Abstract
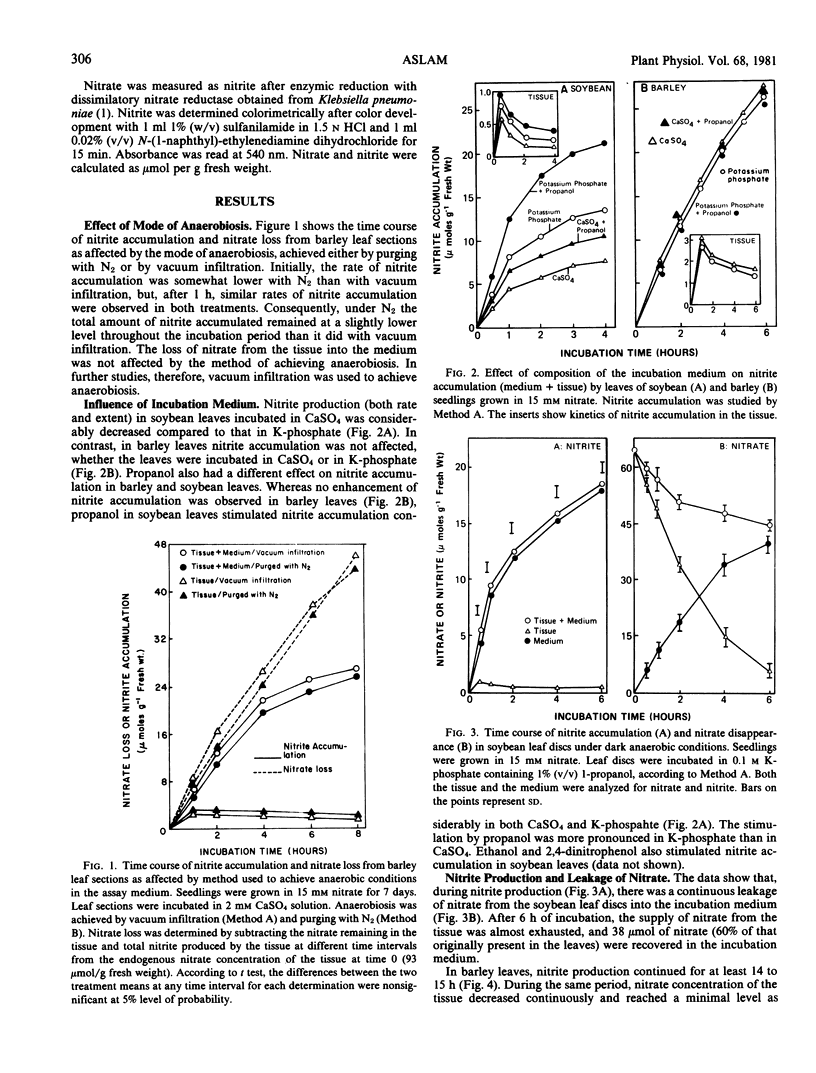
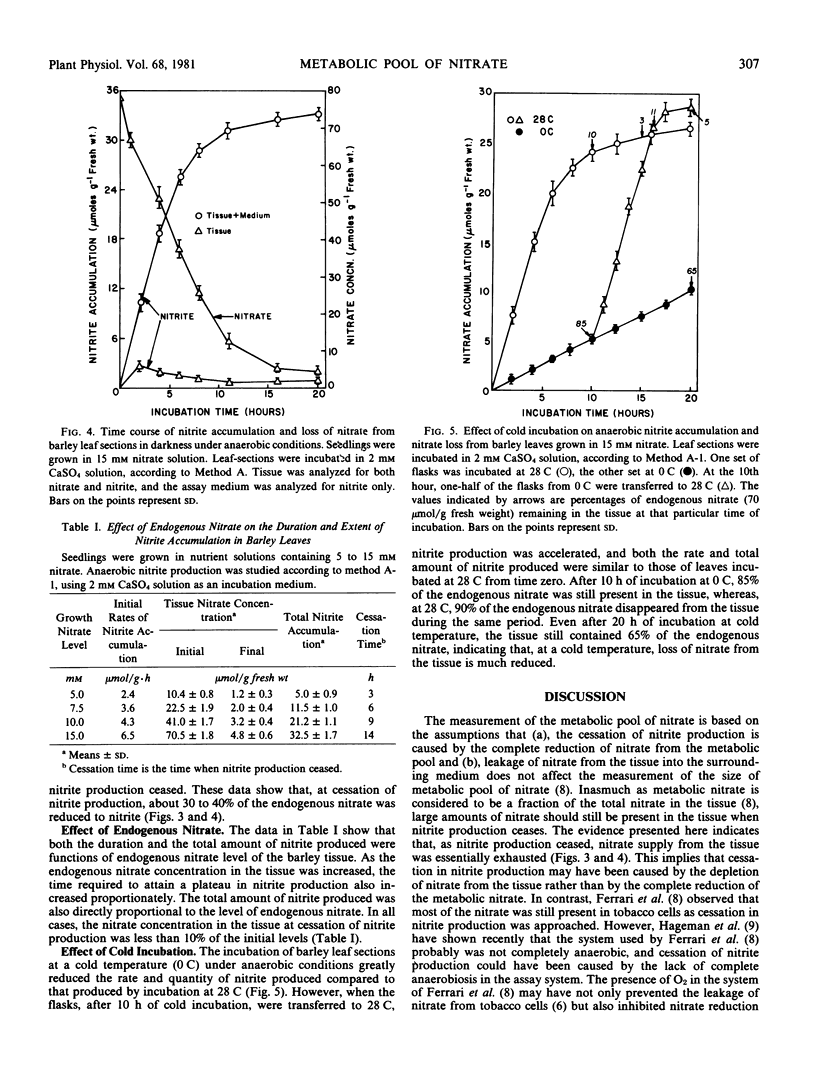
The use of anaerobic nitrite production as an index for the measurement of metabolic pool of nitrate was reevaluated using primary leaves of 7-day-old barley and 10-day-old soybean seedlings. The seedlings were grown in nutrient solutions containing 5 to 15 millimolar nitrate. The nitrate-free in vivo assay system of nitrate reductase was used for measuring the production of nitrite. Both the duration and extent of nitrite production were dependent on the level of endogenous nitrate in the tissue. At cessation of nitrite production, 30 to 50% of the endogenous nitrate was reduced to nitrite. Nitrate from the tissue leaked continuously into the surrounding medium so that, at cessation of nitrite production, nitrate supply from the tissue was exhausted. The cessation of nitrite production, therefore, may have been caused by the depletion of endogenous nitrate from the tissue. It is concluded that anaerobic nitrite production is not a valid index for the measurement of the size of the metabolic pool of nitrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A. Effect of glucose on the induction of nitrate reductase in corn roots. Plant Physiol. 1975 Nov;56(5):634–639. doi: 10.1104/pp.56.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A., Huffaker R. C. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiol. 1976 Oct;58(4):588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen M. N., Carns H. R., Slyter D. J. Stimulation of Solute Loss from Radicles of Gossypium hirsutum L. by Chilling, Anaerobiosis, and Low pH. Plant Physiol. 1970 Jul;46(1):53–56. doi: 10.1104/pp.46.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Reed A. J., Femmer R. A., Sherrard J. H., Dalling M. J. Some New Aspects of the in Vivo Assay for Nitrate Reductase in Wheat (Triticum aestivum L.) Leaves: I. REEVALUATION OF NITRATE POOL SIZES. Plant Physiol. 1980 Jan;65(1):27–32. doi: 10.1104/pp.65.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel K., Pflüger R. The Release of Potassium and Sodium from Young Excised Roots of Zea mays under Various Efflux Conditions. Plant Physiol. 1972 Jan;49(1):16–19. doi: 10.1104/pp.49.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): I. Effects of Light and Temperature. Plant Physiol. 1976 Dec;58(6):731–735. doi: 10.1104/pp.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]



