Abstract
Six skin collagen advanced glycation end products (AGEs) originally measured near to the time of the Diabetes Control and Complications Trial (DCCT) closeout in 1993 may contribute to the “metabolic memory” phenomenon reported in the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study. We have now investigated whether the addition of four originally unavailable AGEs (i.e., glucosepane [GSPNE], hydroimidazolones of methylglyoxal [MG-H1] and glyoxal, and carboxyethyl-lysine) improves associations with incident retinopathy, nephropathy, and neuropathy events during 13–17 years after DCCT. The complete 10-AGE panel is associated with three-step Early Treatment of Diabetic Retinopathy Study scale worsening of retinopathy (P ≤ 0.002), independent of either mean DCCT or EDIC study A1C level. GSPNE and fructose-lysine (furosine [FUR]) correlate with retinopathy progression, independently of A1C level. The complete panel also correlates with microalbuminuria (P = 0.008) and FUR with nephropathy independently of A1C level (P ≤ 0.02). Neuropathy correlates with the complete panel despite adjustment for A1C level (P ≤ 0.005). MG-H1 and FUR are dominant, independent of A1C level (P < 0.0001), whereas A1C loses significance after adjustment for the AGEs. Overall, the added set of four AGEs enhances the association of the original panel with progression risk of retinopathy and neuropathy (P < 0.04) but not nephropathy, while GSPNE and MG-H1 emerge as the principal new risk factors. Skin AGEs are robust long-term markers of microvascular disease progression, emphasizing the importance of early and sustained implementation of intensive therapy.
Introduction
A large body of epidemiological evidence and animal experimentation underlay the hypothesis that hyperglycemia is a causative factor in the development of microvascular and neuropathic complications in diabetes (1). The demonstration by the Diabetes Control and Complications Trial (DCCT) that intensive treatment, which lowered the mean blood glucose level by ∼75 mg/dL and the mean A1C level by ∼2.0% (21.9 mmol/mol), compared with conventional treatment, sharply and significantly reduced the development and progression of these complications (2), confirmed this hypothesis. Nonetheless, the mechanistic connection of hyperglycemia to complications appears complex, containing multiple strands, even if glycemic exposure is primary (3). One path from hyperglycemia to complications is the formation of advanced glycation end products (AGEs), which can affect protein structure and function both directly and by cross-linking (4).
We have previously demonstrated cross-sectional associations between a panel of four AGEs and two solubility abnormalities in collagen from a skin biopsy sample obtained prior to DCCT closeout and the presence of retinopathy, nephropathy, and neuropathy in DCCT subjects (5). These associations included the early glycation product fructose-lysine (FL) (fructosamine) measured in acid hydrolysate as furosine (FUR), pentosidine, and carboxymethyl-lysine (CML). Furthermore, we subsequently demonstrated that these AGE abnormalities, especially FUR and CML, were highly correlated with the progression of retinopathy and nephropathy over the ensuing 10 years in the Epidemiology of Diabetes Interventions and Complications (EDIC) observational study of the DCCT cohort of patients (6).
Using a simultaneously obtained skin biopsy sample near DCCT closeout, which was stored at −80°C awaiting the discovery of AGEs not available in 1993, we have now tested the hypothesis that the previously not available AGEs glucosepane (GSPNE) (7), carboxyethyl-lysine (CEL) (8), and hydroimidazolones of glyoxal (G-H1) and methylglyoxal (MG-H1) (9) determined by liquid chromatography tandem mass spectrometry, as described by Ahmed and Thornalley (10), are correlated with complication risk progression compared with the original set. In that regard, we recently reported the association between these markers and the concurrent progression of diabetes complications during the DCCT (7), which revealed that GSPNE was a particularly robust indicator of past cumulative glycemia that was strongly associated with the presence of nephropathic, retinopathic, and neuropathic outcomes at the time of the biopsy (7). The current study was consequently undertaken to determine whether GSPNE or any of the added AGEs were also associated with the long-term prospective risk of retinopathy, nephropathy, and neuropathy progression, individually or in combination with the originally measured AGEs and solubility markers during the 17 years of follow-up of this cohort during the EDIC study.
Research Design and Methods
Subjects
Two hundred sixteen participants in the DCCT study from eight clinics volunteered for a skin biopsy, as previously described (5), 1–2 years prior to DCCT closeout. One hundred twenty-three participants were from the primary cohort (1–5 years diabetes duration and no retinopathy seen on fundus photography), and 93 were from the secondary cohort (1–15 years diabetes duration and at least one retinal microaneurysm seen on fundus photography). Retinopathy, nephropathy, and neuropathy data at 10–13 years of the EDIC follow-up were available in 196 of those participants originally biopsied. During the early EDIC study period, mean A1C levels of the DCCT intensive and conventional treatment groups began to converge and became indistinguishable at ∼7.9% by 5 years into the EDIC study (11,12).
Outcomes
Retinopathy
During the EDIC study, retinopathy was assessed by standardized seven-field fundus photography in one-quarter of the cohort each year and in the entire cohort at EDIC study years 4 and 10. All photographs were graded centrally, with graders masked to the therapy assignment, using the final Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale (13) and DCCT methods (14). The progression of retinopathy during the EDIC study was defined as a three-step or greater worsening at any time from the DCCT closeout up to EDIC study year 16 on the final ETDRS scale of retinopathy severity (13) or initial scatter photocoagulation therapy during this interval, among those free of scatter laser in DCCT.
Nephropathy
Albumin excretion rate (AER) (15) was measured every 2 years in 1,399 participants during the EDIC study. Progression of nephropathy was defined as the initial development of microalbuminuria (AER ≥40 mg/24 h, n = 50), dialysis (n = 3), or kidney transplantation (n = 3) at any time between the DCCT closeout and the EDIC study year 15 or 16, among those participants free of microalbuminuria in the DCCT. Since microalbuminuria is known to regress (16), sustained microalbuminuria (AER ≥30 mg/24 h) at two or more consecutive visits was used in a sensitivity analysis (Supplementary Tables 1–3) to verify the results of a single AER ≥40 mg/24 h.
Neuropathy
Clinical and nerve conduction studies performed during the DCCT were repeated in the EDIC study during years 13–14 (17). Likewise, cardiovascular autonomic neuropathy assessment performed in the DCCT was reassessed in the EDIC study, first during EDIC study years 13–14 (18) and repeated in years 16–17. Progression of neuropathy was defined as the initial development of confirmed clinical neuropathy or cardiac autonomic neuropathy in the EDIC study, among those free of both complications in the DCCT. Confirmed clinical neuropathy was defined as the presence of both definite clinical neuropathy (the presence of signs and symptoms consistent with distal symmetrical polyneuropathy based on examination by a board-certified neurologist) and abnormal nerve conduction (one or more abnormal attributes in at least two anatomically distinct nerves among the sural, peroneal, or median nerves) (17). Cardiac autonomic neuropathy was defined as either an R-R interval variation of <15 or an R-R interval variation between 15 and 19.9 in combination with a Valsalva ratio of ≤1.5 or a decrease of >10 mmHg in diastolic blood pressure upon standing (17).
Biomedical Evaluations
Demographics were assessed by annual questionnaires. Blood pressure and A1C level were measured annually in the EDIC study. Plasma lipid concentration (cholesterol and triglycerides) was measured biannually during the EDIC study.
AGEs in Skin Biopsies Samples
The AGE measurements used for this prospective longitudinal study have been previously described in detail (7). Briefly recapitulated, the skin biopsy sample used in these studies was stored since 1993 at −80°C under argon. Insoluble collagen was prepared and enzymatically digested to release the free glycation products that were quantitated by liquid chromatography mass spectrometry using isotope dilution assays for GSPNE, CEL, G-H1, and MG-H1. This set of AGEs is referred to as the “added set” (n = 4) in contrast to the “original set” of six AGEs/markers that included collagen fluorescence, acid solubility, pepsin digestibility, CML, pentosidine, and FUR (5,6).
Although native FL was automatically included in the new analytical system, we report here only the values of the originally measured acid hydrolytic conversion product FUR, but also provide some details on how FUR versus FL behaves as a prospective risk marker in the study below.
Statistical Methods
Univariate differences between groups were assessed using Wilcoxon rank sum test for quantitative or ordinal variables and the χ2 test for categorical variables. Skin collagen variables were adjusted for age and diabetes duration using residuals from a simple linear regression model. Spearman correlation was used to evaluate a monotone relationship among skin collagen variables and between A1C and skin collagens. Multivariable logistic regression models were used to assess the effect of skin collagen AGE variables on the odds of each complication in the EDIC study. The significance of effects of specific covariate blocks, unadjusted as well as adjusted for other covariates, were evaluated using the likelihood ratio test (LRT) (19). Backward elimination was used starting with all 10 AGEs (the original and new sets combined) to select the subset of skin collagen variables nominally significant for the risk of complications at the level of 0.05. Entropy R2 was used to assess the strength of association in logistic regression models (19). The odds ratio per SD increase in the skin collagen AGE variables and A1C level was reported. For univariate AGE effects, the Benjamini and Hochberg false discovery rate (FDR) alpha adjustment for multiple tests was conducted to control the overall FDR at a level of 0.05 (20). Unless otherwise noted, nominal P values were presented. SAS was used to perform these analyses.
Results
Clinical Characteristics of Participants
As previously reported (7), Table 1 presents the characteristics of the participants in the skin biopsy study. At DCCT baseline, there was a slight but significant difference in age between the intensive and conventional treatment groups in the primary cohort, and there was a 0.6% (6.6 mmol/mol) A1C difference and a 5 mmHg difference in systolic blood pressure in the secondary cohort. At DCCT closeout, mean A1C level, mean blood glucose, extent of retinopathy, AER, and neuropathy were all lower in the intensive group than in the conventional group in the primary cohort, but only neuropathy was less in the secondary cohort. That the beneficial effect of intensive treatment was less evidenced in the secondary cohort may reflect a somewhat smaller sample size, a slightly higher baseline A1C level in the intensive group, and less of a difference in A1C level (1.7%; 18.6 mmol/mol) between the intensive and conventional groups in the secondary cohort compared with a difference of 2.4% (26.2 mmol/mol) in the primary cohort at DCCT closeout.
Table 1.
Clinical characteristics of DCCT participants at DCCT baseline and closeout
| Primary cohort |
Secondary cohort |
|||||
|---|---|---|---|---|---|---|
| Intensive (N = 65) | Conventional (N = 58) | P value | Intensive (N = 57) | Conventional (N = 36) | P value | |
| DCCT baseline | ||||||
| Age (years) | 28 ± 6 | 26 ± 7 | 0.016 | 30 ± 7 | 30 ± 6 | |
| Duration (months) | 31 ± 19 | 27 ± 14 | 108 ± 51 | 90 ± 43 | ||
| A1C | ||||||
| % | 8.8 ± 1.9 | 9.1 ± 1.9 | 8.9 ± 1.5 | 8.3 ± 1.2 | 0.022 | |
| mmol/mol | 73 ± 20.8 | 76 ± 20.8 | 74 ± 16.2 | 67 ± 13.1 | ||
| Mean blood glucose (mg/dL) | 221 ± 83 | 245 ± 85 | 237 ± 76 | 214 ± 72 | ||
| Triglycerides (mg/dL) | 79 ± 66 | 65 ± 23 | 97 ± 48 | 86 ± 56 | ||
| Cholesterol (mg/dL) | 182 ± 33 | 174 ± 32 | 181 ± 36 | 176 ± 37 | ||
| Retinopathy (%) | ||||||
| 10/10: none | 100 | 100 | 0 | 0 | ||
| 20/≤20: microaneurysms only | 0 | 0 | 58 | 61 | ||
| 30/≤30: mild NPDR | 0 | 0 | 23 | 25 | ||
| 45/≤45: moderate NPDR | 0 | 0 | 19 | 14 | ||
| AER >40 mg/24 h (%) | 1.5 | 0.0 | 10.5 | 8.3 | ||
| Confirmed clinical neuropathy (%) | 6.2 | 1.7 | 17.9 | 11.1 | ||
| SBP (mmHg) | 116 ± 10 | 114 ± 11 | 115 ± 12 | 120 ± 12 | 0.042 | |
| DBP (mmHg) | 73 ± 9 | 72 ± 9 | 73 ± 8 | 76 ± 10 | ||
| DCCT closeout | ||||||
| Age (years) | 34 ± 6 | 31 ± 7 | 0.013 | 36 ± 7 | 36 ± 6 | |
| Duration (months) | 101 ± 31 | 94 ± 23 | 189 ± 55 | 169 ± 46 | ||
| DCCT mean A1C | ||||||
| % | 7.1 ± 0.8 | 9.5 ± 1.4 | <0.001 | 7.1 ± 0.8 | 8.8 ± 1.5 | <0.001 |
| mmol/mol | 54 ± 8.7 | 80 ± 15.3 | 54 ± 8.7 | 73 ± 16.4 | ||
| Mean blood glucose (mg/dL) | 149 ± 48 | 239 ± 76 | <0.001 | 152 ± 46 | 215 ± 83 | <0.001 |
| Triglycerides (mg/dL) | 72 ± 35 | 76 ± 34 | 91 ± 57 | 78 ± 36 | ||
| Cholesterol (mg/dL) | 175 ± 31 | 178 ± 33 | 181 ± 33 | 178 ± 33 | ||
| Retinopathy (%) | 0.005 | |||||
| 10/10: none | 58 | 36 | 4 | 3 | ||
| 20/≤20: microaneurysms only | 37 | 41 | 30 | 36 | ||
| 30/≤30: mild NPDR | 2 | 19 | 44 | 36 | ||
| 45/≤45: moderate NPDR | 3 | 3 | 23 | 25 | ||
| AER >40 mg/24 h | 3.1 | 10.3 | 14.0 | 11.1 | ||
| Confirmed clinical neuropathy (%) | 2.3 | 4.8 | 12.7 | 17.1 | ||
| SBP (mmHg) | 115 ± 9 | 115 ± 11 | 118 ± 9 | 121 ± 14 | ||
| DBP (mmHg) | 75 ± 9 | 74 ± 8 | 77 ± 8 | 77 ± 11 | ||
Data are mean ± SD, unless otherwise indicated. DBP, diastolic blood pressure; NPDR, nonproliferative diabetic retinopathy; SBP, systolic blood pressure.
Univariate Analysis
Table 2 presents univariate risk factors for the progression of various complications from DCCT closeout to years 13–16 of the EDIC study. Mean DCCT and EDIC study A1C levels were highly statistically significantly associated with greater progression of retinopathy (P < 0.0001), nephropathy (<0.0001), and neuropathy (P = 0.04–0.004). Of note, the EDIC study baseline AER was significantly greater in those participants who later showed progression of all three complications during the EDIC study (each P < 0.008), whereas the degree of retinopathy at EDIC study baseline was associated only with the progression of nephropathy and neuropathy (each P ≤ 0.01).
Table 2.
Univariate analysis of risk factors versus further worsening retinopathy, new onset of nephropathy, or new onset of neuropathy during EDIC study#
| Characteristics | Three-step or more ETDRS scale progression from DCCT closeout through EDIC study years 13–16 |
Development of microalbuminuria or worse (AER ≥ 40 mg/24 h) from DCCT closeout through EDIC study years 15–16 |
Development of confirmed clinical neuropathy or abnormal autonomic function from DCCT closeout to EDIC study years 13–14 or 16–17* |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Event (N = 91) | Nonevent (N = 122) | P value | Event (N = 50) | Nonevent (N = 145) | P value | Event (N = 74) | Nonevent (N = 91) | P value | |
| Demographic | |||||||||
| Age at EDIC study baseline (years) | 33.6 ± 7.0 | 34.3 ± 6.6 | 0.52 | 32.6 ± 6.9 | 34.5 ± 6.6 | 0.09 | 34.7 ± 6.3 | 32.5 ± 6.5 | 0.04 |
| Women (%) | 52.8 | 43.3 | 0.18 | 58.0 | 44.8 | 0.11 | 44.6 | 48.4 | 0.63 |
| Diabetes duration at EDIC study baseline (years) | 10.5 ± 4.4 | 11.0 ± 5.0 | 0.64 | 10.1 ± 3.9 | 10.7 ± 4.8 | 0.70 | 10.8 ± 4.6 | 10.4 ± 4.7 | 0.52 |
| DCCT treatment group intensives (%) | 44.0 | 65.5 | 0.002 | 48.0 | 60.7 | 0.12 | 56.8 | 56.0 | 0.93 |
| Glycemia control | |||||||||
| DCCT mean A1C | |||||||||
| % | 8.7 ± 1.7 | 7.5 ± 1.3 | <0.0001 | 8.8 ± 1.6 | 7.7 ± 1.4 | <0.0001 | 8.3 ± 1.7 | 7.7 ± 1.3 | 0.04 |
| mmol/mol | 72 ± 18.6 | 58 ± 14.2 | 73 ± 17.5 | 61 ± 15.3 | 67 ± 18.6 | 61 ± 14.2 | |||
| EDIC study mean A1C up to EDIC study year 16 | |||||||||
| % | 8.6 ± 1.2 | 7.6 ± 0.9 | <0.0001 | 8.9 ± 1.2 | 7.7 ± 1.0 | <0.0001 | 8.3 ± 1.3 | 7.7 ± 0.9 | 0.004 |
| mmol/mol | 70 ± 13.1 | 60 ± 9.8 | 74 ± 13.1 | 61 ± 10.9 | 67 ± 14.2 | 61 ± 9.8 | |||
| Medical at EDIC study baseline | |||||||||
| Mean blood pressure (mmHg) | 90.2 ± 10 | 88.5 ± 7.9 | 0.23 | 90.9 ± 8.7 | 88.4 ± 8.2 | 0.10 | 89.9 ± 7.4 | 87.9 ± 8.7 | 0.19 |
| Hypertension (%)† | 8.8 | 1.6 | 0.017 | 8.0 | 3.5 | 0.19 | 2.7 | 5.5 | 0.38 |
| Triglycerides (mg/dL) | 81.3 ± 40 | 77.6 ± 44 | 0.39 | 82.9 ± 42 | 74.5 ± 40 | 0.21 | 86.3 ± 57 | 75.8 ± 34 | 0.63 |
| HDL cholesterol (mg/dL) | 52.9 ± 13 | 50.3 ± 13 | 0.15 | 52.1 ± 12 | 51.5 ± 13 | 0.74 | 50.8 ± 15 | 50.6 ± 11 | 0.67 |
| LDL cholesterol (mg/dL) | 113 ± 28 | 108 ± 28 | 0.16 | 114 ± 27 | 108 ± 28 | 0.08 | 119 ± 31 | 106 ± 27 | 0.004 |
| Overweight (%)‡ | 55.0 | 54.1 | 0.90 | 62.0 | 53.1 | 0.28 | 60.8 | 49.5 | 0.15 |
| Smoker (%) | 28.6 | 18.9 | 0.10 | 26.0 | 18.6 | 0.27 | 29.7 | 18.7 | 0.10 |
| Retinopathy at EDIC study baseline (%) | |||||||||
| No retinopathy (10/10) | 30.8 | 27.9 | 20.0 | 34.5 | 21.6 | 35.2 | |||
| Microaneurysm only (20/<20) | 30.8 | 41.0 | 0.22 | 34.0 | 39.3 | 0.008 | 40.5 | 42.9 | 0.01 |
| Mild NPDR (35/<35) | 20.0 | 24.6 | 30.0 | 17.9 | 24.3 | 15.4 | |||
| Moderate NPDR or worse (43/≤43+) | 18.9 | 6.6 | 16.0 | 8.3 | 13.5 | 6.6 | |||
| Renal at EDIC study baseline | |||||||||
| AER (mg/24 h) | 10.1 | 7.2 | 0.0002 | 10.1 | 7.2 | 0.0009 | 101.1 | 7.2 | 0.007 |
| Median (interquartiles) | (6, 20) | (4, 12) | (7, 17) | (4, 10) | (6, 20) | (6, 12) | |||
| AER ≥40 (%) | 14.3 | 4.1 | 0.008 | 0 ∼ | 0 ∼ | n/a | 16.2 | 2.2 | 0.001 |
| Neuropathy at EDIC study baseline (%) | |||||||||
| Confirmed clinical neuropathy | 12.2 | 6.6 | 0.15 | 12.0 | 7.6 | 0.35 | 0 | 0 | n/a |
| Abnormal autonomic function | 15.1 | 2.8 | 0.003 | 9.1 | 5.7 | 0.44 | 0 | 0 | n/a |
| Skin collagens at EDIC study baseline | |||||||||
| FUR (pmol/mg) | 878 ± 251 | 686 ± 179 | <0.0001 | 875 ± 236 | 706 ± 194 | <0.0001 | 821 ± 233 | 691 ± 212 | 0.0001 |
| CML (pmol/mg) | 580 ± 138 | 503 ± 117 | <0.0001 | 569 ± 125 | 513 ± 130 | 0.009 | 571 ± 119 | 502 ± 130 | <0.0001 |
| Pentosidine (pmol/mg) | 27.7 ± 7.6 | 24.6 ± 6.9 | 0.002 | 27.5 ± 8.0 | 24.8 ± 6.8 | 0.02 | 27.1 ± 6.9 | 24.0 ± 6.7 | 0.005 |
| Fluorescence (pmol/mg) | 193 ± 43 | 183 ± 52 | 0.01 | 194 ± 42 | 181 ± 51 | 0.02 | 192 ± 46 | 175 ± 40 | 0.007 |
| Acid-soluble collagen (%) | 0.54 ± 0.4 | 0.56 ± 0.3 | 0.03 | 0.54 ± 0.4 | 0.57 ± 0.3 | 0.11 | 0.50 ± 0.2 | 0.57 ± 0.3 | 0.10 |
| Pepsin-soluble collagen (%) | 6.0 ± 3.5 | 7.2 ± 3.0 | 0.0001 | 6.1 ± 2.7 | 7.2 ± 3.4 | 0.02 | 6.0 ± 2.4 | 7.3 ± 3.5 | 0.02 |
| GSPNE (nmoles/mg) | 2.8 ± 0.7 | 2.3 ± 0.5 | <0.0001 | 2.7 ± 0.5 | 2.4 ± 0.6 | 0.0001 | 2.7 ± 0.7 | 2.3 ± 0.6 | <0.0001 |
| CEL (pmol/mg) | 164 ± 120 | 134 ± 96 | 0.06 | 147 ± 83 | 144 ± 116 | 0.14 | 161 ± 115 | 135 ± 94 | 0.09 |
| G-H1 (pmol/mg) | 66 ± 42 | 65 ± 27 | 0.40 | 74 ± 47 | 64 ± 29 | 0.42 | 62 ± 36 | 62 ± 26 | 0.55 |
| MG-H1 (nmol/mg) | 0.91 ± 0.5 | 0.73 ± 0.4 | 0.004 | 0.90 ± 0.6 | 0.76 ± 0.4 | 0.10 | 0.92 ± 0.6 | 0.68 ± 0.3 | 0.006 |
Data are mean ± SD, unless otherwise indicated.
#Analyses only included subjects who were at risk for an outcome during the EDIC study, with those subjects with a pre-existing outcome during the DCCT being excluded. The retinopathy analysis excluded those who underwent scatter photocoagulation during the DCCT (n = 2). The nephropathy analyses excluded those who had AER >40 mg/24 h at DCCT closeout (n = 20). The neuropathy analysis excluded those who had confirmed clinical neuropathy or abnormal autonomic neuropathy (n = 30) at DCCT closeout. NPDR, nonproliferative diabetic retinopathy.
*Confirmed clinical neuropathy at EDIC study years 13–14 or abnormal autonomic function at EDIC study years 13–14 or 16–17.
†Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
‡Overweight was defined as BMI ≥25 kg/m2.
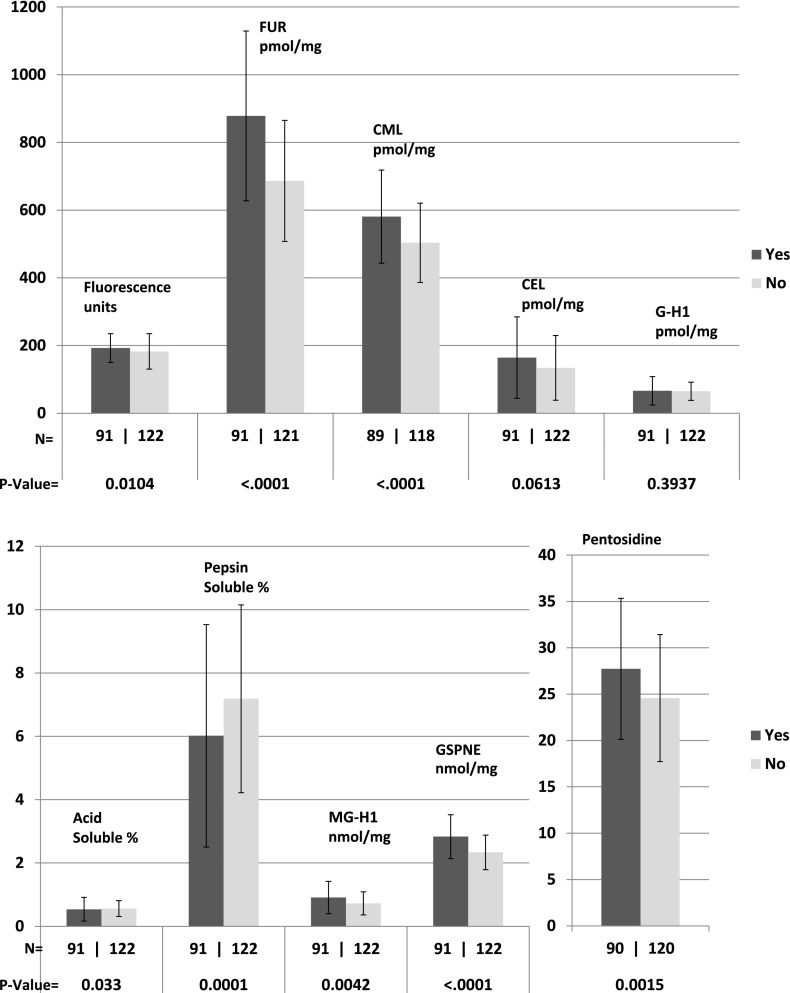
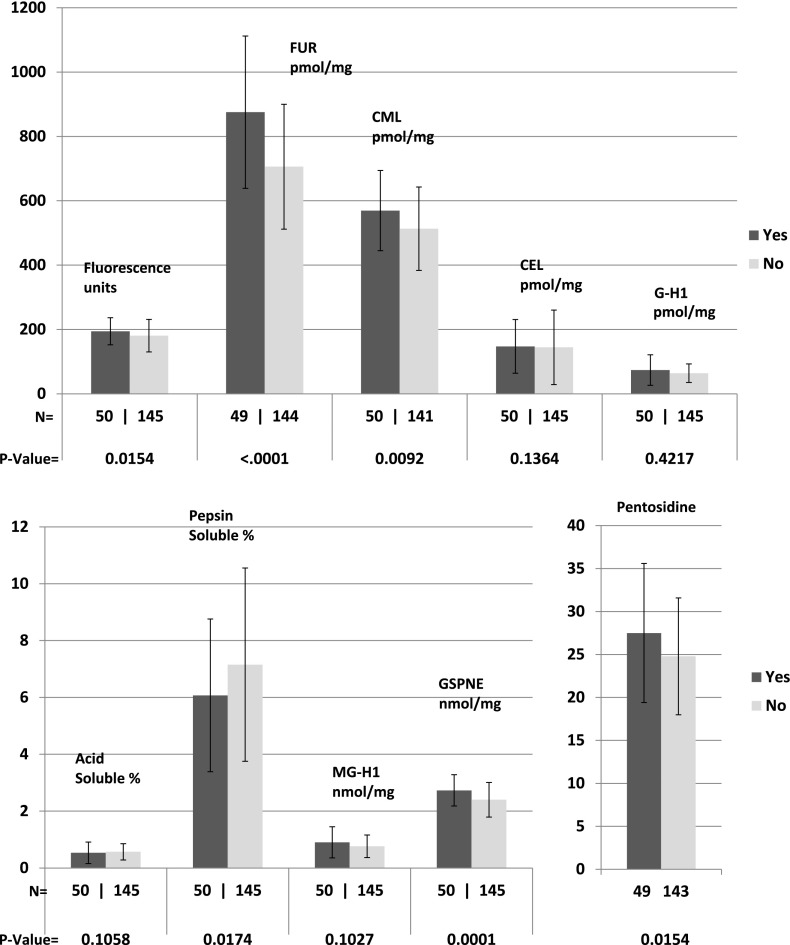
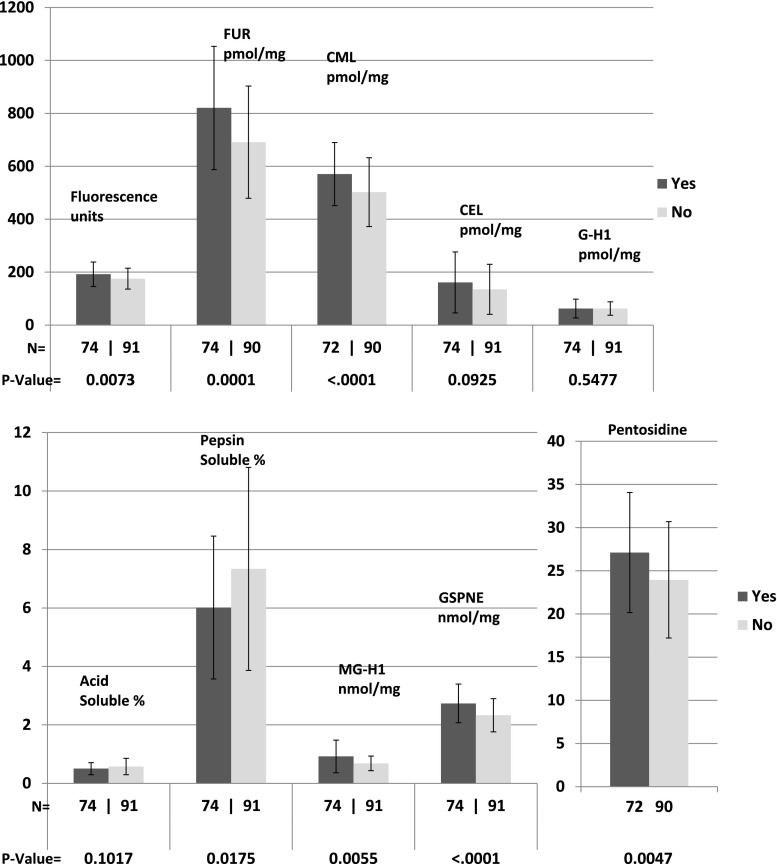
All AGEs except for CEL and G-H1 were associated with progression of retinopathy (each P ≤ 0.01) (Fig. 1); all except acid-soluble collagen, CEL, G-H1, and MG-H1 were associated with progression of nephropathy (Fig. 2) (each P ≤ 0.02); and all except acid-soluble collagen, CEL, and G-H1 were significantly associated with progression of neuropathy (Fig. 3) (each P ≤ 0.02). All of these remained significant after the Benjamini and Hochberg FDR adjustment (each FDR adjusted P < 0.05) (20). As previously reported, FUR, CML, pentosidine, relative fluorescence, and pepsin solubility (negatively) were significantly associated with all three complications, but only GSPNE of the four newly measured AGEs was uniformly associated with future progression of all complications (P < 0.0001).
Figure 1.
AGE levels in those with (yes) or without (no) three-step progression of retinopathy on the ETDRS scale from DCCT closeout up to EDIC study year 16.
Figure 2.
AGE levels in those with (yes) or without (no) development of AER >40 mg/24 h from DCCT closeout to EDIC study years 15–16.
Figure 3.
AGE levels in those with (yes) or without (no) development of confirmed clinical neuropathy or abnormal autonomic nervous system function from DCCT closeout to EDIC study years 13–14.
Multivariable Logistic Regression Analyses
Tables 3–5 present multivariable logistic regression analyses of covariates associated, respectively, with progression of retinopathy, nephropathy, and neuropathy.
Table 3.
Summary of multivariable logistic regressions of AGE associations with the prevalence of retinopathy without and with adjustment for A1C, and vice versa
| Covariate effects evaluated* | df | χ2 | P value | Entropy R2 | OR (95% CI)† |
|---|---|---|---|---|---|
| All AGEs (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE) | |||||
| Unadjusted | 10 | 53.7 | <0.0001 | 0.19 | |
| Adjusted for | |||||
| DCCT mean A1C | 10 | 27.3 | 0.002 | 0.10 | |
| EDIC study mean A1C | 10 | 46.2 | <0.0001 | 0.16 | |
| Original AGEs published in 2005 (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen) | |||||
| Unadjusted | 6 | 42.4 | <0.0001 | 0.15 | |
| Adjusted for | |||||
| DCCT mean A1C | 6 | 16.3 | 0.01 | 0.06 | |
| EDIC study mean A1C | 6 | 33.9 | <0.0001 | 0.12 | |
| Backward selected all AGEs (FUR, GSPNE) | |||||
| Unadjusted | 2 | 45.5 | <0.0001 | 0.16 | |
| Adjusted for | |||||
| DCCT mean A1C | 2 | 17.1 | 0.0002 | 0.06 | |
| EDIC study mean A1C | 2 | 32.9 | <0.0001 | 0.12 | |
| CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1 | 2 | 30.5 | <0.0001 | 0.11 | |
| Selected factors‡ | 2 | 14.5 | 0.0006 | 0.06 | |
| FUR | |||||
| Unadjusted | 1 | 37.4 | <0.0001 | 0.13 | 2.6 (1.8–3.6) |
| Adjusted for | |||||
| DCCT mean A1C | 1 | 9.8 | 0.002 | 0.03 | 2.0 (1.3–3.2) |
| EDIC study mean A1C | 1 | 25.1 | <0.0001 | 0.09 | 2.5 (1.6–3.2) |
| Selected factors‡ | 1 | 5.9 | 0.01 | 0.03 | 2.5 (1.3–4.0) |
| GSPNE | |||||
| Unadjusted | 1 | 31.8 | <0.0001 | 0.11 | 2.4 (1.7–3.4) |
| Adjusted for | |||||
| DCCT mean A1C | 1 | 12.2 | 0.0005 | 0.04 | 2.0 (1.4–2.9) |
| EDIC study mean A1C | 1 | 24.2 | <0.0001 | 0.08 | 2.3 (1.6–3.2) |
| Selected factors‡ | 1 | 11.5 | 0.0007 | 0.05 | 2.2 (1.3–3.5) |
| DCCT mean A1C effect | |||||
| Unadjusted | 1 | 30.4 | <0.0001 | 0.10 | 2.3 (1.7–3.3) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 0.23 | 0.64 | 0.0 | 1.1 (0.7–1.9) |
| Backward-selected AGEs (FUR and GSPNE) | 1 | 0.86 | 0.35 | 0.0 | 1.3 (0.8–2.0) |
| FUR alone | 1 | 1.65 | 0.20 | 0.0 | 1.4 (0.9–2.2) |
| GSPNE alone | 1 | 10.8 | 0.001 | 0.04 | 1.8 (1.3–2.6) |
| EDIC study mean A1C effect | |||||
| Unadjusted | 1 | 34.7 | <0.0001 | 0.12 | 2.4 (1.7–3.3) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 24.3 | <0.0001 | 0.09 | 2.4 (1.6–3.6) |
| Backward-selected AGEs (FUR and GSPNE) | 1 | 20.0 | <0.0001 | 0.07 | 2.1 (1.5–3.0) |
*Within each block, multiple model effects are presented using the set of variables named within each block. For the first block, the joint effect of all 10 AGEs is assessed. The unadjusted model shows the effect of the named variables without adjustment for other factors (e.g., the effect of the 10 AGEs in combination). This is followed by the effect of the covariates after adjustment for one or more specified factors, each from a separate model (e.g., the effect of the 10 AGEs in a model adjusted for the DCCT mean A1C and in a model adjusted for the EDIC study mean A1C).
†Odds ratio (OR) is based on 1 SD increase in the continuous variables (DCCT mean A1C 1.6% [17.5 mmol/mol], EDIC study mean A1C 1.1% [12.0 mmol/mol], FUR 231, GSPNE 0.66, MG-H1 0.44).
‡A model adjusted for the following factors that were nominally, statistically, and significantly associated with the outcome in univariate analyses in Table 2: DCCT mean A1C, EDIC study mean A1C up to year 16, DCCT treatment group, and both the log(AER) and abnormal autonomic function at EDIC study baseline.
Table 5.
Summary of multivariable logistic regressions of AGE associations with the prevalence of neuropathy without and with adjustment for A1C, and vice versa
| Covariate effects evaluated* | df | χ2 | P value | Entropy R2 | OR (95% CI)† |
|---|---|---|---|---|---|
| All AGEs (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE) | |||||
| Unadjusted | 10 | 29.7 | 0.0009 | 0.13 | |
| Adjusted for | |||||
| DCCT mean A1C | 10 | 25.4 | 0.005 | 0.11 | |
| EDIC study mean A1C | 10 | 28.3 | 0.002 | 0.13 | |
| Original AGEs published in 2005 (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen) | |||||
| Unadjusted | 6 | 19.6 | 0.003 | 0.09 | |
| Adjusted for | |||||
| DCCT mean A1C | 6 | 15.2 | 0.02 | 0.07 | |
| EDIC study mean A1C | 6 | 16.4 | 0.01 | 0.07 | |
| Backward-selected AGEs (MG-H1, FUR) | |||||
| Unadjusted | 2 | 26.5 | <0.0001 | 0.12 | |
| Adjusted for | |||||
| DCCT mean A1C | 2 | 21.1 | <0.0001 | 0.09 | |
| EDIC study mean A1C | 2 | 24.0 | <0.0001 | 0.11 | |
| CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1 | 2 | 8.2 | 0.02 | 0.04 | |
| Selected factors‡ | 2 | 18.5 | <0.0001 | 0.08 | |
| MG-H1 | |||||
| Unadjusted | 1 | 13.9 | 0.0002 | 0.06 | 2.0 (1.3–3.1) |
| Adjusted for | |||||
| DCCT mean A1C | 1 | 13.7 | 0.0002 | 0.06 | 2.0 (1.3–3.0) |
| EDIC study mean A1C | 1 | 18.3 | <0.0001 | 0.08 | 2.3 (1.5–3.5) |
| Selected factors‡ | 1 | 16.1 | <0.0001 | 0.07 | 2.3 (1.4–3.7) |
| FUR | |||||
| Unadjusted | 1 | 13.3 | 0.0003 | 0.06 | 1.8 (1.3–2.6) |
| Adjusted for | |||||
| DCCT mean A1C | 1 | 7.8 | 0.005 | 0.03 | 2.0 (1.3–3.2) |
| EDIC study mean A1C | 1 | 7.8 | 0.005 | 0.03 | 1.6 (1.3–2.5) |
| Selected factors‡ | 1 | 2.3 | 0.13 | 0.01 | 1.6 (0.8–3.2) |
| DCCT mean A1C effect | |||||
| Unadjusted | 1 | 5.7 | 0.02 | 0.03 | 1.5 (1.1–2.1) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 1.0 | 0.31 | 0.0 | 0.7 (0.4–1.3) |
| Backward-selected AGEs (FUR, MG-H1) | 1 | 0.4 | 0.51 | 0.0 | 0.8 (0.5–1.5) |
| FUR | 1 | 0.3 | 0.56 | 0.0 | 0.9 (0.5–1.5) |
| MG-H1 | 1 | 5.5 | 0.02 | 0.02 | 1.5 (1.1–2.1) |
| EDIC study mean A1C effect | |||||
| Unadjusted | 1 | 10.0 | 0.002 | 0.04 | 1.7 (1.2–2.3) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 7.4 | 0.007 | 0.03 | 1.6 (1.1–2.4) |
| Backward-selected AGEs (FUR, MG-H1) | 1 | 7.7 | 0.006 | 0.03 | 1.7 (1.1–2.5) |
*Within each block, multiple model effects are presented using the set of variables named within each block. For the first block, the joint effect of all 10 AGEs is assessed. The unadjusted model shows the effect of the named variables without adjustment for other factors (e.g., the effect of the 10 AGEs in combination). This is followed by the effect of the covariates after adjustment for one or more specified factors, each from a separate model (e.g., the effect of the 10 AGEs in a model adjusted for the DCCT mean A1C and in a model adjusted for the EDIC study mean A1C).
†Odds ratio (OR) is based on 1 SD increase in the continuous variables (DCCT mean A1C 1.6% [17.5 mmol/mol], EDIC study mean A1C 1.1% [12.0 mmol/mol], FUR 231, GSPNE 0.66, MG-H1 0.44).
‡A model adjusted for the following factors that were nominally, statistically, and significantly associated with the outcome in univariate analyses in Table 2: DCCT mean A1C, EDIC study mean A1C up to year 16, and also age, LDL, and log(AER) at EDIC study baseline.
Retinopathy
The complete panel comprising the original set and the new set of AGEs was significantly associated with progression of retinopathy 16 years after DCCT closeout and remained so after adjustment for mean DCCT A1C level, mean EDIC study A1C level, and all the other significant factors from univariate analyses listed in Tables 2 and 3 (treatment group, AER, and autonomic neuropathy). The same was true for the panel of six original AGEs, and backward-selected AGEs FUR and GSPNE, jointly or individually. The four added AGEs strengthened the association between the original AGEs and retinopathy (LRT P = 0.02). Interestingly, the mean DCCT A1C effect on retinopathy progression during the EDIC study lost significance when adjusted for the whole panel of AGEs, for FUR alone, or for the combination of FUR and GSPNE (i.e., glycated collagen and its downstream cross-linked product).
Nephropathy
The whole panel of 10 AGEs and the panel of 6 original AGEs were both associated with the progression of nephropathy 15–16 years after DCCT closeout (Table 4). However, the added AGEs did not strengthen this association (P = 0.59). FUR was selected as the strongest predictor of nephropathy, which remained significantly associated after adjustment for mean DCCT and mean EDIC study A1C levels, whereas the mean DCCT A1C effect lost significance when adjusted for FUR or the whole panel of AGEs. The nephropathy analysis was repeated by using the outcome of a sustained AER of ≥30 mg/24 h (Supplementary Tables 1 and 2). The results were almost identical to what was reported for AER ≥40 mg/24 h, which confirmed the robustness of these findings.
Table 4.
Summary of multivariable logistic regressions of AGE associations with the prevalence of nephropathy without and with adjustment for A1C level, and vice versa
| Covariate effects evaluated* | df | χ2 | P value | Entropy R2 | OR (95% CI)† |
|---|---|---|---|---|---|
| All AGEs (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE) | |||||
| Unadjusted | 10 | 24.0 | 0.008 | 0.11 | |
| Adjusted for | |||||
| DCCT mean A1C | 10 | 9.6 | 0.48 | 0.04 | |
| EDIC study mean A1C | 10 | 18.1 | 0.05 | 0.08 | |
| Original AGEs published in 2005 (FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen) | |||||
| Unadjusted | 6 | 21.2 | 0.002 | 0.10 | |
| Adjusted for | |||||
| DCCT mean A1C | 6 | 6.8 | 0.34 | 0.03 | |
| EDIC study mean A1C | 6 | 15.3 | 0.02 | 0.07 | |
| Backward-selected AGEs (FUR) | |||||
| Unadjusted | 1 | 21.5 | <0.0001 | 0.10 | 2.3 (1.6–3.3) |
| Adjusted for | |||||
| DCCT mean A1C | 1 | 5.1 | 0.02 | 0.02 | 2.0 (1.0–3.2) |
| EDIC study mean A1C | 1 | 13.1 | 0.0003 | 0.06 | 2.0 (1.3–2.5) |
| CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 11.7 | 0.0006 | 0.05 | 2.0 (1.0–3.2) |
| Selected factors‡ | 1 | 3.9 | 0.049 | 0.02 | 2.5 (1.3–5.0) |
| DCCT mean A1C effect | |||||
| Unadjusted | 1 | 18.8 | <0.0001 | 0.08 | 3.0 (1.5–2.1) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 0.8 | 0.38 | 0.0 | 1.3 (0.7–2.2) |
| Backward-selected AGEs (FUR) | 1 | 1.4 | 0.23 | 0.01 | 1.4 (0.8–2.3) |
| EDIC study mean A1C effect | |||||
| Unadjusted | 1 | 44.0 | <0.0001 | 0.20 | 3.4 (2.2–5.1) |
| Adjusted for | |||||
| FUR, CML, pentosidine, fluorescence, acid-soluble collagen, pepsin-soluble collagen, CEL, G-H1, MG-H1, GSPNE | 1 | 34.1 | <0.0001 | 0.16 | 3.2 (2.0–5.0) |
| Backward-selected AGEs (FUR) | 1 | 33.8 | <0.0001 | 0.15 | 3.1 (2.0–4.8) |
*Within each block, multiple model effects are presented using the set of variables named within each block. For the first block, the joint effect of all 10 AGEs is assessed. The unadjusted model shows the effect of the named variables without adjustment for other factors (e.g., the effect of the 10 AGEs in combination). This is followed by the effect of the covariates after adjustment for one or more specified factors, each from a separate model (e.g., the effect of the 10 AGEs in a model adjusted for the DCCT mean A1C and in a model adjusted for the EDIC study mean A1C).
†Odds ratio (OR) is based on 1 SD increase in the continuous variables (DCCT mean A1C 1.6% [17.5 mmol/mol], EDIC study mean A1C 1.1% [12.0 mmol/mol], FUR 231, GSPNE 0.66, MG-H1 0.44).
‡A model adjusted for the following factors that were nominally, statistically, and significantly associated with the outcome in univariate analyses in Table 2: DCCT mean A1C, EDIC study mean A1C up to year 16, and both retinopathy and log(AER) at EDIC study baseline.
Neuropathy
The entire panel of 10 AGEs, the panel of 6 original AGEs, the combination of backward-selected AGEs FUR and MG-H1, and both of these AGEs individually, remained associated with the progression of neuropathy in the EDIC study after adjustment for mean DCCT A1C and mean EDIC study A1C levels (Table 5). The four new AGEs strengthened the association between the six original AGEs and neuropathy (LRT P = 0.04). Moreover, the effect of mean DCCT A1C level on neuropathy progression lost significance when adjusted for FUR alone or in combination with MG-H1 and when adjusted for the whole panel of AGEs.
FUR Versus FL and Associations Among AGEs and With A1C
We have reported in this study the 13–15 year EDIC study data on FUR from the original set instead of FL, its native precursor that was automatically included in the analytical procedure. Both markers performed about the same in the Spearman correlation analysis with other AGEs (Supplementary Table 3). As expected, the two markers correlated highly with each other (R2 = 0.70, P < 0.001). The R2 values with other AGEs were very similar, in general slightly favoring FUR over FL, except for the correlation with GSPNE (0.52 vs. 0.56) and MG-H1 (0.13 vs. 0.26), whereby only FL was significant (P < 0.01). As expected, FUR correlated better with past (DCCT) than prospective (EDIC study) mean glycemia levels, as reflected by A1C values (0.72 vs. 029, both P < 0.001). Of interest is that collagen pepsin solubility was highly inversely correlated with most other markers, in particular with the matrix cross-link GSPNE, which is the most abundant AGE in skin. GSPNE was the glycation product most strongly correlated with the other AGEs, followed by FUR and MG-H1. FUR (r = 0.72, P < 0.0001) was more strongly associated with mean DCCT A1C level than FL (r = 0.48, P < 0.0001). CEL, G-H1, and MG-H1 were not correlated with mean DCCT A1C level. CML correlated with each component of the original set of AGEs, as previously reported, and also correlated with GSPNE (r = 0.50, P < 0.0001) and with DCCT mean A1C level (r = 0.38, P < 0.0001) but did not correlate with EDIC study mean A1C level.
As for correlation with complications, our previous study on association with the progression of complications during the DCCT up to the time of the biopsy (7) revealed that the order of individual strength of association (entropy R2) for sustained three-step progression was FL > FUR (each P < 0.05), but for sustained progression greater than or equal to 3 microaneurysms, it was FUR > FL (P < 0.05); for nephropathy, it was FUR > FL (each P < 0.05); and for neuropathy, it was FUR >>> FL (each P < 0.05). For the future progression of complications during the EDIC study, we found only trivial differences in the χ2 and R2 effects, leading to the conclusion that both forms of the Amadori product behave very similarly.
Discussion
In univariate analysis of eight AGEs and two collagen solubility markers, four of which we have measured for the first time in our original DCCT biopsy samples (i.e., GSPNE, CEL, G-H1, and MG-H1), GSPNE stands out in its significant association with three-step ETDRS worsening of retinopathy, the development of microalbuminuria, and the development of neuropathy from the closeout of DCCT to 13–16 years later in the EDIC study. In multivariable analysis using backward selection, GSPNE was associated with the subsequent development of retinopathy even after adjustment for DCCT mean A1C and EDIC study mean A1C levels, as well as DCCT treatment group and log AER at EDIC study baseline (Table 3). In addition, it is noteworthy that the content of GSPNE in skin collagen from people with type 1 diabetes is 3–4 times higher than that of FUR, CML, or MG-H1; 17 times higher than that of CEL; 42 times higher than that of G-H1; and 100 times higher than that of pentosidine. GSPNE is also quantitatively both the single most prevalent AGE and protein cross-link, and it is exclusively derived from glucose. These facts support GSPNE as perhaps being the one AGE that plays the most important role in the pathogenesis of retinopathy from hyperglycemia. This is not to deny a role for other AGEs either individually or in combination.
In univariate analysis, FUR, CML, and pentosidine were associated with all three progressive complications, as previously reported (6), and FUR remained significant after adjustment for DCCT mean A1C and for EDIC study mean A1C levels. MG-H1, derived from methylglyoxal, was also strongly associated with retinopathy (P = 0.004) and neuropathy (P = 0.006), but was not significantly associated with nephropathy (P = 0.1).
Not surprisingly, there were numerous correlations between many pairs of AGEs, and between AGEs and DCCT mean A1C level (Supplementary Table 3), which expressed the glycemic exposure prior to the measurement of the AGEs. Again, the correlations between GSPNE and each of the other AGEs as well as with DCCT mean A1C level were most often the strongest of any single AGE. FUR, a measure of glycated collagen, correlated most strongly with DCCT mean A1C level (r = 0.72), while the AGE MG-H1, formed from methylglyoxal originating mainly from the degradation of cellular triosephosphate and influenced by glycolytic activity (21,22), did not correlate at all with mean DCCT A1C level. Within the panel of 10 AGEs reported here, 2 were indirect indices of cross-linking (i.e., acid solubility and pepsin solubility), with each representing physicochemical properties that reflect the incorporation of AGEs into tissue proteins.
Although GSPNE has been known since 1999 (23), its precise role in the development of diabetes complications remains to be understood. Incorporation of GSPNE may decrease the turnover of collagen and increase its accumulation, perhaps contributing to the thickening of basement membranes (24) in the retina, kidney, and peripheral nerves in addition to hyperglycemia-mediated increased extracellular matrix deposition (25). Second, the necessity of arginine residues for the formation of GSPNE (Fig. 1) may compete with integrin-binding sites to basement membrane (23,26), and the resultant loss of proper attachment of endothelial cells to basement membrane may promote programmed death of these cells (27).
An unexpected finding was that the methylglyoxal AGE MG-H1 remained strongly associated with neuropathy progression despite adjustment for all other risk factors. This provides crucial support for the recently reported association between neuropathy and methylglyoxal in 27 participants of the Oslo Study (28). These clinical findings, together with preclinical data, provide a rationale for in-depth studies on the role of methylglyoxal in diabetic neuropathy.
The strengths of this study are the availability of tissue specimens from an exceptionally well-characterized type 1 diabetes cohort with repeated measurements of retinopathy, nephropathy, and neuropathy by the latest and most modern validated methods for up to 17 years after the skin biopsy samples were obtained and stored. There also are limitations to the study. The data are fundamentally associative; even though the AGE measurements preceded the subsequent development of complications and have a predictive quality, this by itself does not prove a causal role. It is conceivable that an antecedent pathogenic factor induces independently both the complications and AGEs in parallel fashion. It is also not certain that qualitative or quantitative findings in skin collagen AGEs reflect those of retinal, renal, or neural tissue.
In conclusion, skin collagen AGEs are strongly associated with the future development of diabetic retinopathy, nephropathy, and neuropathy up to 17 years later. GSPNE is the single most prevalent measured AGE in human skin collagen and is systematically most associated with the progression of these complications, along with its immediate glucose-derived precursor, FL, judged herein by the presence of the surrogate marker FUR.
Supplementary Material
Article Information
Acknowledgments. The authors thank Mary Hawkins of the Biostatistics Center, The George Washington University, for technical assistance and for editorial compliance during manuscript submission. The authors also thank the DCCT participants who volunteered for the skin biopsies that made this study possible.
Funding. This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant DK-79432 to D.R.S. and Juvenile Diabetes Research Foundation grant 17-2010-318 to V.M.M. The DCCT/EDIC Study has been supported by multiple grants from the National Institutes of Health, including U01 Cooperative Agreement grants (1982–1993, 2011–2016) and contracts (1982–2011) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the NIDDK (current grants U01 DK094176 and U01 DK094157); and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006 to present), Bethesda, MD.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Industry contributors have had no role in the DCCT/EDIC Study but have provided free or discounted supplies or equipment to support participants’ adherence to the study.
Author Contributions. S.G. drafted the manuscript and interpreted the results. W.S. and X.G. performed the statistical analyses and cowrote the manuscript. P.C. and J.L. reviewed the manuscript. D.R.S. performed the laboratory analyses and reviewed the manuscript. The DCCT/EDIC Research Group collected the data and reviewed the manuscript. V.M.M. interpreted the results and cowrote the manuscript. P.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0215/-/DC1.
*A complete list of participants and industry contributors for the DCCT/EDIC Research Group can be found at http://www.nejm.org/doi/full/10.1056/NEJMoa1111732.
See accompanying article, p. 9.
References
- 1.Genuth SM. The case for blood glucose control. Adv Intern Med 1995;40:573–623 [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.The DCCT Research Group . The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44:968–983 [PubMed] [Google Scholar]
- 4.Monnier VM, Sell DR, Genuth S. Glycation products as markers and predictors of the progression of diabetic complications. Ann N Y Acad Sci 2005;1043:567–581 [DOI] [PubMed] [Google Scholar]
- 5.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genuth S, Sun W, Cleary P, et al. DCCT Skin Collagen Ancillary Study Group . Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnier VM, Sell DR, Strauch C, et al. DCCT Research Group . The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J Diabetes Complications 2013;27:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J 1997;324:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J 2002;364:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans 2003;31:1417–1422 [DOI] [PubMed] [Google Scholar]
- 11.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White NH, Sun W, Cleary PA, et al. DCCT-EDIC Research Group . Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes 2010;59:1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EDTRS . Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 1991;98(Suppl.):741–756 [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Research Group . The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Arch Ophthalmol 1995;113:36–51 [DOI] [PubMed] [Google Scholar]
- 15.Molitch ME, Steffes MW, Cleary PA, Nathan DM. Baseline analysis of renal function in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group [corrected]. Kidney Int 1993;43:668–674 [DOI] [PubMed] [Google Scholar]
- 16.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 17.Martin CL, Albers J, Herman WH, et al. DCCT/EDIC Research Group . Neuropathy among the Diabetes Control and Complications Trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pop-Busui R, Lu J, Lopes N, Jones TL, BARI 2D Investigators . Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst 2009;14:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachin J. Biostatistical Methods: The Assessment of Relative Risks. New York, John Wiley & Sons, 2000 [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;54:289–300 [Google Scholar]
- 21.Thornalley PJ. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 1988;254:751–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 1993;212:101–105 [DOI] [PubMed] [Google Scholar]
- 23.Lederer MO, Klaiber RG. Cross-linking of proteins by Maillard processes: characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorg Med Chem 1999;7:2499–2507 [DOI] [PubMed] [Google Scholar]
- 24.Kilo C, Vogler N, Williamson JR. Muscle capillary basement membrane changes related to aging and to diabetes mellitus. Diabetes 1972;21:881–905 [DOI] [PubMed] [Google Scholar]
- 25.Muona P, Jaakkola S, Zhang RZ, et al. Hyperglycemic glucose concentrations up-regulate the expression of type VI collagen in vitro. Relevance to alterations of peripheral nerves in diabetes mellitus. Am J Pathol 1993;142:1586–1597 [PMC free article] [PubMed] [Google Scholar]
- 26.Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 2006;55:1961–1969 [DOI] [PubMed] [Google Scholar]
- 27.Bhatwadekar AD, Glenn JV, Li G, Curtis TM, Gardiner TA, Stitt AW. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci 2008;49:1232–1241 [DOI] [PubMed] [Google Scholar]
- 28.Sveen KA, Karimé B, Jørum E, et al. Small- and large-fiber neuropathy after 40 years of type 1 diabetes: associations with glycemic control and advanced protein glycation: the Oslo Study. Diabetes Care 2013;36:3712–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.