Google Scholar]
- 44.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006;93:491–507 [Google Scholar]
- 45.Lu LH, Zhou XJ, Fitzgerald J, et al. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Disord 2012;14:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beasley CL, Dwork AJ, Rosoklija G, et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res 2009;109:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]