Abstract
Tomato plants overexpressing a prosystemin gene that encodes the precursor of a mobile wound signal called systemin have been shown previously to constitutively synthesize extraordinarily high levels of two defensive proteinase inhibitor proteins in leaves in the absence of wounding. We herein report that leaves of these transgenic plants possess enhanced levels of another defensive protein, polyphenol oxidase (PPO) at levels that are up to 70-fold higher than levels found in leaves of wild-type plants. Supplying young wild-type tomato plants with systemin through cut stems induced PPO activity in leaves, and wounding lower leaves of young tomato plants induced PPO activity in both wounded and unwounded leaves to levels equal to those induced by systemin. Exposing young tomato plants to methyl jasmonate vapor caused an increase in PPO activity equivalent to levels found in plants overexpressing the prosystemin gene. The data indicate that PPO and proteinase inhibitor genes are coactivated systemically by wounding via the octadecanoid signal transduction pathway and that systemin has a much broader role in signaling plant defensive genes than was previously known.
Full text
PDF

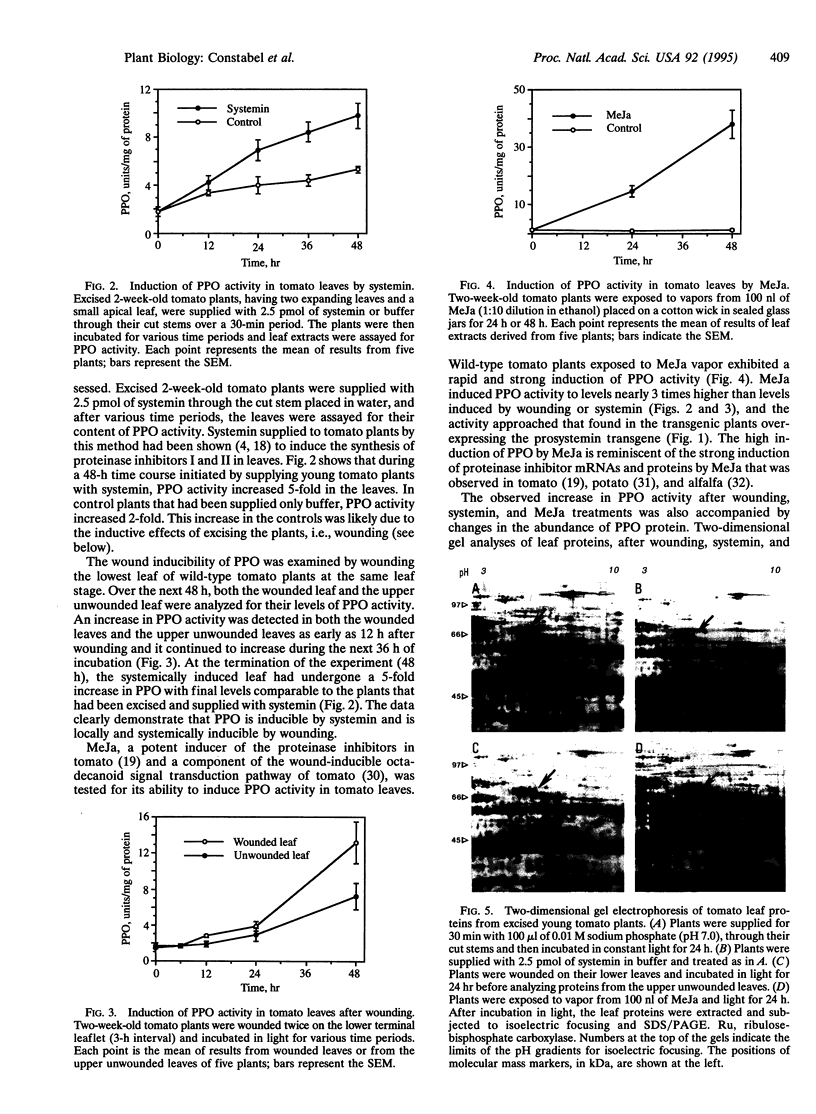


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Makus D. J., Pearce G., Ryan C. A. Proteinase inhibitor-inducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell walls. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3536–3540. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolter C. J. Methyl Jasmonate Induces Papain Inhibitor(s) in Tomato Leaves. Plant Physiol. 1993 Dec;103(4):1347–1353. doi: 10.1104/pp.103.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schwarzl E., Hayn M. A rapid assay for catechol oxidase and lactase using 2-nitro-5-thiobenzoic acid. Anal Biochem. 1977 Feb;77(2):486–494. doi: 10.1016/0003-2697(77)90262-7. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Johnson R. R., Ryan C. A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic Acid. Plant Physiol. 1992 Mar;98(3):995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Hildmann T., Ebneth M., Peña-Cortés H., Sánchez-Serrano J. J., Willmitzer L., Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992 Sep;4(9):1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Narvaez J., An G., Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski S. P., Eannetta N. T., Hirzel A. T., Steffens J. C. Purification and Characterization of Polyphenol Oxidase from Glandular Trichomes of Solanum berthaultii. Plant Physiol. 1992 Oct;100(2):677–684. doi: 10.1104/pp.100.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGurl B., Orozco-Cardenas M., Pearce G., Ryan C. A. Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9799–9802. doi: 10.1073/pnas.91.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B., Pearce G., Orozco-Cardenas M., Ryan C. A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992 Mar 20;255(5051):1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Narvaez-Vasquez J., Orozco-Cardenas M. L., Ryan C. A. A Sulfhydryl Reagent Modulates Systemic Signaling for Wound-Induced and Systemin-Induced Proteinase Inhibitor Synthesis. Plant Physiol. 1994 Jun;105(2):725–730. doi: 10.1104/pp.105.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. M., Eannetta N. T., Yu H., Prince J. P., de Vicente M. C., Tanksley S. D., Steffens J. C. Organisation of the tomato polyphenol oxidase gene family. Plant Mol Biol. 1993 Mar;21(6):1035–1051. doi: 10.1007/BF00023601. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas M., McGurl B., Ryan C. A. Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8273–8276. doi: 10.1073/pnas.90.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot V., Holzer F. M., Reisch B., Walling L. L. Leucine aminopeptidase: an inducible component of the defense response in Lycopersicon esculentum (tomato). Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9906–9910. doi: 10.1073/pnas.90.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Johnson S., Ryan C. A. Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem. 1993 Jan 5;268(1):212–216. [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Proteinase inhibitor synthesis in tomato leaves : induction by chitosan oligomers and chemically modified chitosan and chitin. Plant Physiol. 1984 Nov;76(3):787–790. doi: 10.1104/pp.76.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kowalski S. P., Steffens J. C. Comparison of polyphenol oxidase expression in glandular trichomes of solanum and lycopersicon species. Plant Physiol. 1992 Dec;100(4):1885–1890. doi: 10.1104/pp.100.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]





