Abstract
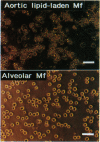
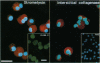
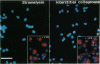
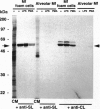
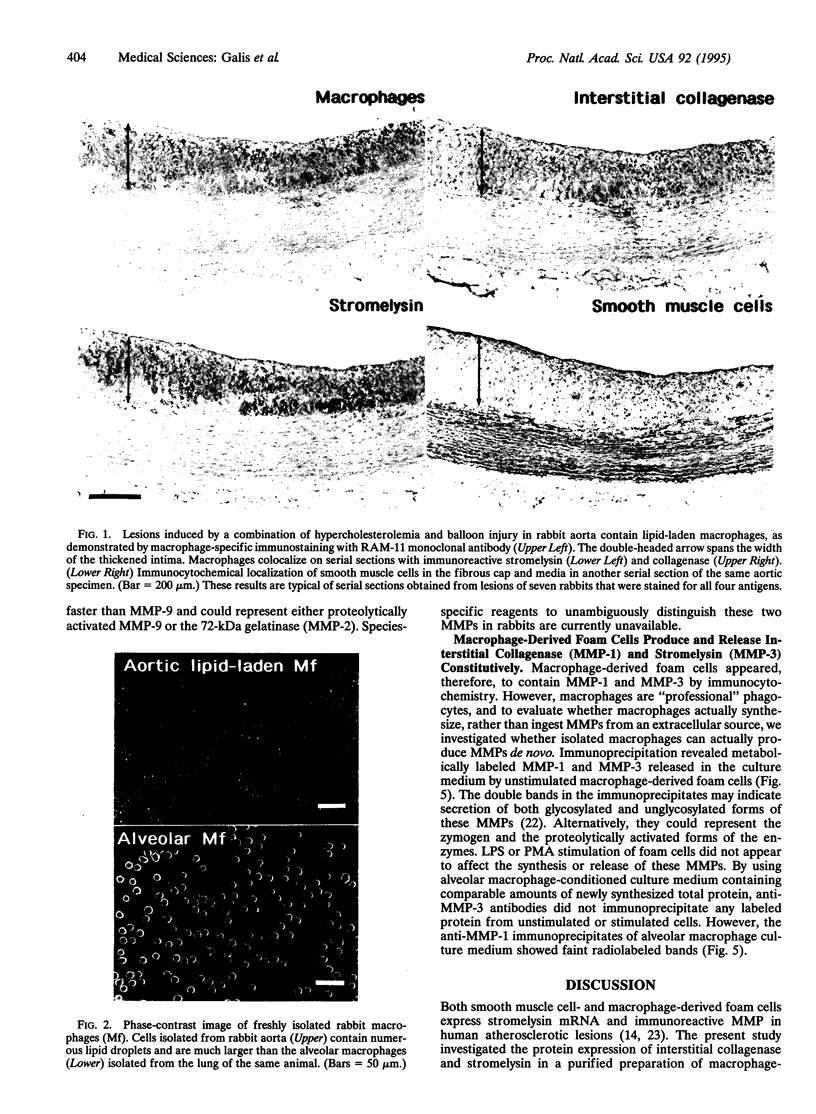
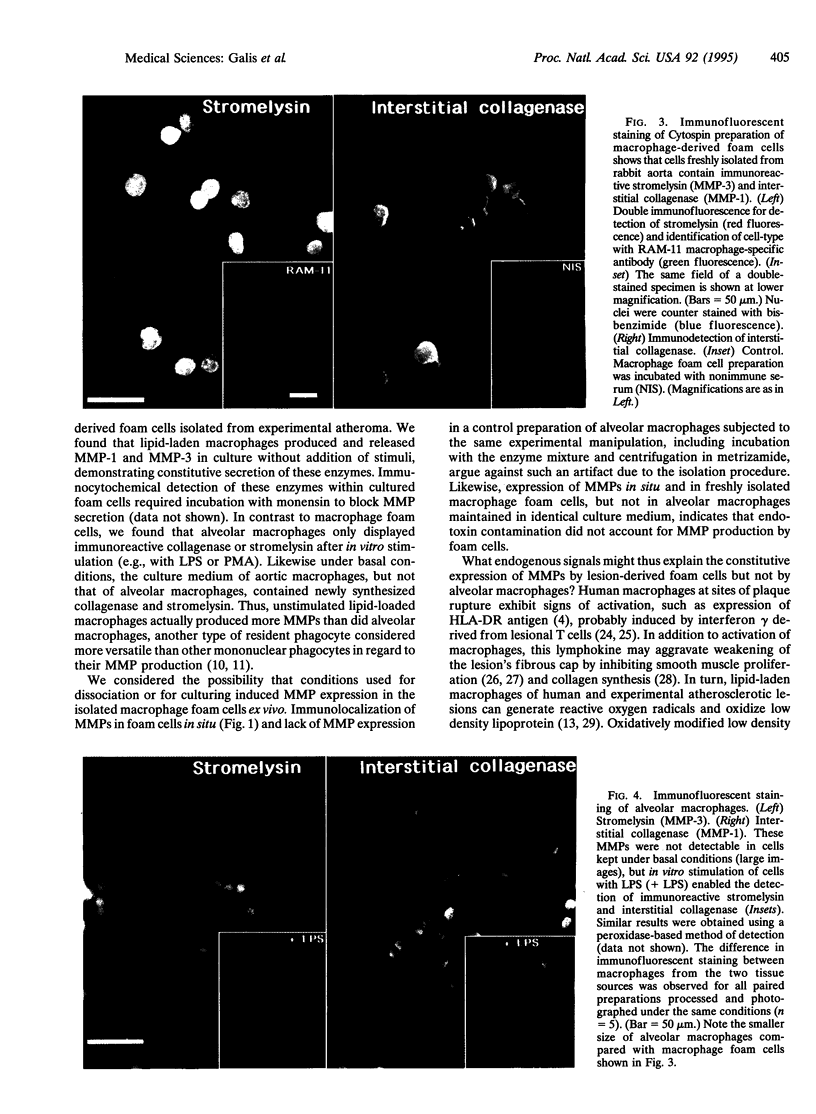
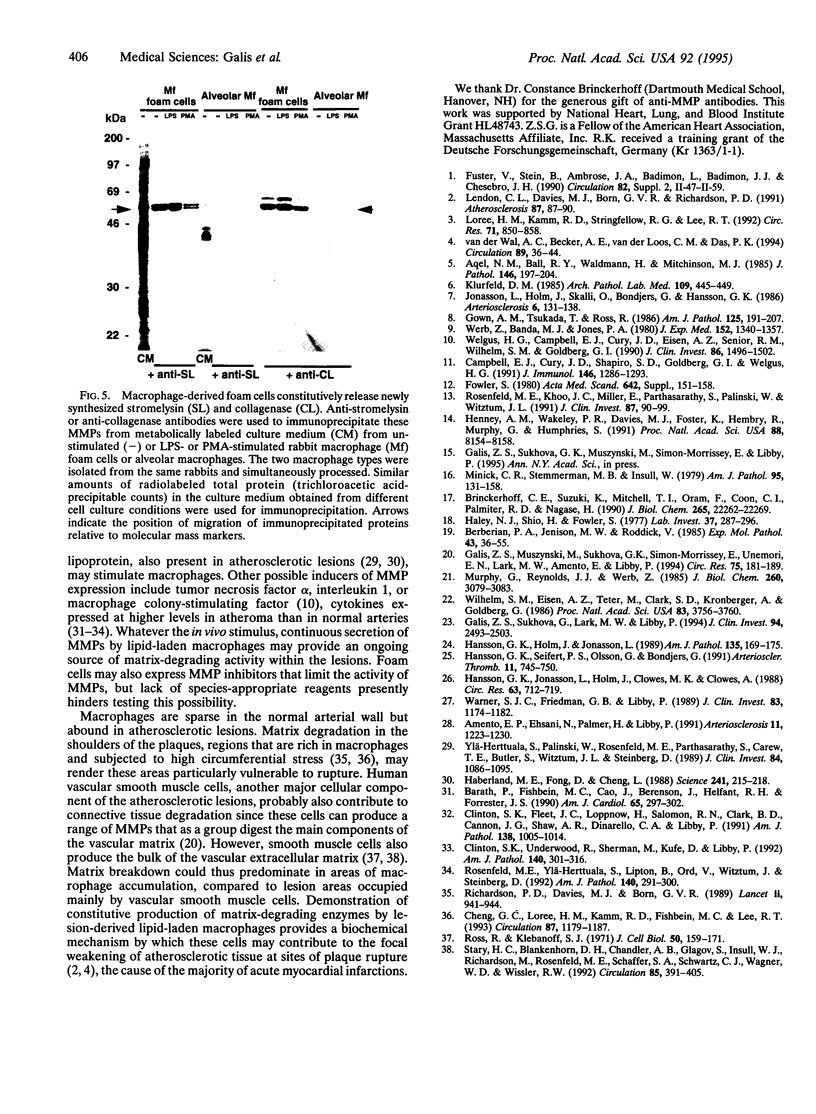
Monocyte-derived foam cells figure prominently in rupture-prone regions of atherosclerotic plaques. Peripheral blood monocytes in culture can produce certain enzymes that degrade extracellular matrix, known as matrix metalloproteinases (MMPs). Lipid-laden macrophages may thus contribute to weakening of extracellular matrix of rupture-prone atherosclerotic plaques. However, the spectrum and regulation of MMP production by foam cells remain unknown. To investigate this issue, we isolated lipid-laden macrophages from rabbit aortic lesions produced by a combination of hypercholesterolemia and balloon injury. Freshly isolated aortic macrophage foam cells, identified using cell-specific antibodies, contained immunoreactive stromelysin and interstitial collagenase, whereas alveolar macrophages isolated from the lungs of same rabbits did not. Macrophages from both tissue sources released gelatinolytic activity consistent with the 92-kDa gelatinase. In vitro, lipid-laden aortic macrophages, but not alveolar macrophages, synthesized de novo and released immunoprecipitable stromelysin and collagenase, with or without stimulation by phorbol ester or bacterial lipopolysaccharide. These stimuli caused foam cells to release additional gelatinolytic activity that migrated faster than a purified preparation of 92-kDa gelatinase in substrate-containing polyacrylamide gels, indicating activation of the 92-kDa gelatinase or induction of the 72-kDa gelatinase. Our results show that lipid-laden macrophages elaborate MMPs capable of degrading the major constituents of vascular extracellular matrix even without further stimulation. Therefore, these cells may contribute to remodeling of the extracellular matrix during atherogenesis and to the disruption of plaques often responsible for acute clinical manifestations of atherosclerosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Ehsani N., Palmer H., Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991 Sep-Oct;11(5):1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990 Feb 1;65(5):297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- Berberian P. A., Jenison M. W., Roddick V. Arterial prostaglandins and lysosomal function during atherogenesis. II. Isolated cells of diet-induced atherosclerotic aortas of rabbit. Exp Mol Pathol. 1985 Aug;43(1):36–55. doi: 10.1016/0014-4800(85)90053-x. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Suzuki K., Mitchell T. I., Oram F., Coon C. I., Palmiter R. D., Nagase H. Rabbit procollagenase synthesized and secreted by a high-yield mammalian expression vector requires stromelysin (matrix metalloproteinase-3) for maximal activation. J Biol Chem. 1990 Dec 25;265(36):22262–22269. [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Shapiro S. D., Goldberg G. I., Welgus H. G. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991 Feb 15;146(4):1286–1293. [PubMed] [Google Scholar]
- Cheng G. C., Loree H. M., Kamm R. D., Fishbein M. C., Lee R. T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993 Apr;87(4):1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Fleet J. C., Loppnow H., Salomon R. N., Clark B. D., Cannon J. G., Shaw A. R., Dinarello C. A., Libby P. Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol. 1991 Apr;138(4):1005–1014. [PMC free article] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Fowler S. Characterization of foam cells in experimental atherosclerosis. Acta Med Scand Suppl. 1980;642:151–158. doi: 10.1111/j.0954-6820.1980.tb10947.x. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Muszynski M., Sukhova G. K., Simon-Morrissey E., Unemori E. N., Lark M. W., Amento E., Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994 Jul;75(1):181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Haley N. J., Shio H., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. I. Resolution of aortic cell populations by metrizamide density gradient centrifugation. Lab Invest. 1977 Sep;37(3):287–296. [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Henney A. M., Wakeley P. R., Davies M. J., Foster K., Hembry R., Murphy G., Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Klurfeld D. M. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985 May;109(5):445–449. [PubMed] [Google Scholar]
- Lendon C. L., Davies M. J., Born G. V., Richardson P. D. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991 Mar;87(1):87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- Loree H. M., Kamm R. D., Stringfellow R. G., Lee R. T. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ Res. 1992 Oct;71(4):850–858. doi: 10.1161/01.res.71.4.850. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. B., Insull W., Jr Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol. 1979 Apr;95(1):131–158. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ylä-Herttuala S., Lipton B. A., Ord V. A., Witztum J. L., Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992 Feb;140(2):291–300. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary H. C., Blankenhorn D. H., Chandler A. B., Glagov S., Insull W., Jr, Richardson M., Rosenfeld M. E., Schaffer S. A., Schwartz C. J., Wagner W. D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992 Jan;85(1):391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Friedman G. B., Libby P. Immune interferon inhibits proliferation and induces 2'-5'-oligoadenylate synthetase gene expression in human vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1174–1182. doi: 10.1172/JCI113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Banda M. J., Jones P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980 Nov 1;152(5):1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Eisen A. Z., Teter M., Clark S. D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal A. C., Becker A. E., van der Loos C. M., Das P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994 Jan;89(1):36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]