Abstract
Alzheimer's disease (AD) is the most common cause of dementia worldwide with no curative therapies currently available. Previously, global transcriptome analysis of AD brains by microarray failed to identify the set of consistently deregulated genes for biomarker development of AD. Therefore, the molecular pathogenesis of AD remains largely unknown. Whole RNA sequencing (RNA-Seq) is an innovative technology for the comprehensive transcriptome profiling on a genome-wide scale that overcomes several drawbacks of the microarray-based approach. To identify biomarker genes for AD, we analyzed a RNA-Seq dataset composed of the comprehensive transcriptome of autopsized AD brains derived from two independent cohorts. We identified the core set of 522 genes deregulated in AD brains shared between both, compared with normal control subjects. They included downregulation of neuronal differentiation 6 (NeuroD6), a basic helix-loop-helix (bHLH) transcription factor involved in neuronal development, differentiation, and survival in AD brains of both cohorts. We verified the results of RNA-Seq by analyzing three microarray datasets of AD brains different in brain regions, ethnicities, and microarray platforms. Thus, both RNA-Seq and microarray data analysis indicated consistent downregulation of NeuroD6 in AD brains. These results suggested that downregulation of NeuroD6 serves as a possible biomarker for AD brains.
1. Introduction
Alzheimer's disease (AD) is the most common cause of dementia worldwide affecting the elderly population, characterized by the hallmark pathology of amyloid-β (Aβ) deposition, neurofibrillary tangle (NFT) formation, and extensive neurodegeneration in the brain. The complex interaction between multiple genetic and environmental factors affecting various molecular pathways plays a key role in the pathogenesis of AD [1]. With regard to environmental factors, disturbed homeostasis of dietary metals, such as copper, aluminum, and iron, confers an increased risk of AD [2, 3]. With respect to genetic factors, genome-wide association studies (GWAS), composed of large cohorts of AD and controls, identified numerous common variants but with smaller risks associated with development of late-onset AD [4]. They include complement component receptor 1 (CR1), bridging integrator 1 (BIN1), clusterin (CLU), phosphatidylinositol binding clathrin assembly protein (PICALM), membrane-spanning 4-domains, subfamily A, member 4A/membrane-spanning 4-domains, subfamily A, member 6E (MS4A4/MS4A6E), CD2-associated protein (CD2AP), CD33 molecule (CD33), EPH receptor A1 (EPHA1), and ATP-binding cassette, subfamily A, member 7 (ABCA7) [4]. More recently, whole-exome sequencing (WES) studies discovered rare functional variants located in the genes encoding Aβ precursor protein (APP), triggering receptor expressed on myeloid cells 2 (TREM2), and phospholipase D3 (PLD3), exhibiting a much greater contribution to protection or development of AD [5–7]. However, at present, the central molecular mechanism underlying neurodegeneration in AD remains largely unknown. Therefore, no curative therapies based on the molecular pathogenesis of AD are currently available.
The completion of the Human Genome Project in 2003 allows us to systematically study disease-associated profiles of the whole human genome. Particularly, microarray technologies enable us not only to identify disease-specific molecular signatures and biomarkers for diagnosis and prediction of prognosis but also to characterize druggable targets for effective therapy. Actually, global transcriptome analysis of postmortem AD brains by microarray has identified a battery of genes aberrantly regulated in AD, whose role has not been previously predicted in its pathogenesis [8]. They include reduced expression of kinases/phosphatases, cytoskeletal proteins, synaptic proteins, and neurotransmitter receptors in NFT-bearing CA1 neurons [9], downregulation of neurotrophic factors and upregulation of proapoptotic molecules in the hippocampal CA1 region [10], disturbed sphingolipid metabolism in various brain regions during progression of AD [11], and overexpression of the AMPA receptor GluR2 subunit in synaptosomes of the prefrontal cortex [12]. However, previous studies failed to identify the set of definite biomarker genes, whose expression is consistently deregulated in AD brains across different studies [8]. The failure in reproducibility of the results is attributable to differences in study designs and samples, including the quality of RNA, disease stages, brain regions, cellular diversities, ethnicities, and microarray platforms [13].
Recently, the revolution of the next-generation sequencing (NGS) technology has made a great impact on the field of genome research. Whole RNA sequencing (RNA-Seq) serves as an innovative tool for the comprehensive transcriptome profiling on a genome scale in a high-throughput and quantitative manner [14, 15]. RNA-Seq clarifies the unbiased expression of the complete set of transcripts at a single base resolution, including splice junctions and fusion genes, by providing digital gene expression levels with high reproducibility. RNA-Seq enables us to characterize the complex transcriptome, composed of mRNAs, noncoding RNAs, and small RNAs, theoretically at a single cell level, by aligning sequencing reads on reference genomes or assembling them de novo without references. For these reasons, RNA-Seq overcomes several drawbacks intrinsic to the microarray-based approach that is hampered by the difficulty in detection of novel transcripts and splice variants, the poor sensitivity of rare transcripts, and high backgrounds due to cross hybridization.
To identify biomarker genes relevant to the molecular pathogenesis of AD, we analyzed publicly available RNA-Seq datasets, composed of the comprehensive transcriptome of autopsied AD brains derived from two independent cohorts. First, we identified the core set of 522 genes deregulated in AD brains overlapping between both. Then, we verified the results of RNA-Seq by analyzing three independent microarray datasets of AD brains that are different in brain regions, ethnicities, and microarray platforms. Consequently, we found consistent downregulation of neuronal differentiation 6 (NeuroD6), a bHLH transcription factor involved in neuronal development and differentiation, serving as a possible biomarker for AD brains.
2. Materials and Methods
2.1. RNA-Seq Datasets of AD Brains
To identify a comprehensive set of differentially expressed genes (DEGs) in the brains of AD patients compared with normal control (NC) subjects, we investigated FASTQ-formatted files of RNA-Seq datasets retrieved from the DDBJ Sequence Read Archive (DRA) (https://trace.ddbj.nig.ac.jp/DRASearch) under the accession number of SRA060572. It consisted of 15 separate samples derived from two independent cohorts, studied by the researchers in Emory University, Atlanta, here abbreviated as EMU, and by those in the University of Kentucky, Lexington, abbreviated as UKY [16]. The EMU dataset contains transcriptome of the frontal cortex isolated from three male and two female AD patients with age = 71.0 ± 8.2 years and postmortem interval (PMI) = 13.8 ± 7.2 hours and two male and two female NC subjects with age = 60.8 ± 3.3 years and PMI = 9.0 ± 2.6 hours. The UKY dataset contains transcriptome of the frontal cortex isolated from three female AD patients with age = 81.7 ± 3.2 years and PMI = 2.2 ± 0.4 hours and three female NC subjects with age = 85.0 ± 1.0 years and PMI = 2.8 ± 0.6 hours. The information on the Braak stage of AD pathology [17] is not available for any cases. In these experiments, total RNA was purified by oligo (dT) beads and converted to cDNA for PCR amplification using SMARTer PCR cDNA Synthesis Kit (Clontech). Then, PCR products were fragmented and processed for DNA library preparation via PCR amplification using NEBNext DNA Library Prep Master Mix Set for Illumina (New England BioLabs). The final DNA library products with size ~200 bp were prepared for paired-end sequencing on HiSeq 2000 (Illumina).
After removing poly-A tails and low quality reads from the original data, we mapped short read data on the human genome reference sequence hg19 by using TopHat2.0.9 (http://ccb.jhu.edu/software/tophat/index.shtml). The expression levels were transformed into fragments per kilobase of exon per million mapped fragments (FPKM). We identified DEGs that satisfy the significance expressed as q-value representing FDR-adjusted P value < 0.05 by using Cufflinks2.1.1 (http://cufflinks.cbcb.umd.edu).
2.2. Microarray Datasets of AD Brains
To verify the results of RNA-Seq data analysis, we investigated three distinct microarray datasets of AD brains retrieved from Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers of GSE1297, GSE5281, and GSE11829. The GSE1297 dataset contains transcriptome of postmortem hippocampal CA1 tissues studied on a Human Genome U133A Array containing 22,215 transcripts (Affymetrix), and the data were normalized by the Microarray Analysis Suite 5.0 (MAS5) algorithm [18]. The samples were collected by the researchers in UKY. They were prepared from 31 age-matched individuals, composed of nine NC subjects (age = 85.3 ± 8.0 years; male = 7, female = 2), seven patients with incipient AD (age = 91.9 ± 6.2 years; male = 2, female = 5), eight with moderate AD (age = 83.4 ± 3.2 years; male = 2, female = 6), and seven with severe AD (age = 84.0 ± 10.6 years; male = 2, female = 5). The clinical stage of AD was defined by the Mini-Mental State Examination (MMSE) score as follows: the control (the score > 25), incipient (20–26), moderate (14–19), and severe (<14) AD. The information on the Braak stage of AD pathology is not available for any cases.
The GSE5281 dataset, alternatively named steph-affy-human 433773, contains transcriptome of laser microdissection (LCM)-captured layer III neurons derived from various brain regions studied on a Human Genome U133 Plus 2.0 Array containing 47,400 transcripts (Affymetrix), and the data were normalized by MAS5 [19]. The samples were collected by the researchers in AD Centers of Arizona, Duke University, and Washington University. We studied the gene expression profile of cortical neurons in the superior frontal gyrus, which were isolated from 11 age-matched NC subjects (age = 79.3 ± 10.2 years; male = 7, female = 4) and 23 AD patients (age = 79.2 ± 7.5 years; male = 13, female = 10). The information on the Braak stage of AD pathology is not available for any cases.
The GSE36980 dataset contains transcriptome of postmortem brain tissues isolated from frontal and temporal cortices and the hippocampus studied on a Human Gene 1.0 ST Array containing 28,869 genes (Affymetrix), and the data were normalized by the robust multiarray average (RMA) algorithm [20]. The samples were collected by the researchers in Kyushu University, Japan, for the Hisayama study. We studied the gene expression profile of the hippocampus, derived from ten non-AD controls (age = 77.0 ± 9.0 years; male = 5, female = 5) and seven AD patients (age = 92.9 ± 6.1 years; male = 3, female = 4). The information on the Braak stage of AD pathology is not available for any cases.
To evaluate the statistically significant difference in gene expression levels between AD and NC or non-AD groups, we performed a two-tailed Welch t-test by using TTEST function of Excel. In some experiments, we performed receiver operating characteristic (ROC) analysis by using SPSS version 19 (IBM).
2.3. Molecular Network Analysis
We imported Entrez Gene IDs of DEGs into the Functional Annotation tool of Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 [21]. DAVID extracts gene ontology (GO) terms enriched in the set of imported genes and identifies relevant pathways constructed by Kyoto Encyclopedia of Genes and Genomes (KEGG). The results are followed by statistical evaluation with the modified Fisher exact test corrected by multiple comparison tests. We considered P value < 0.05 after Bonferroni's correction as significant. KEGG is a publicly accessible knowledgebase that contains 337,524 manually curated pathways that cover a wide range of metabolic, genetic, environmental, and cellular processes and human diseases [22].
We also imported Entrez Gene IDs of DEGs into the Core Analysis tool of Ingenuity Pathways Analysis (IPA) (Ingenuity Systems). IPA is a commercial knowledgebase that contains approximately 3,000,000 biological and chemical interactions with definite scientific evidence. By uploading the list of Gene IDs, the network-generation algorithm identifies focused genes integrated in global molecular pathways and networks. IPA calculates the score P value that reflects the statistical significance of association between the genes and the pathways and networks by Fisher's exact test. We considered P value < 0.05 by Fisher's exact test as significant.
3. Results
3.1. RNA-Seq Data Analysis of AD Brains
By RNA-Seq data analysis with the combination of TopHat and Cufflinks, we studied transcriptome of the frontal cortex of AD and NC derived from two distinct cohorts named EMU and UKY. We identified 587,301 and 766,998 consensus transcripts in total from datasets of EMU and UKY, respectively. Among them, we identified 1,226 DEGs for EMU and 2,625 DEGs for UKY that satisfy q-value (FDR-corrected P value) < 0.05 and fold change greater than 2.0 or smaller than 0.5, when compared between AD and NC groups. Then, we extracted the core set of 522 DEGs overlapping between both cohorts, composed of 470 downregulated and 52 upregulated genes in AD (see Supplementary Table 1 of the Supplementary Material available online at http://dx.doi.org/10.1155/2014/123165). Thus, downregulated genes greatly outnumbered upregulated ones in AD brains. Top 20 genes are listed in Table 1. Notably, the expression of neuronal differentiation 6 (NeuroD6), a brain-specific basic helix-loop-helix (bHLH) transcription factor [23], is greatly reduced at fold changes 0.095 for EMU and 0.159 for UKY in AD brains (q = 0.0023 for EMU and 0.0006 for UKY) (Table 1, italicized). Furthermore, lipid phosphate phosphatase-related protein type 4 (LPPR4; PRG1), a direct target gene of NeuroD6 [24], was also downregulated in AD brains of both cohorts (Supplementary Table 1). We identified totally 60 differentially spliced genes in the frontal cortex of AD, when the data derived from both cohorts were combined, although none of them were shared between both (Supplementary Table 2).
Table 1.
Top 20 DEGs in the frontal cortex of AD overlapping between two cohorts identified by RNA-Seq data analysis of SRA060752.
| Entrez Gene ID |
Gene symbol | Gene name | Chromosome locus | Fold change (AD versus NC: EMU) |
q-value (EMU) |
Fold change (AD versus NC: UKY) |
q-value (UKY) |
|---|---|---|---|---|---|---|---|
| 6863 | TAC1 | Tachykinin, precursor 1 | chr7: 97361270–97369784 | 0.028013577 | 0.00232257 | 0.189297859 | 0.000638422 |
| 5121 | PCP4 | Purkinje cell protein 4 | chr21: 41239346–41301322 | 0.060477403 | 0.00232257 | 0.263070248 | 0.000638422 |
| 793 | CALB1 | Calbindin 1, 28 kDa | chr8: 91070837–91095107 | 0.068756027 | 0.00232257 | 0.229478357 | 0.000638422 |
| 54112 | GPR88 | G protein-coupled receptor 88 | chr1: 101002397–101008223 | 0.083735314 | 0.00232257 | 0.380695653 | 0.000638422 |
| 143162 | FRMPD2 | FERM and PDZ domain containing 2 | chr10: 49364156–49482941 | 0.086014876 | 0.00232257 | 0.344904773 | 0.00248927 |
| 6588 | SLN | Sarcolipin | chr11: 107578100–107582787 | 0.091647542 | 0.00398798 | 0.110806929 | 0.00396642 |
| 6750 | SST | Somatostatin | chr3: 187386693–187388201 | 0.094938693 | 0.00232257 | 0.042406817 | 0.000638422 |
| 63974 | NeuroD6 | Neurogenic differentiation 6 | chr7: 31377079–31380538 | 0.095418314 | 0.00232257 | 0.159354316 | 0.000638422 |
| 3358 | HTR2C | 5-Hydroxytryptamine (serotonin) receptor 2C | chrX: 113818550–114144627 | 0.09625865 | 0.00232257 | 0.211803744 | 0.000638422 |
| 771 | CA12 | Carbonic anhydrase XII | chr15: 63615729–63674075 | 0.104187926 | 0.00232257 | 0.453231488 | 0.0218249 |
|
| |||||||
| 845 | CASQ2 | Calsequestrin 2 (cardiac muscle) | chr1: 116242519–116345204 | 3.491410523 | 0.00962874 | 5.322706439 | 0.000638422 |
| 23704 | KCNE4 | Potassium voltage-gated channel, Isk-related family, member 4 | chr2: 223916861–223920355 | 4.436801472 | 0.00232257 | 4.699574041 | 0.000638422 |
| 6279 | S100A8 | S100 calcium binding protein A8 | chr1: 153362507–153363664 | 5.018965399 | 0.00232257 | 2.142150734 | 0.0151369 |
| 871 | SERPINH1 | Serpin peptidase inhibitor, clade H (heat shock protein 47), member 1 (collagen binding protein 1) | chr11: 75273100–75283870 | 5.437863735 | 0.00232257 | 2.129345418 | 0.0101566 |
| 6275 | S100A4 | S100 calcium binding protein A4 | chr1: 153516094–153518282 | 5.52659158 | 0.00232257 | 4.606560625 | 0.000638422 |
| 26266 | SLC13A4 | Solute carrier family 13 (sodium/sulfate symporters), member 4 | chr7: 135365246–135412933 | 6.580968881 | 0.00232257 | 2.996233377 | 0.000638422 |
| 3303 | HSPA1A | Heat shock 70 kDa protein 1A | chr6: 31777395–31785719 | 6.599057552 | 0.00232257 | 3.8880048 | 0.000638422 |
| 3304 | HSPA1B | Heat shock 70 kDa protein 1B | chr6: 31795511–31798031 | 6.599057552 | 0.00232257 | 2.366327899 | 0.000638422 |
| 375061 | FAM89A | Family with sequence similarity 89, member A | chr1: 231154703–231175995 | 8.186858725 | 0.00232257 | 2.253222839 | 0.00248927 |
| 100500849 | MIR3916 | MicroRNA 3916 | chr1: 247342111–247374105 | 17.2291545 | 0.00762148 | 2.127294836 | 0.00287725 |
The core set of 522 DEGs in the frontal cortex of AD overlapping between EMU and UKY satisfying q-value (FDR-corrected P value) <0.05 and fold change greater than 2.0 or smaller than 0.5 were extracted by RNA-Seq data analysis of SRA060572. Top 10 downregulated and top 10 upregulated genes based on fold change in EMU are listed with Entrez Gene ID, gene symbol, gene name, chromosomal locus, fold change, and q-value. NeuroD6 is italicized. The complete list of 522 DEGs is shown in Supplementary Table 1.
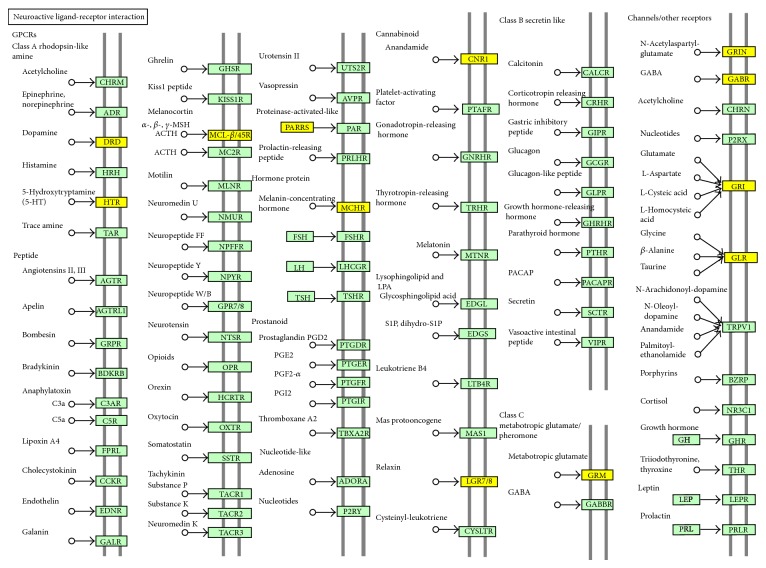
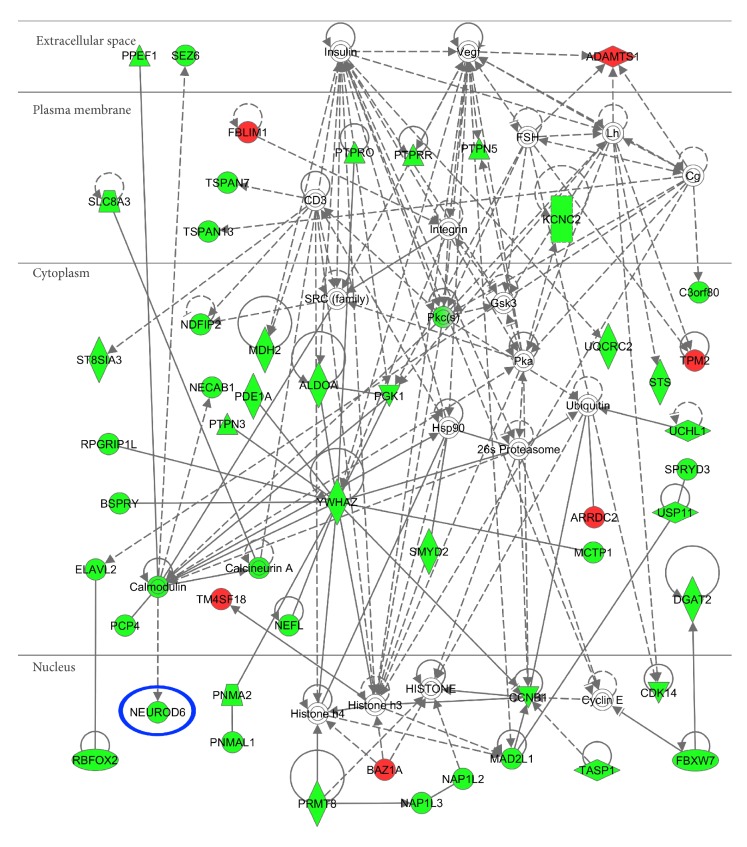
DAVID revealed that the set of 470 DEGs downregulated in the frontal cortex of AD are relevant to GO terms of “synaptic transmission” (GO:0007268; P = 2.545E − 17 corrected by Bonferroni) and “transmission of nerve impulse” (GO:0019226; P = 1.778E − 15 corrected by Bonferroni). They are also relevant to the KEGG pathway named “neuroactive ligand-receptor interaction” (hsa04080; P = 0.0004 corrected by Bonferroni) (Figure 1). In contrast, the set of 52 genes upregulated in AD were not significantly associated with any GO terms or KEGG pathways. IPA showed that the core set of 522 DEGs have a significant relationship with top two different functional networks defined as “Cell-To-Cell Signaling and Interaction, Nervous System Development and Function, Neurological Disease” (P = 1.00E − 71) and “Hereditary Disorder, Neurological Disease, Psychological Disorders” (P = 1.00E − 71) (Figure 2). Taken together, these results suggest that a battery of the genes essential for neuronal interactions is coordinately downregulated in AD brains.
Figure 1.
KEGG pathway of 470 DEGs downregulated in the frontal cortex of AD identified by RNA-Seq data analysis. Entrez Gene IDs of 470 DEGs downregulated in the frontal cortex of AD identified by RNA-Seq data analysis of SRA060572 were imported into the Functional Annotation tool of DAVID. It extracted the most significant KEGG pathway termed “neuroactive ligand-receptor interaction” (hsa04080) relevant to the set of imported genes. Downregulated genes are colored yellow.
Figure 2.
IPA pathways of the core set of 522 DEGs in the frontal cortex of AD identified by RNA-Seq data analysis. Entrez Gene IDs of 522 DEGs in the frontal cortex of AD identified by RNA-Seq data analysis of SRA060572 were imported into the Core Analysis tool of IPA. It extracted the most significant functional network termed “Hereditary Disorder, Neurological Disease, Psychological Disorders” relevant to the set of imported genes. Downregulated DEGs are colored green, while upregulated DEGs are colored red. NeuroD6 is highlighted by a blue circle.
3.2. Microarray Data Analysis of AD Brains
To verify the results of RNA-Seq data analysis, we studied three distinct microarray datasets of AD brains numbered GSE1297, GSE5281, and GSE36980. We compared the core set of 522 DEGs of RNA-Seq with DEGs extracted from microarray datasets. First, we studied the GSE5281 dataset composed of transcriptome of LCM-captured cortical neurons in the superior frontal gyrus. We identified the set of 215 DEGs compared between AD and NC groups, including 210 downregulated and 5 upregulated genes in AD (Supplementary Table 3). Because downregulated genes greatly outnumbered upregulated classes, we thereafter focused on the downregulated set. Among them, we found that 15 genes correspond to the core set of 522 DEGs of RNA-Seq (Table 2). Notably, the expression of NeuroD6 was reduced at fold change = 0.238 in purified cortical neurons of the superior frontal gyrus in AD brains (P = 0.000066, Table 2, italicized). In contrast, the levels of expression of NeuroD1 were not significantly different between AD and NC brains (P = 0.530, not shown). ROC analysis indicated that the area under the ROC curve (AUC) is 0.893 for NeuroD6 and 0.474 for NeuroD1, and the levels of sensitivity and specificity for discrimination between AD and NC are acceptable for NeuroD6 (P = 2.494E − 04) but unacceptable for NeuroD1 (P = 0.811) (Supplementary Figure 1).
Table 2.
The set of 15 genes DEGs downregulated in cortical neurons of the superior frontal gyrus of AD identified by microarray data analysis of GSE5281 corresponding to RNA-Seq data analysis of SRA060752.
| Entrez Gene ID |
Gene symbol | Gene name | Fold change (AD versus NC) |
P value |
|---|---|---|---|---|
| 116 | ADCYAP1 | Adenylate cyclase activating polypeptide 1 (pituitary) | 0.069636923 | 1.26039E − 07 |
| 6750 | SST | Somatostatin | 0.078547047 | 2.49899E − 05 |
| 10777 | ARPP21 | cAMP-regulated phosphoprotein, 21 kDa | 0.1474134 | 4.52823E − 06 |
| 6511 | SLC1A6 | Solute carrier family 1 (high affinity aspartate/glutamate transporter), member 6 | 0.156759363 | 4.74541E − 05 |
| 728192 | LINC00460 | Long intergenic non-protein-coding RNA 460 | 0.171656016 | 8.49002E − 07 |
| 891 | CCNB1 | Cyclin B1 | 0.182817517 | 1.34014E − 05 |
| 523 | ATP6V1A | ATPase, H+ transporting, lysosomal 70 kDa, V1 subunit A | 0.190442377 | 8.45909E − 05 |
| 7991 | TUSC3 | Tumor suppressor candidate 3 | 0.200399391 | 3.74354E − 05 |
| 1741 | DLG3 | Discs, large homolog 3 (Drosophila) | 0.222930685 | 8.59311E − 06 |
| 63974 | NeuroD6 | Neurogenic differentiation 6 | 0.237572737 | 6.60728E − 05 |
| 3382 | ICA1 | Islet cell autoantigen 1, 69 kDa | 0.249762845 | 2.41638E − 05 |
| 844 | CASQ1 | Calsequestrin 1 (fast-twitch, skeletal muscle) | 0.271852315 | 3.03481E − 05 |
| 9577 | BRE | Brain and reproductive organ-expressed (TNFRSF1A modulator) | 0.306075086 | 9.92354E − 05 |
| 84900 | RNFT2 | Ring finger protein, transmembrane 2 | 0.306171729 | 4.52424E − 05 |
| 9515 | STXBP5L | Syntaxin binding protein 5-like | 0.360338998 | 8.58457E − 05 |
The set of 215 DEGs in LCM-captured frontal cortex neurons of AD satisfying P value <0.0001 by two-tailed t-test and fold change greater than 2 or smaller than 0.5 were extracted by microarray data analysis of GSE5281. Among them, the set of 15 genes corresponding to the core set of 522 DEGs identified by RNA-Seq data analysis of SRA060572 are listed with Entrez Gene ID, gene symbol, gene name, fold change, and P value. NeuroD6 is italicized. The complete set of 215 DEGs are shown in Supplementary Table 3.
Next, we attempted to answer the question whether downregulation of NeuroD6 serves as a possible biomarker for diagnosis of AD by brain transcriptome profiling, regardless of differences in brain regions, microarray platforms, or ethnicities of samples. We analyzed two more datasets of transcriptome of postmortem hippocampal tissues isolated from Caucasian (GSE1297 on Human Genome U133A Array) or Japanese (GSE36980 on Human Gene 1.0 ST Array) AD patients. From the GSE1297 dataset, we identified the set of 131 DEGs downregulated in the hippocampal CA1 region at fold change of severe AD versus NC < 0.6 (Supplementary Table 4). We found that 25 genes of 131 DEGs correspond to the core set of 522 DEGs of RNA-Seq (Table 3). They also included NeuroD6 whose expression levels are reduced in Caucasian AD brains during progression of AD at fold change = 0.569 for the comparison between severe AD and NC (P = 0.0072, Table 3, italicized). From the GSE11829 dataset, we identified the set of 31 DEGs downregulated in the region-unrestricted hippocampus of Japanese AD patients, compared with non-AD controls (Supplementary Table 5). We found that 12 genes of 31 DEGs correspond to the core set of 522 DEGs of RNA-Seq (Table 4). Again, they included NeuroD6 whose expression levels are reduced in AD brains at fold change = 0.433 for the comparison between AD and non-AD (P = 0.0016, Table 4, italicized). Taken together, these observations suggest that downregulation of NeuroD6 serves as a fairly universal biomarker for diagnosis of AD by brain transcriptome profiling, regardless of differences in brain regions, microarray platforms, or ethnicities of samples.
Table 3.
The set of 25 DEGs downregulated in the hippocampal CA1 region during progression of AD identified by microarray data analysis of GSE1297 corresponding to RNA-Seq data analysis of SRA060752.
| Entrez Gene ID |
Gene symbol | Gene name | Fold change (severe AD versus NC) |
P value |
|---|---|---|---|---|
| 57172 | CAMK1G | Calcium/calmodulin-dependent protein kinase IG | 0.119791216 | 0.00015044 |
| 7447 | VSNL1 | Visinin-like 1 | 0.176659556 | 0.003811085 |
| 10368 | CACNG3 | Calcium channel, voltage-dependent, gamma subunit 3 | 0.212906942 | 0.000645324 |
| 55711 | FAR2 | Fatty acyl CoA reductase 2 | 0.230090829 | 0.00066547 |
| 10769 | PLK2 | Polo-like kinase 2 | 0.264329482 | 0.001588852 |
| 9331 | B4GALT6 | UDP-Gal: betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 6 | 0.284246871 | 0.005450676 |
| 55312 | RFK | Riboflavin kinase | 0.326351561 | 0.000250327 |
| 5274 | SERPINI1 | Serpin peptidase inhibitor, clade I (neuroserpin), member 1 | 0.337526915 | 0.006332563 |
| 9079 | LDB2 | LIM domain binding 2 | 0.340999225 | 0.003326867 |
| 1268 | CNR1 | Cannabinoid receptor 1 (brain) | 0.344653589 | 0.008057005 |
| 5579 | PRKCB | Protein kinase C, beta | 0.345004889 | 0.002186833 |
| 63982 | ANO3 | Anoctamin 3 | 0.372084936 | 0.008600399 |
| 81831 | NETO2 | Neuropilin (NRP) and tolloid- (TLL-) like 2 | 0.393977566 | 0.000965658 |
| 440270 | GOLGA8B | Golgin A8 family, member B | 0.422538873 | 0.004711934 |
| 23236 | PLCB1 | Phospholipase C, beta 1 (phosphoinositide-specific) | 0.43589904 | 0.001873881 |
| 27324 | TOX3 | TOX high mobility group box family member 3 | 0.450425735 | 0.002724595 |
| 6000 | RGS7 | Regulator of G-protein signaling 7 | 0.451971533 | 0.006045425 |
| 138046 | RALYL | RALY RNA binding protein-like | 0.453463038 | 0.001029166 |
| 5530 | PPP3CA | Protein phosphatase 3, catalytic subunit, alpha isozyme | 0.461727097 | 0.001385837 |
| 1020 | CDK5 | Cyclin-dependent kinase 5 | 0.464742579 | 0.00340998 |
| 3751 | KCND2 | Potassium voltage-gated channel, Shal-related subfamily, member 2 | 0.489787298 | 0.004105453 |
| 29114 | TAGLN3 | Transgelin 3 | 0.536481218 | 0.00215522 |
| 7534 | YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | 0.556167619 | 0.006101886 |
| 63974 | NeuroD6 | Neurogenic differentiation 6 | 0.568549476 | 0.007199422 |
| 2744 | GLS | Glutaminase | 0.591660135 | 0.007897416 |
The set of 131 DEGs downregulated in the hippocampal CA1 region among incipient, moderate, and severe AD and NC groups by one-way ANOVA satisfying P value <0.01 and fold change of severe AD versus NC smaller than 0.6 were extracted by microarray data analysis of GSE1297. Among them, the set of 25 genes corresponding to the core set of 522 DEGs identified by RNA-Seq data analysis of SRA060572 are listed with Entrez Gene ID, gene symbol, gene name, fold change, and P value. NeuroD6 is italicized. The complete list of 131 DEGs are shown in Supplementary Table 4.
Table 4.
The set of 12 DEGs downregulated in the hippocampus of Japanese AD patients identified by microarray data analysis of GSE36980 corresponding to RNA-Seq data analysis of SRA060752.
| Entrez Gene ID |
Gene symbol | Gene name | Fold change (AD versus non-AD) |
P value |
|---|---|---|---|---|
| 63974 | NeuroD6 | Neurogenic differentiation 6 | 0.433171474 | 0.001616741 |
| 10368 | CACNG3 | Calcium channel, voltage-dependent, gamma subunit 3 | 0.496685808 | 0.004499281 |
| 5176 | SERPINF1 | Serpin peptidase inhibitor, clade F (alpha-2 antiplasmin, pigment epithelium derived factor), member 1 | 0.522226034 | 0.00019849 |
| 348980 | HCN1 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 1 | 0.523810594 | 0.004508668 |
| 5774 | PTPN3 | Protein tyrosine phosphatase, nonreceptor type 3 | 0.54910964 | 0.001537752 |
| 8507 | ENC1 | Ectodermal-neural cortex (with BTB-like domain) | 0.559652363 | 0.003288053 |
| 266722 | HS6ST3 | Heparan sulfate 6-O-sulfotransferase 3 | 0.560440342 | 0.001753288 |
| 2903 | GRIN2A | Glutamate receptor, ionotropic, N-methyl D-aspartate 2A | 0.581203611 | 0.002390123 |
| 51299 | NRN1 | Neuritin 1 | 0.590013553 | 0.001828335 |
| 125113 | KRT222 | Keratin 222 pseudogene | 0.592296133 | 0.004941811 |
| 1428 | CRYM | Crystallin, mu | 0.594516424 | 0.002824762 |
| 221692 | PHACTR1 | Phosphatase and actin regulator 1 | 0.597310446 | 0.002464967 |
The set of 31 DEGs downregulated in the hippocampus of Japanese AD patients satisfying P value <0.005 by two-tailed t-test and fold change smaller than 0.6 were extracted by microarray data analysis of GSE36980. Among them, the set of 12 genes corresponding to the core set of 522 DEGs identified by RNA-Seq data analysis of SRA060572 are listed with Entrez Gene ID, gene symbol, gene name, fold change, and P value. NeuroD6 is italicized. The complete list of 31 DEGs are shown in Supplementary Table 5.
4. Discussion
Previously, a number of microarray-based transcriptome studies of AD brains failed to identify the set of consistently deregulated genes across different studies [8]. RNA-Seq serves as an innovative technology for the comprehensive transcriptome profiling on a genome scale in a high-throughput and quantitative manner [14, 15]. To identify biomarker genes relevant to the molecular pathogenesis of AD, we first studied publicly available RNA-Seq datasets of AD brain transcriptome derived from two independent cohorts named EMU and UKY. We identified the core set of 522 DEGs consistently deregulated in AD brains of both cohorts. They include 470 downregulated and 52 upregulated genes in AD brains, relevant to synaptic transmission, neuroactive ligand-receptor interaction, nervous system development, and pathological processes of neuropsychiatric diseases by GO and pathway analysis. Then, we compared the results of RNA-Seq data analysis with those of three distinct microarray datasets of AD brains, which are different in brain regions, ethnicities, and microarray platforms. As a result, we identified consistent downregulation of NeuroD6 in AD brains throughout the datasets studied.
The NeuroD family of bHLH transcription factors, composed of three major members, such as NeuroD1 (BETA2), NeuroD2 (NDRF), and NeuroD6 (NEX1, MATH2, and ATOH2), acts as a differentiation factor for neural precursor cells in the developing central nervous system (CNS) [23]. Each member exhibits an overlapping but distinct spatiotemporal expression profile with partially redundant function in the formation of subpopulations of neurons. A previous study by in situ hybridization showed that NeuroD6 is expressed abundantly in mature adult neurons of the cerebral cortex, the hippocampus, and the cerebellum [25]. Although NeuroD6-deficient mice exhibit no obvious defect in development, NeuroD1/NeuroD6 double knockout mice show arrest of terminal differentiation of granule cells in the hippocampus [26]. NeuroD6-expressing progenitor cells located in the subventricular zone have a capacity to differentiate into pyramidal glutamatergic neurons in upper cortical layers [27]. Both NeuroD2 and NeuroD6 regulate axonal fasciculation and proper formation of callosal fiber tracts [28]. NeuroD6 plays a key role in cell fate decision of subtypes of amacrine cells in the retina [29]. Constitutive expression of NeuroD6 triggers neuronal differentiation of PC12 cells, originated from a pheochromocytoma of the rat adrenal medulla, without requirement of nerve growth factor (NGF) [30]. NeuroD6 plays a decisive role in the switch from proapoptotic to antiapoptotic pathways during neuronal differentiation of PC12 cells [31]. Furthermore, NeuroD6 confers tolerance to oxidative stress by inducing antioxidant responses and by increasing the mitochondrial biomass [32]. Importantly, NeuroD6, by forming a coexpression network module with TBR1, FEZF2, FOXG1, SATB2, and EMX1, plays a key role in development of the human neocortex and hippocampus projection neurons that are severely degenerated in AD brains [33]. All of these observations suggest that NeuroD6 acts as a key regulator of neuronal development, differentiation, and survival.
Previous studies identified LPPR4 and growth associated protein 43 (GAP43) as direct target genes for NeuroD6 by binding assay to E-boxes located in target gene promoters [24, 34]. Importantly, we found that the core set of 522 DEGs of RNA-Seq include LPPR as one of the downregulated genes in AD brains of both cohorts, and the study also identified GAP43 as a downregulated gene in AD brains of the UKY cohort (not shown). Although the precise biological role of NeuroD6 and its target genes in adult human brains remains unknown, the present observations suggest that downregulation of NeuroD6 might be detrimental for neuronal survival under stressful conditions caused by extensive accumulation of extracellular Aβ and intracellular NFT in AD.
In conclusion, the present study using bioinformatics data mining approach suggested that downregulation of NeuroD6 serves as a possible biomarker for diagnosis of AD by brain transcriptome profiling. Since the sample sizes we studied are apparently small, these findings warrant further validation in larger cohorts of AD patients and adequate controls performed in a blinded manner.
Supplementary Material
Supplementary Figure 1: ROC analysis of microarray data GSE5281 by using NeuroD6 as a classifier. Transcriptome of LCM-captured frontal cortex neurons of age-matched AD patients and NC subjects was studied on a Human Genome U133 Plus 2.0 Array. The data normalized by MAS5 were transformed to Log2 values. ROC analysis was performed by importing the expression levels of NeuroD6 (panel a) or NeuroD1 (panel b) into SPSS in the setting of the group discrimination number 0 for AD and 1 for NC. The area under the ROC curve (AUC) is 0.893 for NeuroD6 and 0.474 for NeuroD1.
Supplementary Table 1: The core set of 522 DEGs in the frontal cortex of AD overlapping between two cohorts identified by RNA-Seq data analysis of SRA060752.
Supplementary Table 2: The set of 60 differentially spliced genes in the frontal cortex of AD identified by RNA-Seq data analysis of SRA060752.
Supplementary Table 3: The set of 215 DEGs in cortical neurons of the superior frontal gyrus of AD identified by microarray data analysis of GSE5281.
Supplementary Table 4: The set of 131 DEGs downregulated in the hippocampal CA1 region during progression of AD identified by microarray data analysis of GSE1297.
Supplementary Table 5: The set of 31 DEGs downregulated in the hippocampus of Japanese AD patients identified by microarray data analysis of GSE36980.
Acknowledgments
The present study was supported by the JSPS KAKENHI (C22500322 and C25430054), the Dementia Drug Development Research Center (DRC) Project, the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and the grant from the National Center for Geriatrics and Gerontology (NCGC26-20).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Gatz M., Reynolds C. A., Fratiglioni L., Johansson B., Mortimer J. A., Berg S., Fiske A., Pedersen N. L. Role of genes and environments for explaining Alzheimer disease. Archives of General Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 2.Walton J. R. Aluminum's involvement in the progression of alzheimer's disease. Journal of Alzheimer's Disease. 2013;35(1):7–43. doi: 10.3233/JAD-121909. [DOI] [PubMed] [Google Scholar]
- 3.Squitti R., Siotto M., Polimanti R. Low-copper diet as a preventive strategy for Alzheimer's disease. Neurobiology of Aging. 2014;35(2):S40–S50. doi: 10.1016/j.neurobiolaging.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal S. L., Kamboth M. I. Late-onset Alzheimer’s disease genes and the potentially implicated pathways. Current Genetic Medicine Reports. 2014;2:85–101. doi: 10.1007/s40142-014-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson T., Atwal J. K., Steinberg S., Snaedal J., Jonsson P. V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., Hoyte K., Gustafson A., Liu Y., Lu Y., Bhangale T., Graham R. R., Huttenlocher J., Bjornsdottir G., Andreassen O. A., Jönsson E. G., Palotie A., Behrens T. W., Magnusson O. T., Kong A., Thorsteinsdottir U., Watts R. J., Stefansson K. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;487(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 6.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J. S. K., Younkin S., Hazrati L., Collinge J., Pocock J., Lashley T., Williams J., Lambert J.-C., Amouyel P., Goate A., Rademakers R., Morgan K., Powell J., George-Hyslop P. S., Singleton A., Hardy J. TREM2 variants in Alzheimer's disease. The New England Journal of Medicine. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruchaga C., Karch C. M., Jin S. C., et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer's disease. Nature. 2014;505(7484):550–554. doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper-Knock J., Kirby J., Ferraiuolo L., Heath P. R., Rattray M., Shaw P. J. Gene expression profiling in human neurodegenerative disease. Nature Reviews Neurology. 2012;8(9):518–530. doi: 10.1038/nrneurol.2012.156. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg S. D., Hemby S. E., Lee V. M., Eberwine J. H., Trojanowski J. Q. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Annals of Neurology. 2000;48(1):77–87. [PubMed] [Google Scholar]
- 10.Colangelo V., Schurr J., Ball M. J., Pelaez R. P., Bazan N. G., Lukiw W. J. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. Journal of Neuroscience Research. 2002;70(3):462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 11.Katsel P., Li C., Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer's disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer's disease? Neurochemical Research. 2007;32(4-5):845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- 12.Williams C., Shai R. M., Wu Y., Hsu Y.-H., Sitzer T., Spann B., McCleary C., Mo Y., Miller C. A. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer's disease. PLoS ONE. 2009;4(3) doi: 10.1371/journal.pone.0004936.e4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J. J., Hsueh H.-M., Delongchamp R. R., Lin C.-J., Tsai C.-A. Reproducibility of microarray data: a further analysis of microarray quality control (MAQC) data. BMC Bioinformatics. 2007;8, article 412 doi: 10.1186/1471-2105-8-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland G. T., Janitz M., Kril J. J. Understanding the pathogenesis of Alzheimer's disease: will RNA-Seq realize the promise of transcriptomics? Journal of Neurochemistry. 2011;116(6):937–946. doi: 10.1111/j.1471-4159.2010.07157.x. [DOI] [PubMed] [Google Scholar]
- 16.Bai B., Hales C. M., Chen P.-C., Gozal Y., Dammer E. B., Fritz J. J., Wang X., Xia Q., Duong D. M., Street C., Cantero G., Cheng D., Jones D. R., Wu Z., Li Y., Diner I., Heilman C. J., Rees H. D., Wu H., Lin L., Szulwach K. E., Gearing M., Mufson E. J., Bennett D. A., Montine T. J., Seyfried N. T., Wingo T. S., Sun Y. E., Jin P., Hanfelt J., Willcock D. M., Levey A., Lah J. J., Peng J. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41):16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathologica. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blalock E. M., Geddes J. W., Chen K. C., Porter N. M., Markesbery W. R., Landfield P. W. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W. S., Reiman E. M., Valla J., Dunckley T., Beach T. G., Grover A., Niedzielko T. L., Schneider L. E., Mastroeni D., Caselli R., Kukull W., Morris J. C., Hulette C. M., Schmechel D., Rogers J., Stephan D. A. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hokama M., Oka S., Leon J., et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cerebral Cortex. 2014;24(9):2476–2488. doi: 10.1093/cercor/bht101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang D. W., Sherman B. T., Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Research. 2014;42(1):D199–D205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwab M. H., Druffel-Augustin S., Gass P., et al. Neuronal basic helix-loop-helix proteins (NEX, neuroD, NDRF): spatiotemporal expression and targeted disruption of the NEX gene in transgenic mice. The Journal of Neuroscience. 1998;18(4):1408–1418. doi: 10.1523/JNEUROSCI.18-04-01408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada M., Shida Y., Takahashi K., Tanioka T., Nakano Y., Tobe T., Yamada M. Prg1 is regulated by the basic helix-loop-helix transcription factor Math2. Journal of Neurochemistry. 2008;106(6):2375–2384. doi: 10.1111/j.1471-4159.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomä A., Nave K.-A. NEX-1: a novel brain-specific helix-loop-helix protein with autoregulation and sustained expression in mature cortical neurons. Mechanisms of Development. 1994;48(3):217–228. doi: 10.1016/0925-4773(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwab M. H., Bartholomae A., Heimrich B., Feldmeyer D., Druffel-Augustin S., Goebbels S., Naya F. J., Zhao S., Frotscher M., Tsai M.-J., Nave K.-A. Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. Journal of Neuroscience. 2000;20(10):3714–3724. doi: 10.1523/JNEUROSCI.20-10-03714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S.-X., Goebbels S., Nakamura K., Kometani K., Minato N., Kaneko T., Nave K.-A., Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bormuth I., Yan K., Yonemasu T., Gummert M., Zhang M., Wichert S., Grishina O., Pieper A., Zhang W., Goebbels S., Tarabykin V., Nave K.-A., Schwab M. H. Neuronal basic helix-loop-helix proteins neurod2/6 regulate cortical commissure formation before midline interactions. The Journal of Neuroscience. 2013;33(2):641–651. doi: 10.1523/JNEUROSCI.0899-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay J. N., Voinescu P. E., Chu M. W., Sanes J. R. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nature Neuroscience. 2011;14(8):965–972. doi: 10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uittenbogaard M., Chiaramello A. Constitutive overexpression of the basic helix-loop-helix Nex1/MATH-2 transcription factor promotes neuronal differentiation of PC12 cells and neurite regeneration. Journal of Neuroscience Research. 2002;67(2):235–245. doi: 10.1002/jnr.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uittenbogaard M., Chiaramello A. The basic helix-loop-helix transcription factor Nex-1/Math-2 promotes neuronal survival of PC12 cells by modulating the dynamic expression of anti-apoptotic and cell cycle regulators. Journal of Neurochemistry. 2005;92(3):585–596. doi: 10.1111/j.1471-4159.2004.02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uittenbogaard M., Baxter K. K., Chiaramello A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 confers tolerance to oxidative stress by triggering an antioxidant response and sustaining the mitochondrial biomass. ASN Neuro. 2010;2(2) doi: 10.1042/AN20100005.e00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang H. J., Kawasawa Y. I., Cheng F., Zhu Y., Xu X., Li M., Sousa A. M. M., Pletikos M., Meyer K. A., Sedmak G., Guennel T., Shin Y., Johnson M. B., Krsnik Ž., Mayer S., Fertuzinhos S., Umlauf S., Lisgo S. N., Vortmeyer A., Weinberger D. R., Mane S., Hyde T. M., Huttner A., Reimers M., Kleinman J. E., Šestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uittenbogaard M., Martinka D. L., Chiaramello A. The basic helix-loop-helix differentiation factor Nex1/MATH-2 functions as a key activator of the GAP-43 gene. Journal of Neurochemistry. 2003;84(4):678–688. doi: 10.1046/j.1471-4159.2003.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: ROC analysis of microarray data GSE5281 by using NeuroD6 as a classifier. Transcriptome of LCM-captured frontal cortex neurons of age-matched AD patients and NC subjects was studied on a Human Genome U133 Plus 2.0 Array. The data normalized by MAS5 were transformed to Log2 values. ROC analysis was performed by importing the expression levels of NeuroD6 (panel a) or NeuroD1 (panel b) into SPSS in the setting of the group discrimination number 0 for AD and 1 for NC. The area under the ROC curve (AUC) is 0.893 for NeuroD6 and 0.474 for NeuroD1.
Supplementary Table 1: The core set of 522 DEGs in the frontal cortex of AD overlapping between two cohorts identified by RNA-Seq data analysis of SRA060752.
Supplementary Table 2: The set of 60 differentially spliced genes in the frontal cortex of AD identified by RNA-Seq data analysis of SRA060752.
Supplementary Table 3: The set of 215 DEGs in cortical neurons of the superior frontal gyrus of AD identified by microarray data analysis of GSE5281.
Supplementary Table 4: The set of 131 DEGs downregulated in the hippocampal CA1 region during progression of AD identified by microarray data analysis of GSE1297.
Supplementary Table 5: The set of 31 DEGs downregulated in the hippocampus of Japanese AD patients identified by microarray data analysis of GSE36980.