Abstract
Persistent spiking in response to a discrete stimulus is considered to reflect the active maintenance of a memory for that stimulus until a behavioral response is made. This response pattern has been reported in learning paradigms that impose a temporal gap between stimulus presentation and behavioral response, including trace eyeblink conditioning. However, it is unknown whether persistent responses are acquired as a function of learning or simply represent an already existing category of response type. This fundamental question was addressed by recording single-unit activity in the medial prefrontal cortex (mPFC) of rabbits during the initial learning phase of trace eyeblink conditioning. Persistent responses to the tone conditioned stimulus were observed in the mPFC during the very first training sessions. Further analysis revealed that most cells with persistent responses showed this pattern during the very first training trial, before animals had experienced paired training. However, persistent cells showed reliable decreases in response magnitude over the first training session, which were not observed on the second day of training or for sessions in which learning criterion was met. This modification of response magnitude was specific to persistent responses and was not observed for cells showing phasic tone-evoked responses. The data suggest that persistent responses to discrete stimuli do not require learning but that the ongoing robustness of such responses over the course of training is modified as a result of experience. Putative mechanisms for this modification are discussed, including changes in cellular or network properties, neuromodulatory tone, and/or the synaptic efficacy of tone-associated inputs.
Keywords: delay cells, persistent activity, working memory
persistent neural responses, in which evoked spiking persists beyond stimulus offset, have been observed in the medial prefrontal cortex (mPFC) during tasks that impose a delay interval between stimulus presentation and behavioral response (Baeg et al. 2001; Burgos-Robles et al. 2009; Fritz et al. 2010; Funahashi et al. 1989; Fuster 1973; Fuster and Alexander 1971; Gilmartin and McEchron 2005; Gilmartin et al. 2013; Hattori et al. 2014; Kubota and Niki 1971; Siegel et al. 2012). Persistent responses have been observed across stimulus modalities, including auditory (e.g., Baeg et al. 2001; Gilmartin and McEchron 2005; Siegel et al. 2012), visual (e.g., Funahashi et al. 1989; Fuster 1973; Miller et al. 1996; Procyk and Goldman-Rakic 2006), and somatosensory (whisker stimulation; Hattori et al. 2013). Such responses are considered to reflect the maintenance of a “memory trace” of that stimulus until a behavioral response can be made (Funahashi et al. 1989; Fuster 1973; Goldman-Rakic 1995). Although several cellular and network mechanisms have been proposed to modulate persistent spiking (Dembrow et al. 2010; Fransen et al. 2006; Kalmbach et al. 2013; McCormick et al. 2003; Wang 2003), the fundamental question of whether such responses are acquired as a result of experience in vivo has not been directly addressed. Only a scarce number of studies have recorded single-neuron activity during the acquisition of tasks thought to rely on persistent responses (Erickson and Desimone 1999; Gilmartin and McEchron 2005; Hattori et al. 2014; Miyashita 1988). These authors suggest that persistent responses may not be acquired per se based on session averages or pseudoconditioning. However, these studies did not analyze responses during the first presentation of stimuli for persistent spiking as a pivotal test of this hypothesis, nor did they examine the trial-by-trial development of such responses before the expression of learning.
Recently, persistent responses have been reported in the mPFC during trace eyeblink conditioned responses (CRs), which require 200–500+ training trials to acquire (Hattori et al. 2014; Siegel et al. 2012; Siegel and Mauk 2013; Takehara-Nishiuchi and McNaughton 2008), providing a window of opportunity to examine the possible evolution of persistent responses early in learning. Trace conditioning consists of paired presentations of an initially neutral conditioned stimulus (CS; such as a tone or light) followed by a stimulus-free trace interval and terminating with a reflexive unconditioned stimulus (US; such as a puff of air to the eye; Fig. 1A, top). In rabbits, the cerebellum and mPFC are necessary to learn and express trace eyeblink CRs (Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Siegel and Mauk 2013; Solomon et al. 1986; see also Chen et al. 2014 and Takehara et al. 2003), whereas the role of the hippocampus is restricted to the acquisition phase (Kim et al. 1995; Moyer et al. 1990; Solomon et al. 1986). Because the cerebellum requires near overlap of the CS and US to support learning (Chen et al. 2014; Kalmbach et al. 2010), the hypothesized role of the mPFC in trace conditioning is to provide a CS-evoked input to the cerebellum (via the pons) that persists across the stimulus-free trace interval to overlap with the US (Fig. 1A, bottom; Chen et al. 2014; Kalmbach et al. 2009; Siegel et al. 2012; Weiss and Disterhoft 2011). To directly address whether persistent mPFC responses are acquired as a function of learning, single-unit activity was recorded in the mPFC of rabbits during trace eyeblink conditioning with an emphasis on examining the earliest phases of learning. The data show that persistent responses can be observed before paired training and therefore are not necessarily acquired as a result of experience or learning. However, substantial modification of persistent responses was observed during the earliest stages of learning, which may reflect either intrinsic or network plasticity mechanisms resulting in the development of stronger and more robust persistent responses as a function of training or experience.
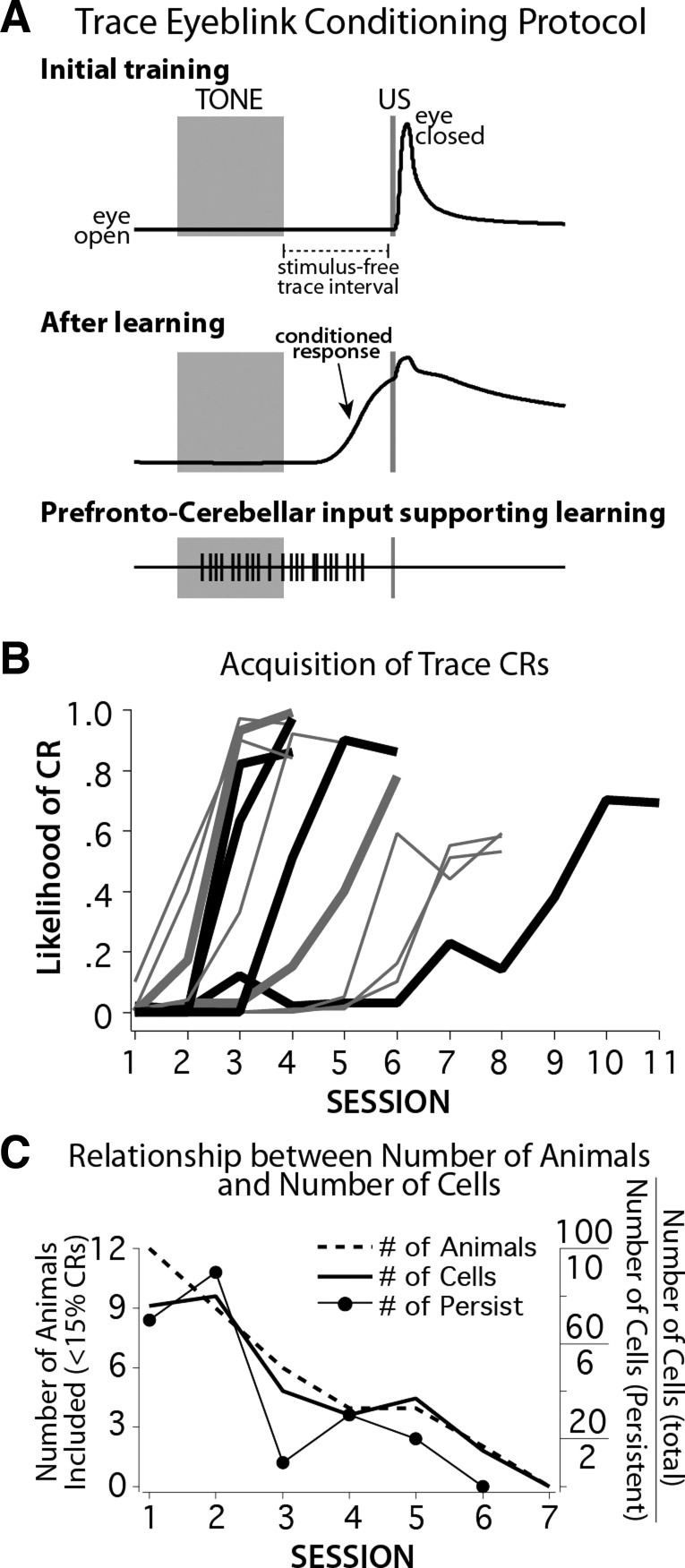
Fig. 1.
A: schematic representation of the trace eyeblink conditioning protocol. Each training trial began with the presentation of a 500-ms tone (conditioned stimulus, CS; wide gray bar) followed by a 500-ms stimulus-free trace interval and terminated with a 50-ms periorbital stimulation (unconditioned stimulus, US; narrow gray bar). Early in training, rabbits responded only with a reflexive eyelid closure to the US (top). With continued training, rabbits learned to close the eyelid in response to the tone and in anticipation of the US (middle). It has been shown that the cerebellum requires an input that nearly bridges the stimulus-free interval to support learning. Such response patterns have recently been observed in the medial prefrontal cortex (mPFC) of trained rabbits (see text). B: conditioned response (CR) likelihood over sessions during learning for 12 rabbits. Heavy lines show animals with persistent responses recorded in the mPFC on the first day of training (ACQ1; heavy gray and black lines), some of which were also observed on the second day (ACQ2; heavy black lines). C: analysis was limited to sessions in which rabbits were still learning, before expression of CRs. Graph shows the relationship between the number of rabbits that had not learned and so could be included in analyses on each day, the total number of cells included in analysis from those rabbits, and the number of persistent responses observed from included cells.
MATERIALS AND METHODS
Subjects and surgical procedures.
All surgical and experimental procedures were approved by The University of Texas at Austin Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health guidelines. Twelve New Zealand albino rabbits (males, 2.5–4 kg) were implanted with a custom-built microdrive housing 18 independently moveable tetrodes targeting the caudal anterior cingulate/medial agranular (ACc/AGm) regions of mPFC, shown previously to specifically play a role in the acquisition (Hattori et al. 2014; Kronforst-Collins and Disterhoft 1998; McLaughlin et al. 2002) and expression (Kalmbach et al. 2009; Powell et al. 2001) of trace eyeblink conditioning. To prepare for sterile surgery, each rabbit was given a subcutaneous injection of ketamine (45 mg/kg) and acepromazine (1.5 mg/kg) and mounted in a specialized stereotaxic apparatus (with lambda 1.5 mm below bregma). Isoflurane gas (1–3% in medical grade oxygen) was used to maintain surgical depth anesthesia levels. Microdrive tetrode bundles (1.5–2.5 mm in diameter) were positioned on the brain's surface over the right posterior mPFC (centered at 3.0 mm anterior to bregma and 1.0–1.5 mm lateral from the midline) and secured to the skull with screws and dental cement. Each rabbit was also prepared with a head bolt fixed in dental cement over the anterior skull to hold the eyeblink detector during conditioning. Finally, two stainless steel stimulating electrodes were implanted subdurally just anterior and posterior to the upper eyelid contralateral to the microdrive. Each rabbit was allowed at least 1 wk of recovery before training began.
Behavioral training and analysis.
Training involved a standard trace eyeblink conditioning protocol as previously described (Siegel et al. 2012). Rabbits were gently restrained using standard procedures and placed in a shielded, sound-attenuating chamber (Gormezano et al. 1966). For restraint, rabbits were placed in a custom Plexiglas container designed to minimize body movement with the head accessible from the outside of the device. Taping the ears back is sufficient to secure the head, and rabbits readily tolerate the restraint after a day or two of acclimation. Daily training sessions were controlled by custom software and consisted of 12 blocks of 9 trials for a total of 108 trials per session, with the first trial of every block being a CS-only probe. Trials were presented at random intertrial intervals drawn from a flat distribution between 25 and 35 s. The CS consisted of a 500-ms tone (1.3-kHz pure tone with rise and fall times of 5 ms to avoid audible onset and offset transients). The US consisted of a 50-ms train of current pulses (1-ms pulse width at 100 Hz) delivered across the periorbital electrodes. The US intensity was carefully adjusted for each rabbit to just above threshold to elicit a full eyeblink closure (between 1 and 3 mA). On paired training trials the CS was followed by a 500-ms stimulus-free period (the trace interval), after which the US was presented (Fig. 1A). For each trial, the position of the external eyelid was measured for 2.5 s, beginning 200 ms before CS onset, using an infrared emitter and collector assembly connected to the head bolt such that it maintained a constant position relative to the eye. Closure of the eyelid resulted in an increase the amount of reflected infrared light, which was detected with an infrared detector and converted to a voltage deflection linearly related to eyelid position. The voltage output of the detector was digitized at 1 kHz and stored for offline analyses. Before each training session, the eyeblink detector was calibrated by measuring the voltage deflection produced by a full eyelid closure and defining that voltage change as 6.0 mm (the amplitude of a full eyelid closure in rabbit). A CR was defined as an eyelid response that exceeded 0.3 mm between CS and US onsets. Trials with eyelid deflections >0.3 mm in the 200 ms preceding CS onset were considered invalid and excluded from behavioral and spike analyses (never more than 5 trials/session).
Rabbits were given one training session per day. The likelihood of CRs was calculated for each training session by dividing the number of trials in which a CR was observed by the total number of valid trials for the session. Rabbits were considered to have met criterion during the session in which they displayed the first instance of eight CRs in nine consecutive trials (CRIT session). This criterion always occurred in the initial session in which total CR likelihood for the session exceeded 0.50. However, in most cases there was at least one session that preceded CRIT in which CR likelihood was between 0.15 and 0.50, which likely represents the initial expression of CRs (Fig. 1B). Because the focus of the study was on the earliest phase of learning prior to any expression of CRs, the data recorded from rabbits with CR rates >0.15 on a given day were not included in analysis for that day. On the basis of this criterion, all 12 rabbits were included for analysis for the first day of training (ACQ1) and 9 rabbits were included for the second day (ACQ2). By the third day of training, only seven of the rabbits could be included, yielding too few persistent cells for analysis (Fig. 1C). All 12 rabbits were included for analysis for the day each met learning criterion (CRIT: 5 learned on ACQ3, 1 on ACQ4, 1 on ACQ5, 2 on ACQ6, 2 on ACQ7, and 1 on ACQ10).
Single-unit recordings.
Neural activity was acquired during training sessions with a Digital Lynx system (Neuralynx). Tetrodes were constructed from polyimide-coated nichrome wire (12-μm diameter; Kanthal Palm Coast) and gold-plated to an impedance of 0.5–1.25 MΩ at 1 kHz. This range of impedance is higher than often employed for single-unit recordings from tetrodes. Although the total number of potential units is less than observed with lower impedances, impedances within this range resulted in a higher quality of unambiguously isolated single-unit activity in the mPFC (Siegel et al. 2012; Siegel and Mauk 2013). Tetrode signals were passed through a multichannel, unity-gain head stage, amplified, bandpass filtered between 600 and 6,000 Hz, and then digitized at 32 kHz for offline spike sorting and analysis. Neural data were synchronized with the presentation of training stimuli by triggering the Digital Lynx I/O port with the same TTL pulses used to trigger CS and US stimuli. For 7 rabbits, training started after 5–7 days of postsurgical recovery, which began with 2 days of acclimation to restraint and to the recording chamber. For these animals tetrodes were adjusted after daily sessions in an attempt to maximize the number of isolated clusters for the following day. However, from these animals it became apparent that neurons showing persistent responses are primarily observed in the deeper cortical layers (Siegel et al. 2012). For the last five rabbits the tetrodes were adjusted before the start of training and were not moved during learning to avoid a potential confound from slowly moving the tetrodes into the deeper layers, which could be interpreted as an increase in the incidence of persistent responses over learning. However, the proportion of cell types observed and histologically verified recording sites was not different between these 5 rabbits and the previous 7, so data from all 12 rabbits were pooled for analysis. For cells that qualified as showing a persistent response to the CS during ACQ1, half came from the first group and half came from the second group of rabbits in which tetrodes were adjusted before training began. It should be noted that for the first 7 rabbits, the goal was not to record the maximum number of cells possible during early learning, and so tetrodes were not adjusted before the start of training to maximize the number of cells observed. However, stable, well-isolated cells were obtained in these animals and so were included in analysis. Histological analysis verified that most single units were recorded in the deeper layers of mPFC for all 12 rabbits (Fig. 2).
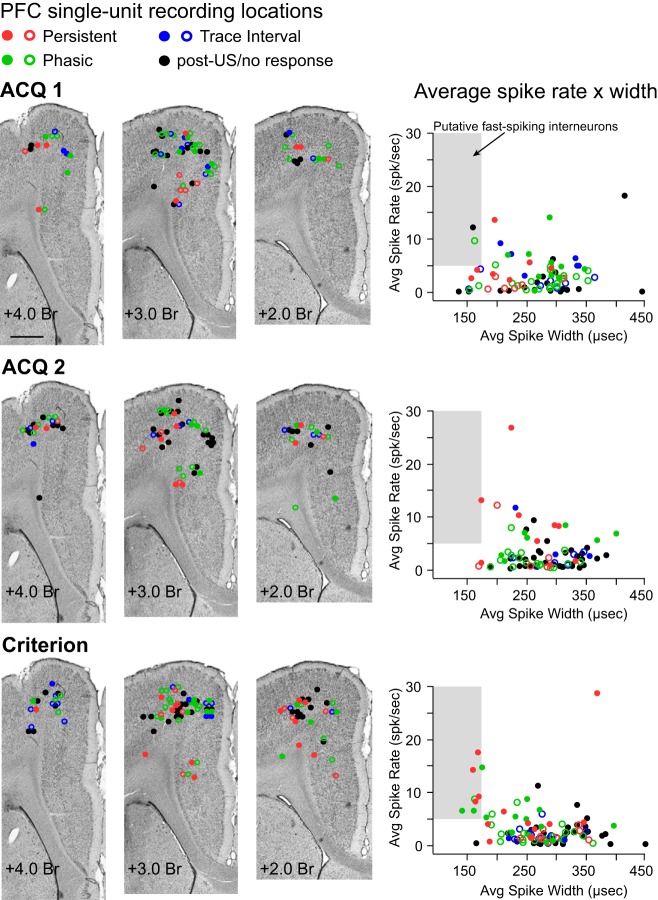
Fig. 2.
Estimated sites of single-unit activity for cells recorded on different training days (ACQ1, ACQ2, and Criterion sessions) and scatterplots of the average firing rate and spike width of each single unit to disambiguate putative pyramidal cells from fast-spiking interneurons. Colored circles represent single units that showed a given response type (red, persistent; green, phasic; blue, trace interval, black, US/posttrial/no response) and indicate whether the cell increased (filled circle) or decreased (open circle) spike activity in response to conditioning stimuli. Left: representative Nissl-stained coronal sections at 3 locations along the rostrocaudal plane of the mPFC, with markers indicating the estimated recording site of each single unit on each training day analyzed. All cells were recorded within the medial agranular or caudal anterior cingulate regions of the mPFC. Scale bar, 1 mm. Right: scatterplots of average spike width × average firing rate for each single unit, plotted separately for each training day (color-coded as described above). Fewer than 15% of cells in each condition would be designated as putative interneurons for this data set (shaded gray area).
The activity of single units was isolated offline using interactive cluster-cutting software (WinClust; Knierim JJ and Mauk MD, adapted from Wilson MA), as previously described (Siegel et al. 2012). Isolated clusters of suprathreshold neural events with common waveform parameters were identified as single units, and the time stamps of those events (spikes) were extracted and analyzed relative to the presentation of training stimuli and behavioral responses. Only single-units with clustered points that showed little or no overlap (<10%) with another cluster and with background activity were included in analysis (Siegel et al. 2008, 2012). Previous work suggests that the spike width of extracellularly recorded action potentials in conjunction with average spike rates can be used to differentiate between prefrontal pyramidal cells and fast-spiking interneurons in vivo (Dilgen et al. 2013; Tierney et al. 2004; Tseng et al. 2006). The spike width for each spike assigned to a cluster was calculated as the time between the peak and valley of the waveform. The average spike rate for each cluster of single-unit activity was calculated as the total number of spikes observed during a session divided by the length of that session (in seconds).
Histological procedures.
At the conclusion of experiments rabbits received marker lesions through a subset of tetrodes (2 mA for 15–20 s) and were euthanized 48–72 h later with an intravenous injection of Euthasol (0.3 ml/kg) and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brains were extracted and postfixed in 4% paraformaldehyde or 10% formalin for 1–4 wk before being cryoprotected in a 30% sucrose solution. The mPFC was sectioned on a freezing microtome (40–50 μm). Sections were mounted on Superfrost Plus slides (Fisher-Scientific), dried overnight, and then stained with cresyl violet to visualize tetrode tracks and surrounding anatomic structures. The approximate recording locations of single-unit activity were determined and plotted on representative images as previously described (Siegel et al. 2012; Fig. 2). In short, the identities of each tetrode track were determined on the basis of relative location within the recording bundle and further disambiguated by the subset that received marker lesions. Approximate recording locations of single-unit activity were determined by measuring the distance moved between the day of the recording and the tetrode's maximum depth, observed histologically as the tip of the tetrode track or marker lesion center. Estimated recording sites were plotted on exemplar coronal sections from the anterior-posterior axis relative to surrounding structures (such as cell layers, brain surface, corpus callosum topography, etc.).
Response categorization and raster plot analysis.
For each isolated single unit, the spikes were assigned to 100-ms time bins, beginning 1 s before CS onset and extending 3 s after US onset (Siegel et al. 2012). The mean number of spikes per bin observed for the 10 bins preceding CS onset was used as the baseline activity for that trial. The statistical reliability of changes in spike activity for each time bin (5 bins during the CS, 5 bins during the trace interval, and 30 US and posttrial bins) was determined with a paired t-test between the number of spikes observed for that bin and the baseline (with Bonferroni correction for the number of time bin comparisons, α = 0.05/40 time bins, P < 0.00125).
Single units were then categorized according to the pattern of significant changes in activity during trace conditioning trials and whether the spike activity significantly increased or decreased relative to baseline (Siegel et al. 2012). “Persistent” responses were defined as a significant increase in spike activity that began before the end of the CS and persisted at least 2 bins (200 ms) into the trace interval (e.g., Fig. 3). Note that this qualification reflects a purely functional definition that is specific to the training paradigm and length of the 500-ms stimulus-free trace interval used in the current study. Previous work used stimulation of cerebellar inputs to determine that the cerebellum can learn only if the CS stimulation terminates within 300 ms of the US (Kalmbach et al. 2009). Therefore, any input that persists to at least 300 ms within the time of the US can support learning in this task (200 ms into the trace interval for the 500-ms interval used here). If a different trace interval were to be utilized, this functional definition would need to be altered to reflect this cerebellar learning rule. However, it is important to note that the majority of persistent cells showed spike responses that persisted across the entire trace interval and sometimes for seconds post-US (Fig. 4; also see Siegel et al. 2012). “Phasic” responses to the CS began during the CS but failed to meet persistent criteria (e.g., Fig. 3). A third “trace interval” response type was defined by increased activity after CS offset and before US onset. To test for changes in activity that may have occurred over trials within a session, the binned activity of each cell was not collapsed across trials. The resulting 30 × 108 bin matrix of each cell (number of time bins × number of trials per session) was normalized by subtracting the mean number of spikes per bin in the pretrial baseline from the entire matrix. Each cell's matrix was smoothed by a single pass with a 3 × 3 median filter. The filter was applied separately to pretrial bins, to bins from CS onset to US onset, and then to posttrial bins to guard against any contamination of activity during the CS and trace intervals from activity before or after these intervals (however, the same results were obtained if no filter mask was used). The matrices of cells from the same response-type category were averaged to characterize the general profile spike responses within training sessions and across days. Statistical comparisons within sessions or between days were made using bootstrap methods applied to the individual cell matrices to account for cell-to-cell variability within response types (see below).
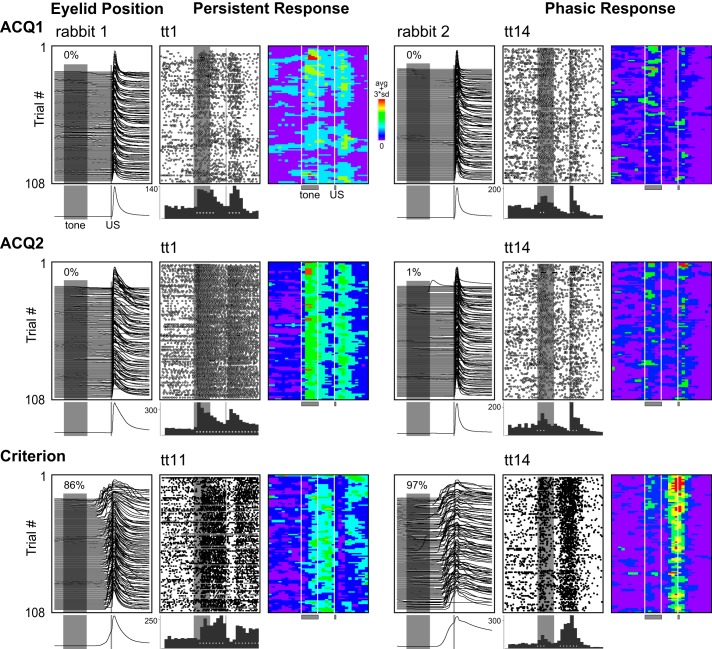
Fig. 3.
Representative examples of behavior and single-unit responses categorized as persistent or phasic on the first (ACQ1) and second (ACQ2) days of training and during sessions in which learning criterion was met (Criterion). Eyeblink responses (waterfall plots) are given for each training day, with session averages shown below. Upward deflection indicates eyelid closure (maximum = 6 mm); gray bars indicate onset and duration of the CS (500 ms) and US (50 ms), with CR likelihood indicated for each session. Upward deflection before US onset reflects learned behavior (compare ACQ1 and ACQ2 sessions with Criterion session). Raster plots represent the activity of one isolated single unit, with each row showing 1 trial (1-s baseline before and after) and dots indicating the occurrence of a single spike. Histograms below show cumulative number of spikes for 100-ms bins, with white symbols indicating time bins with significant increases in activity relative to baseline. Pseudocolored matrices indicate the binned and baseline-subtracted spike activity for each trial, which has been smoothed with a single pass of a 3 × 3-bin median filter (see materials and methods). Note the decrease in response magnitude for the example persistent cell over the session for ACQ1, which was not observed for ACQ2 or Criterion. The learning-related modification between ACQ1 and ACQ2 was typical for persistent cells.
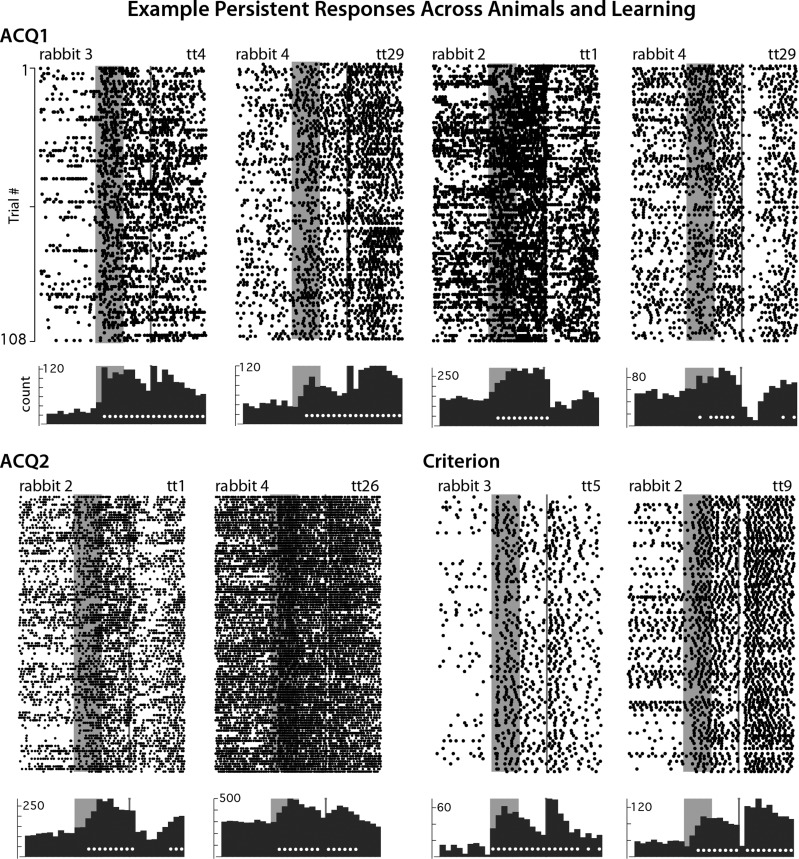
Fig. 4.
Examples of persistent increases in spike activity observed during (ACQ1 and ACQ2) and after learning (Criterion). Raster plots and histograms are as described for Fig. 3. Most persistent mPFC neurons showed reliable increases in spike activity that bridged the entire stimulus-free trace interval to overlap with the US at each phase of learning. However, during ACQ1, persistent mPFC cells showed modest but reliable decreases in response magnitude during the second half of the training session, whereas response magnitudes remained consistent over ACQ2 and Criterion sessions. Note that rabbits included for analysis in ACQ1 and ACQ2 expressed few or no CRs during these sessions (0–15% CR rate).
First trial analysis.
For each cell categorized as “persistent,” the following bootstrap procedure was used to determine whether a reliable persistent response was observed on a given single trial (for these purposes, analysis was applied to the initial CS-only probe trial of the first acquisition session, ACQ1). For each iteration (×1,000), the 10 pretrial bins of the first trial were sampled with replacement (10 samples were drawn for each iteration), and the mean was calculated and stored. The distribution of the resampled pretrial bin averages was used to determine the 95% confidence interval for that single trial. Each of the five CS time bins and five trace interval time bins was evaluated with respect to the confidence interval determined from the pretrial baseline for that cell. A cell was defined as showing a persistent response on the first trial if reliable increases were observed during the tone, with these increases extending to at least 2 bins into the trace interval (as defined above). Using the same bootstrap procedure, the first trial of all nonpersistent cell types recorded during ACQ1 was also analyzed to determine the probability of observing a persistent response, independently of how that cell responded for the remainder of the session. For this analysis, the baseline activity of cells was checked for nonstationarities by applying the same bootstrapping procedure between the first 5 and last 5 pretrial bins. Reliable increases in the 300 ms preceding trial 1 would have been cause to reject the trial, but none were observed for any of the persistent cells.
Comparison of spike activity between the first and second half of training sessions.
To test for changes in spike output between the first and second halves of training sessions, a different bootstrap analysis was applied. This analysis was based on the individual cell matrices for each session (30 time bins × 108 trials) and was performed separately for persistent, phasic, and trace interval response types. For a given training day, the matrices of all cells of a given response type were averaged, and a difference score was calculated for each time bin by subtracting the average observed during the first half (trials 1–54) from the average observed for the second half (trials 55–108). To test whether the observed difference scores for each time bin were reliably different from zero, a bootstrapping procedure that replicated the same procedures from which the original observed differences were derived was used. For each iteration (×1,000), the first half of each cell's unsmoothed matrix was sampled with replacement (54 samples) and the second half of each cell's unsmoothed matrix was also sampled with replacement (54 samples). The resampled matrices of each cell were smoothed in the same fashion as the original data and averaged, and then the difference scores for each time bin were calculated and stored. Resampling from the original unsmoothed matrices and then smoothing ensured that the original smoothing procedure did not somehow bias the original observed differences. A 99.5% confidence interval was then calculated from the distribution of resampled difference scores for each time bin (α = 0.05/10 trial bins as a Bonferroni correction). Time bins for which zero fell outside the confidence interval were considered to show difference scores that were reliably different from zero (i.e., were significant at P < 0.005). This analysis was performed for each response type separately for ACQ1, ACQ2, and CRIT. The same analysis was also applied between training days (e.g., between the first or second halves of the training sessions on ACQ1 and ACQ2).
Spike response analysis.
The primary analyses based on the bootstrapping procedure described above utilized trial activity that was normalized to a pretrial baseline. To check whether changes in baseline activity were observed for persistent cells during and/or between sessions, a parametric analysis of the raw spike activity was performed (without normalization). The average number of spikes observed during the 500-ms pretrial window was calculated for the first and second halves of both ACQ1 and ACQ2 for each persistent cell. The average number of spikes observed for pretrial time windows were compared between session halves as a repeated measure and between training days as independent samples using a two-way mixed-effects ANOVA (α = 0.05). As an additional analysis to show that the bootstrapping results for persistent and phasic cells were robust, the average number of spikes observed for the first and second halves of both ACQ1 and ACQ2 was calculated for the appropriate time windows (between CS onset and US onset for persistent cells, and between CS onset and offset for phasic cells). Each response type was tested separately with a two-way mixed ANOVA.
RESULTS
The activity of 564 well-isolated single units in the mPFC of 12 rabbits was recorded during the acquisition of trace eyeblink conditioned responses (72 sessions, 7.83 units per session). Rabbits learned at different rates, with individual animals reaching learning criterion between 3 and 10 sessions (Fig. 1B). Because the primary question was in regard to whether persistent responses were observed during the earliest phases of learning, analysis was focused primarily on the first two training sessions from rabbits that showed few if any CRs during those sessions (ACQ1: 12 rabbits, n = 76 cells; ACQ2: 9 rabbits, n = 80 cells). Comparisons were also made between early learning mPFC responses to sessions in which learning criterion was met (CRIT: 12 rabbits, n = 105 cells).
All cells included in analysis were recorded from the ACc and AGm regions of the mPFC, primarily from layers 5/6 (Fig. 2). Scatterplots of the average width of the recorded spikes vs. the average baseline firing rate for each unit suggest only a small percentage of recorded cells (<15%) may have been fast-spiking interneurons (Fig. 2; Dilgen et al. 2013; Tierney et al. 2004; Tseng et al. 2006), consistent with previous reports for cortex (e.g., Hengen et al. 2013).
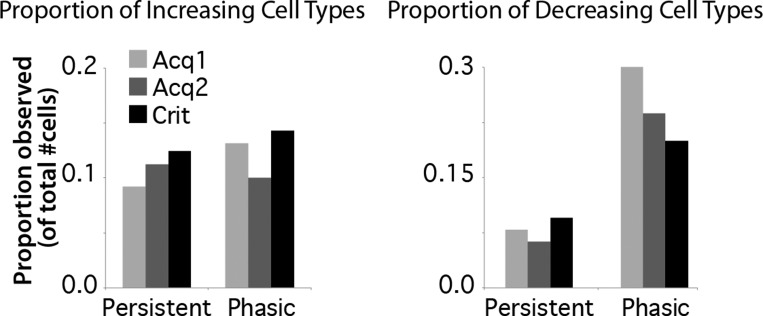
Cells were initially categorized according to the trial-associated response patterns observed during a given session, as previously described (see materials and methods; Siegel et al. 2012; Siegel and Mauk 2013). As previously reported for postlearning training sessions (Siegel et al. 2012; Siegel and Mauk 2013), over half of mPFC cells showed a significant change in spike response to the tone CS during early training sessions (59/76 cells during ACQ1, 42/80 cells during ACQ2) and for sessions in which animals met learning criterion (59/105 cells during CRIT). Also similar to previously reported postlearning data, a subset of cells showed changes in activity that persisted beyond CS offset into the trace interval during early learning sessions (“persistent”; e.g., Fig. 3, left, and Fig. 4; ACQ1: 7 increasing and 6 decreasing persistent cells, 17% of all cells recorded; ACQ2: 9 increasing and 5 decreasing, 18% of all cells recorded; CRIT: 13 increasing and 10 decreasing, 22% of all cells recorded). The proportion of persistent cells observed from the total recorded was similar to that previously reported in the mPFC of rabbits during trace conditioning (25%, Siegel et al. 2012; 14%, Siegel and Mauk 2013). Also as previously reported, persistent increases in activity showed a variety of response patterns, including increasing or decreasing trends over the trial period (Fig. 4; Siegel et al. 2012). These various patterns of persistent responses are similar to that reported for the primate mPFC (e.g., Miller et al. 1996; Procyk and Goldman-Rakic 2006). Although some cells only met the minimal criteria to be categorized as persistent (e.g., Fig. 3A, top left), most persistent cells showed significant changes in activity for the duration of the trial period (e.g., Fig. 4). During learning in the current study, the proportion of cells that showed persistent responses was not different across ACQ1, ACQ2, and CRIT for either increasing (df = 2, χ2 = 0.45, P = 0.80; Fig. 5, left) or decreasing response types (χ2 = 0.66, P = 0.72; Fig. 5, right), suggesting that such responses did not become more prevalent with learning.
Fig. 5.
Incidence of cells showing increasing (left) or decreasing (right) spike responses that were persistent or phasic during ACQ1, ACQ2, and Criterion (Crit) sessions. The proportions observed were not different across training days for either response type (increasing: χ2 = 0.45, P = 0.80; decreasing: χ2 = 0.66, P = 0.72).
Phasic responses to the CS were also observed both during early learning and at CRIT (e.g., Fig. 3, right; ACQ1: 10 increasing and 23 decreasing phasic cells, 43% of all cells recorded; ACQ2: 8 increasing and 19 decreasing, 34% of all cells recorded; compared with CRIT: 15 increasing and 21 decreasing, 34% of all cells recorded), as previously reported (Hattori et al. 2014; Siegel et al. 2012). Similar to that of persistent cells, the proportion of phasic cells was not different across ACQ1, ACQ2, and CRIT for either increasing (df = 2, χ2 = 0.78, P = 0.68; Fig. 5, left) or decreasing response types (χ2 = 2.55, P = 0.28; Fig. 5, right).
These data suggest that it is unlikely that phasic responses in mPFC cells are converted to persistent responses during learning because the proportion of each response type was not different across these training days, particularly for increasing cell types, which are hypothesized to drive cerebellar learning. However, it is possible that a phasic-to-persistent modification occurred within each session. To address this, each cell was recategorized separately for the first and second halves of training sessions to see if such changes may have occurred. The analysis did not detect any mPFC cells that changed from the phasic to the persistent category during ACQ1 (0/12 phasic cells) and revealed only one cell that did so during ACQ2 (1/10 phasic cells). The data suggest that if persistent responses were acquired as a result of training, it must have occurred early in the first training session.
Persistent responses on the first acquisition trial.
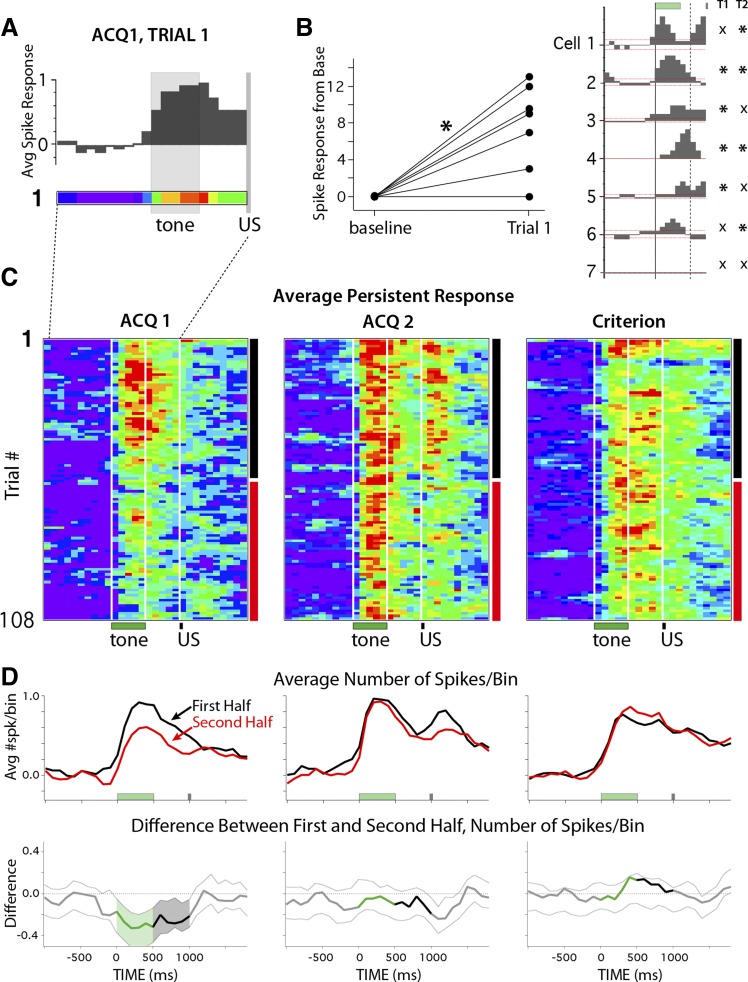
The primary goal was to determine whether persistent increases in spike activity in the mPFC were acquired during training. One approach to answer this question was to determine whether these persistent responses were present on the very first training trial before a paired presentation was experienced. To this end, the spike activity of each persistent increasing cell was binned (100 ms) and averaged for trial 1 of the first training session (ACQ1). On average, cells categorized as persistent for ACQ1 showed this response on the very first presentation of the tone CS, before any pairing with the US occurred (Fig. 6A). Initially, the number of spikes observed for each cell during a 1-s pretrial baseline was compared with the number of spikes observed during the trial to test whether the average really reflected an increase in spiking for individual cells (compared with the activity of 1 or 2 very robustly responding cells). As a group, these mPFC cells showed a significant increase in spike activity relative to baseline activity during trial 1 [Fig. 6B, left, shown normalized to baseline; paired t(6) = 4.28, P = 0.005]. However, it is possible that the increased activity did not reflect a persistent pattern of spiking during the trial. To address this directly, a single-trial bootstrap procedure was applied to trial 1 of each persistent cell to determine whether individual cells showed a persistent response pattern or if the increased spiking only occurred during the CS and did not reflect a persistent response. Four of the seven mPFC cells that showed a persistent response during ACQ1 responded in this way on the very first trial (57%; Fig. 6B, right, asterisks below T1 denote a persistent pattern). Because the first trial of every session is a CS-only probe trial, on trial 2 rabbits still had not yet experienced a CS-US paired trial. The same result was observed for trial 2, but for a different (overlapping) subset of cells (asterisks below T2 in Fig. 6B, right). The data for the first two trials indicate that 6/7 persistent cells (85%) showed a persistent spike response to the tone prior to the rabbit ever experiencing a CS-US paired trial (note that the cell that did not show a persistent pattern on the first 2 trials is the exemplar persistent cell shown in Fig. 3). To test the likelihood of observing a persistent response pattern by chance, the single-trial analysis was applied to all cells recorded during ACQ1 that were not categorized as persistent. Only 5 of 69 (7%) of these cells met the criteria for persistent responses during trial 1 of ACQ1, which was significantly lower than the 57% observed for persistent categorized cells [Yates-corrected χ2(1) = 10.75, P = 0.001]. The control analysis shows that the likelihood of observing a persistent pattern of response by chance is extremely unlikely, whereas almost all of the persistent cells (85%) showed this pattern before rabbits ever experienced a CS-US paired trial. The data suggest that mPFC cells can show persistent spiking to stimulus presentation as an innate response property and that such response patterns are not necessarily acquired as a function of learning or experience.
Fig. 6.
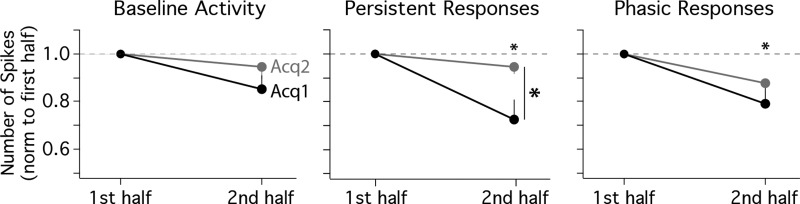
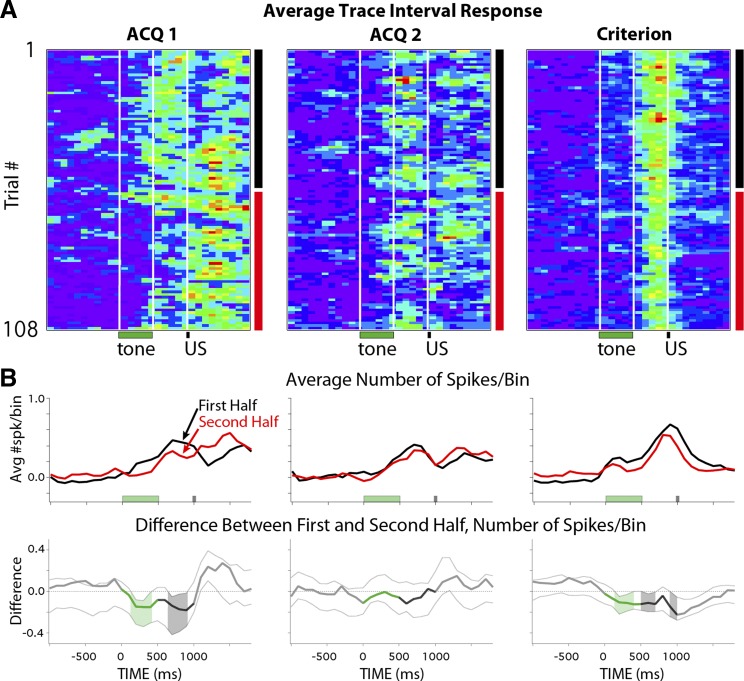
Activity profiles of persistent cells on the first training trials and over training sessions for the first (ACQ1, n = 7 cells) and second (ACQ2, n = 9 cells) days of training and during Criterion sessions (n = 13 cells). A: histogram and pseudocolored spike train (100-ms bins) of the averaged response of persistent cells for the very first presentation of the tone stimulus (normalized to the pretrial average of that trial; color scale: 0–1 spikes above baseline). Note that the first trial of each session is a tone-only probe, so the timing of the US is indicated but was not presented on this trial. B, left: persistent cells showed a significant increase in spike activity in response to tone presentation on the first trial (shown normalized to baseline; paired t-test: *P = 0.005). Right: histograms of the response patterns of persistent cells on the first trial (green and gray bars indicate timing of stimuli, dashed vertical line indicates minimum length of persistent response that could support cerebellar learning, and dashed horizontal red line indicates 95% confidence intervals for reliable changes from baseline). Asterisks denote cells that showed persistent response patterns on trial 1 (TI) and trial 2 (T2). Six of the 7 cells showed a persistent response to tone presentation before the animal had ever experienced a paired trial. C: pseudocolored matrices (color scale = 0–1 spikes above baseline) of the average spike response of persistent cells for each trial (100-ms bins) of a given session (108 trials) for ACQ1, ACQ2, and Criterion sessions. For each trial bin, the average response was compared between the first half (black vertical bars) and second half (red vertical bars) of sessions. White vertical lines indicate onset/offset of training stimuli (with every 9th trial as a tone-only probe beginning with the first trial). D, top: line graphs showing average change in spike activity from baseline for persistent cells during the first half (black lines) and second half (red lines) of sessions. Bottom: line graphs showing the difference between session halves (downward deflection indicates decrease), with dashed lines indicating 99.5% confidence intervals at each time bin. Shaded regions show reliable changes from zero, color-coded for trial epoch (green, tone CS; black, trace interval). Persistent responses showed reliable decreases in magnitude on the first day of training, which was not observed in the following sessions.
Persistent responses show within-session decreases during ACQ1, but not during ACQ2 or criterion sessions.
Although mPFC cells showed persistent responses beginning with the very first trials, of interest was whether that activity was modulated as a function of experience across sessions. To visualize the activity of persistent cells over all trials of a session, matrices of binned spike activity were created for each cell during each session (trial time × number of trials, normalized to the average pretrial baseline observed for a given cell; e.g., Fig. 3). The matrices of persistent cells were averaged to yield the mean persistent response for each trial of a given session (in 100-ms bins; Fig. 6C). On average, the spike output of persistent cells appeared to decrease in magnitude after the first half of the first training session (Fig. 6C, ACQ1). However, a similar decrease was not apparent for the second day of training (Fig. 6C, ACQ2) or after the rabbits had learned and were expressing CRs (Fig. 6C, criterion). For each time bin, a difference score quantifying this change (Fig. 6D, bottom) was calculated by subtracting the average spike output observed for the first half of sessions (trials 1–54; Fig. 6D, top, black lines) from that observed for the second half (trials 55–108; Fig. 6D, top, red lines). A bootstrap analysis was applied to each time bin to determine whether observed differences between session halves were reliable (α = 0.05/10 trial bins of interest = P < 0.005 per bin as a Bonferroni correction, with pre- and posttrial analysis also given for comparison). Note that for this bootstrap analysis the iterative resampling was performed using the same procedures from which the averaged matrices were derived (i.e., the matrices of individual cells were resampled before difference scores were averaged and calculated, and so this analysis accounts for individual cell variability). The results from ACQ1 indicate that persistent mPFC cells showed reliable decreases in spike output for each bin of the entire trial during the second half of the session (Fig. 6D, bottom left; shading indicates trial bins with reliable decreases). In contrast, comparisons between the first and second half of ACQ2 indicated that persistent cells did not change and were able to maintain response levels through the second half of the session (Fig. 6D, middle). The same results were obtained for ACQ1 and ACQ2 if the data set was reduced to cells that significantly increased activity across all 5 bins of the trace interval (n = 4 cells in ACQ1 and 5 cells in ACQ2; example raster plots for these ACQ1 cells and a subset for ACQ2 are shown in Fig. 4). Also in contrast to ACQ1 and similar to ACQ2, persistent cells did not show changes in response level during sessions in which rabbits met a learning criterion (Fig. 6D, bottom right; examples shown in Fig. 4). Taken together, the data reveal that mPFC neurons can generate persistent spiking to the tone CS before any paired training, but the magnitude of the response declined significantly over the first training session. However, on subsequent training days persistent responses remained robust during the entire training session, suggesting a change in the modulation of persistent spike output between the first and second day of training.
Phasic responses show within-session decreases during and after learning.
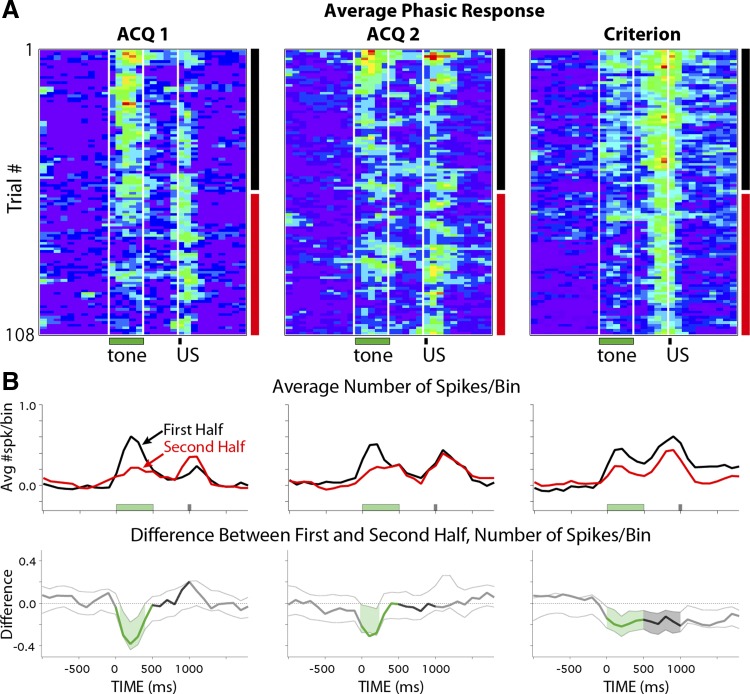
A subset of mPFC cells showed phasic response patterns to the CS that are not capable of supporting cerebellar learning for the trace interval used in the current study (Kalmbach et al. 2009; see also Chen et al. 2014). To determine whether the increased robustness of persistent responses observed between the second halves of ACQ1 and ACQ2 was specific to persistent cells, the same bootstrap analysis was applied to mPFC cells that showed phasic responses. Similar to persistent cells, phasic cells showed a decrease in spike output in response to the CS between the first and second halves of training sessions on ACQ1 (Fig. 7, A and B, left). However, in contrast to persistent cells, phasic cells also showed reliable decreases during ACQ2 and CRIT sessions (Fig. 7, A and B, middle and right, respectively). The data suggest that the ability of persistent cells to maintain robust responses during the entire session after the first day of training was specific to that response type and does not represent a global change across the mPFC network.
Fig. 7.
Pseudocolored matrices of the averaged spike response of phasic mPFC cells during training and when animals met criterion (A) and average responses for session halves and difference scores with confidence intervals (B), as described for Fig. 6, C and D. In contrast to persistent responses, phasic responses showed reliable decreases during the tone between the first and second halves of each training session analyzed, independent of previous experience or learning.
Persistent and phasic cells did not show increases in spike response between training days.
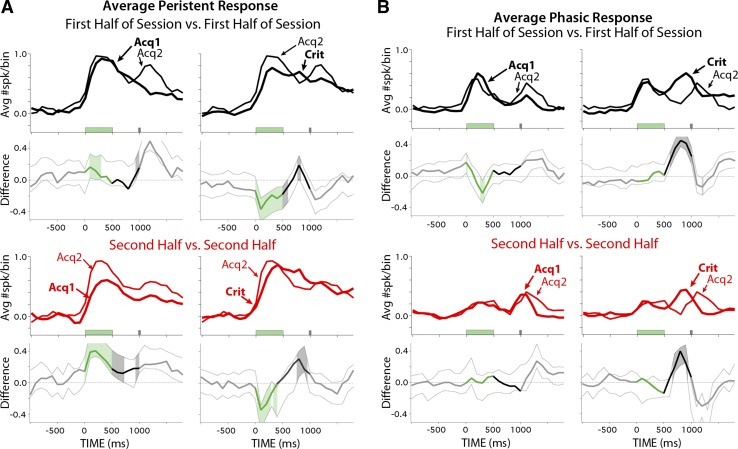
It is possible that the increased robustness of persistent responses between ACQ1 and ACQ2 was due to stronger CS-associated inputs and/or mPFC postsynaptic responses. Such a change could be reflected as an increase in the initial spike output in response to tone presentation during the first half of sessions between ACQ1 and ACQ2. To test this, the same bootstrap analysis was used to compare the first half of ACQ1 with the first half of ACQ2 for the persistent cells recorded on each of those days. The maximum spike output in response to the CS was not different between the two days (i.e., both lines reach the same magnitude, Fig. 8A, top left). Interestingly, the magnitude of the average spike response is reached sooner after CS onset on ACQ2, suggesting a modestly faster but reliable spike response to the CS between ACQ1 and ACQ2 (Fig. 8A, top left, black lines). Analysis comparing the second halves of sessions between training days supported previous analysis: persistent cells showed reliably higher spike responses on ACQ2 relative to those observed on ACQ1 (Fig. 8A, bottom left, red lines). Interestingly, persistent cells showed a reliable decrease during the CS and initial part of the trace interval between ACQ2 and CRIT sessions (Fig. 8A, top right, black lines), suggesting the magnitude of persistent spike output after learning is less than observed during early training. An increase in activity is also noted during the trace interval just before the US between ACQ2 and CRIT (Fig. 8A, right). However, it was previously shown that this difference is largely due to the absence of behavior-associated feedback before the expression of CRs (compare with Fig. 9B of Siegel and Mauk 2013, before and after the abolition of CRs with muscimol). In contrast, phasic mPFC cells showed little difference in spike response to the tone CS between training days for the first or second halves of sessions (Fig. 8B). The data indicate that persistent mPFC cells did not show an overall increase in spike output between the first halves of ACQ1 and ACQ2 and that the increased robustness of within-session persistent responses was likely due to a modification that influenced the spike output of mPFC cells over the course of the session (see discussion). The data further show that such modulation was specific to persistent cells.
Fig. 8.
Comparison of the average spike response above baseline of persistent (A) and phasic cells (B) for the first half of ACQ2 (thin black lines) relative to ACQ1 or Criterion (heavy black lines) and difference scores and confidence intervals for each comparison, as described for Fig. 6D. The second halves of sessions were also compared across training days (red lines). A, top graphs: during the first half of sessions, persistent cells showed shorter latencies to initial spike response but were of similar magnitude between the first 2 days of training and showed a decrease in magnitude between ACQ2 and Criterion sessions. Bottom graphs: persistent cells showed an increase in response magnitude during the last half of sessions between the first and second days of training, supporting previous analysis. B: phasic cells showed similar response magnitudes to the tone during the first and second halves of sessions between all training days analyzed.
Fig. 9.
Parametric analysis of raw spike counts for the baseline activity of persistent cells (left; 1-s pretrial average) during the first and second halves of training sessions on ACQ1 (black lines) and ACQ2 (gray lines) and for trial-associated activity of persistent cells (middle; average number of spikes between tone and US onset) and phasic cells (right; average number of spikes between tone onset and offset). Pretrial baseline activity of persistent cells was not different between session halves or between training days and so was unlikely to have influenced the previous bootstrap analyses that used baseline-normalized data (2-way ANOVA, P > 0.05, shown normalized to the first half of sessions for simplicity). Analysis of trial-associated activity based on raw spike counts was in agreement with previous analyses for persistent cells (2-way ANOVA: *P < 0.05, main effect and interaction) and for phasic cells (2-way ANOVA: *P < 0.05, main effect only).
Baseline activity levels were not different between ACQ1 and ACQ2.
The previous bootstrap analyses were based on spike output that was normalized to the baseline (pretrial) activity observed for each cell. It is possible that systematic changes in the pretrial spike activity of mPFC cells during a session or between days could have influenced the normalization, and therefore the results. To address this, a second analysis based on the average raw spike counts observed for each persistent cell during the first vs. second halves of sessions on ACQ1 and ACQ2 was performed (using a mixed-effects repeated-measures ANOVA). No significant changes in baseline spike activity were observed between session halves or between training days for ACQ1 or ACQ2 [training day: F(1) = 1.36, P = 0.26; session half: F(1) = 3.05, P = 0.10; training day × session half: F(1) = 0.03, P = 0.86; Fig. 9, left]. To further substantiate the results of previous bootstrap analyses, phasic and persistent cells were also analyzed. Similar to the previous results, persistent cells showed significant decreases in spike number between the first and second halves of sessions [F(1) = 18.25, P < 0.001], with a significant interaction for training day due to spike activity that decreased significantly more on ACQ1 than on ACQ2 [F(1) = 9.30, P = 0.04; Fig. 9, middle]. Also similar to the previous results, phasic mPFC cells showed significant decreases in spike activity during the tone CS between the first and second halves of both ACQ1 and ACQ2 [F(1) = 11.07, P = 0.004], with no significant interaction with training day [F(1) = 0.71, P = 0.41; Fig. 9, right], suggesting that spike activity decreased similarly for phasic cells on both training days.
Trace interval responses show different patterns of activity before and after learning.
A previous study demonstrated that mPFC responses that were restricted to the trace interval (i.e., significantly increased after CS offset) reflected feedback from the cerebellar output that drives CRs (Siegel and Mauk 2013). Therefore, it was hypothesized that such activity should not be observed early in learning, before the expression of CRs. However, a proportion of mPFC cells showed significant trace interval activity early in learning before the expression of CRs (ACQ1: 7 increasing and 17 decreasing, 32% of cells; ACQ2: 7 increasing and 15 decreasing, 28% of cells). Additionally, the proportion observed was not significantly different than that observed after learning for increasing or decreasing response types [CRIT: 13 increasing and 28 decreasing, 39% of cells; increasing: χ2(2) = 0.76, P = 0.68; decreasing: χ2(2) = 1.63, P = 0.44].
To compare trace interval responses early in learning with those observed after learning, binned matrices were created for each trace interval cell and were averaged based on training day. Note that some trace interval cells also show phasic responses to the CS, as previously reported and observed here in the averaged binned matrices of trace cells (Fig. 10A, right; Siegel et al. 2012). On average, the temporal structure of spike output during the trace interval was clearly different on ACQ1 and ACQ2 compared with criterion sessions (Fig. 10A). During ACQ1 and ACQ2, increased spike activity was typically observed near the end or just after CS offset and did not show a consistent temporal structure between training trials (Fig. 10A, left and middle). In contrast, after animals were expressing CRs, trace interval cells showed increased spike output later in the trace interval, coinciding with the topography of behavioral CRs as previously reported (Fig. 10A, right; example behavior shown in Fig. 3 waterfall plots; Siegel et al. 2012; Siegel and Mauk 2013). Similar to persistent cells, trace interval cells showed decreased spike output during the tone and trace interval between the first and second half of the training session on ACQ1, which was not observed on ACQ2 (Fig. 10B, left and middle). However, decreasing spike output was again observed for trace interval cells during the stimulus-free interval for criterion sessions, in addition to reliable decreases during the tone interval (likely due to the fact that many trace interval cells also show phasic responses to the CS; Siegel et al. 2012; Fig. 10B, right). The data suggest that mPFC cells that displayed trace interval responses during early learning showed response changes that were reminiscent of weakly persistent cells with fairly late onsets and/or unreliable spike responses. However, after learning, the majority of trace interval cells showed activity that reflected the CR-associated feedback from cerebellar output, as previously demonstrated (Siegel and Mauk 2013).
Fig. 10.
Pseudocolored matrices of the averaged spike response of mPFC cells with responses limited to the trace interval during training and when animals met criterion (A) and average responses for session halves and difference scores with confidence intervals (B), as described for Fig. 6, C and D. Trace interval activity had a different temporal structure early in learning (ACQ1 and ACQ2) relative to that typically observed after learning (Criterion). After learning, trace interval responses reflect the topography of behavioral CRs (compare with Criterion waterfall plots in Fig. 3) and previously have been shown to represent feedback from cerebellar output (see text).
DISCUSSION
The data reveal that mPFC cells can respond with persistent spiking to a neutral stimulus, in that it was observed in 85% of persistent cells prior to experiencing a single pairing of the conditioning stimuli. However, on the first day of training the magnitude of persistent responses reliably decreased after ∼50 conditioning trials. By the second training session persistent responses were modified, showing shorter latencies to spike in response to the CS and the ability to maintain robust responses throughout the session (Fig. 11). Furthermore, this experience-related modification was specific to mPFC cells that showed persistent responses; cells showing phasic responses reliably decreased in response magnitude during each daily training session, even after animals had learned.
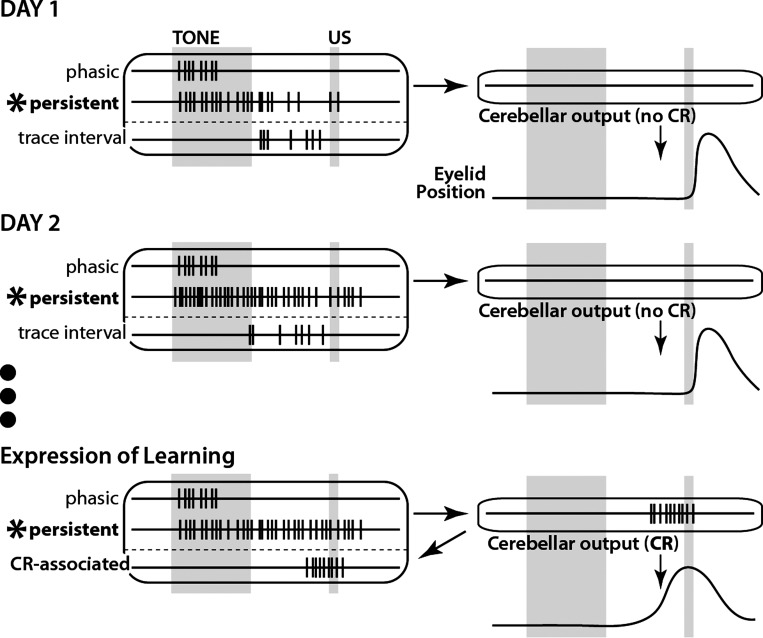
Fig. 11.
Schematic representation of typical patterns of mPFC single-neuron responses to a tone CS over learning in trace eyeblink conditioning revealed in this study. Day 1: persistent responses are observed from the start of training but decrease in magnitude over the training session (similar to phasic responses). Trace interval responses show variable spike patterns across trials. Day 2: persistent responses show slightly shorter latencies to onset relative to day 1 and maintain a similar magnitude of response level throughout the session. Phasic and trace interval responses are similar to those on Day 1. Expression of learning: persistent responses show a lower response magnitude relative to day 2 but remain robust throughout training sessions. Trace interval activity primarily reflects cerebellar feedback to the mPFC and so is topographically similar to behavioral CRs. Phasic responses, which are not sufficient to support cerebellar expression of CRs, remain unchanged.
The proportion of mPFC cells showing persistent spiking to conditioned stimuli appears highly variable across species and tasks (15–40% in primate studies, typically referred to as “delay” cells, and ∼25% in rat trace fear conditioning, vs. ∼10% in rabbits during trace eyeblink conditioning; e.g., Baeg et al. 2001; Funahashi et al. 1989; Fuster 1973; Gilmartin and McEchron 2005; Procyk and Goldman-Rakic 2006). However, the actual proportions of persistent (i.e., “delay”) cells observed in previous studies are often difficult to discern for two reasons. First, more inclusive response categories are typically used. If similar criteria were applied to the current study, cells in the persistent and trace interval categories would have been categorized together, increasing the proportion of “persistent” cells from 10% to 20–30%. Second, the majority of studies tested the spike activity during the entire delay interval (as a single bin) relative to a baseline epoch of similar duration and so do not use criteria in which increased spike activity must be elevated for any given duration (an exception is Procyk and Goldman-Rakic 2006). Interestingly, the overall proportion of mPFC cells that respond to trial-associated stimuli appears to be largely similar across these and other studies (typically >50% of recorded neurons, and much higher if US or behavior-associated responses are included). Nevertheless, an important aspect of the current work is not how many persistent cells were observed, but that such responses were observed at all during the earliest phases of training, particularly during the first trials before a given rabbit experienced a single CS-US pairing (i.e., 57% of persistent and only 7% of nonpersistent cells met our stringent criteria on trial 1). A perceived issue when moving on to analyze the trial-by-trial nature of these persistent cells is that the number of observed cells may be considered modest. However, on the basis of statistical formulas, this is only an issue when interpreting a negative result, given a lack of power. Although the number of persistently increasing cells was close to the minimum necessary to allow for statistical analysis on ACQ1, a clear and reliable decrease in response magnitude was observed over the session using both bootstrapping and parametric statistics, and was not observed during ACQ2 or CRIT when greater numbers of cells were included. Notably, the data included for a given training day were recorded from different rabbits (a majority of which contributed persistent cells on both ACQ1 and ACQ2), further supporting the generalizability of the observed modulation of persistent responses across training days.
Although changes in the proportions of persistent or phasic cell types were not observed across training days in the current study (see also Hattori et al. 2014), a nonsignificant trend is noted. One hypothesis is that phasic responses become persistent during training via synaptic and/or neuromodulatory changes. Although no evidence was found in the current study to support this hypothesis, the question of whether at least some persistent responses are acquired with training was not addressed conclusively here. It is possible that the aforementioned trend might reflect real increases in the proportions of persistent cells observed over training if sample sizes were increased. A power analysis indicated that the required number of cells necessary to detect this modest effect would be greater than a factor of 10 above that reported in this article.
Few studies of persistent responses specifically analyzed the earliest stages of learning. A recent study by Disterhoft and colleagues (Hattori et al. 2014) also observed persistent responses during the first training session, but an analysis of the very first training trials was not reported. One study of trace fear conditioning in rats recorded single neurons from the mPFC and reported persistent responses to the auditory CS during the very first training session (Gilmartin and McEchron 2005). Likewise, an analysis of response patterns for individual cells on the very first trial was not reported and included the additional caveat that animals showed significant learning during the first session. Interestingly, pseudoconditioned animals in both of the latter studies also showed persistent responses to conditioning stimuli throughout those sessions. The authors of these studies suggested that experiencing the CS-US pairing was not a prerequisite to observe persistent responses and that these were likely an innate property of a given subset of mPFC cells. Two other studies have looked at changes in persistent responses in regions outside of mPFC. The first found an increase in the likelihood of perirhinal cells to respond persistently between associated stimulus pairs after learning; however, the robustness of the activity could not be analyzed before and after learning (Erickson and Desimone 1999). Miyashita (1988) found that neurons in the temporal cortex showed a similar effect of learning, with responses to new training stimuli showing weak but stationary persistent spiking over the first 10 presentations that, overall, was not different from the responses to well-learned stimuli. The current study directly tested and supports the suggestions of previous work with analysis of the very first training trials. Persistent responses were observed before the first CS-US pairing and showed training-related modifications on subsequent days in that cells were able to maintain robust responses throughout training sessions after ∼50 trial presentations. Although pseudoconditioning was not used in this or the latter two studies, it is hypothesized that pseudoconditioned rabbits would also show persistent responses on the first trials of the first session but would not show the increased robustness on the second day of training and thereafter. A similar result has been reported in rats and rabbits, in which persistent responses in the mPFC were observed in session-averaged data but were weaker in pseudoconditioned animals relative to animals that experienced paired trials (Hattori et al. 2014; Takehara-Nishiuchi and McNaughton 2008).
Potential role of increased robustness of persistent spike responses during training.
The hypothesized role of the mPFC during trace eyeblink conditioning is to provide an input to the cerebellum, via the pons, in response to the CS that persists to bridge the stimulus-free trace interval until the time of the US (Fig. 1A; Kalmbach et al. 2009; Siegel et al. 2012; Weiss and Disterhoft 2011). The increased robustness of mPFC persistent responses on the second day of training could result in cerebellar input that is more faithful on a trial-to-trial basis throughout a session and may more effectively drive cerebellar learning. However, the data presented in this article are from animals that learned at different rates, and so the changes between ACQ1 and ACQ2 are unlikely to fully account for the difference in learning rates observed across animals (Fig. 1B, where bold black lines are learning curves from rabbits that contributed persistent cells on ACQ1 and ACQ2). It is possible that the decrease in spike response during ACQ1 may reflect neural evidence of fatigue or a decrease in attentional processes during the first session, after which rabbits show increased stamina during subsequent sessions. An additional possibility is that during an animal's first training session, an adjacent mPFC region not typically involved with the expression of trace eyeblink conditioning, such as the prelimbic area, could influence the initial ACc or AGm responses to the tone. The strong reciprocal connectivity between adjacent regions of mPFC has been well-established (e.g., Hoover and Vertes 2007; Weible et al. 2007). Previous work suggests that mPFC regions may influence activity in adjacent areas and that this influence can change with experience (Chang et al. 2010). The data reported in this article indicate that ACc/AGm persistent cells would be able to overcome this influence after 1 training day, whereas phasic responses are similarly effected by such inputs across training days.
Four hypotheses are proposed regarding the training-related modification of persistent tone-evoked responses observed in the mPFC during trace eyeblink conditioning, which are not mutually exclusive.
Hypothesis 1: persistent activity may result from as yet unmeasured CS-evoked responses, which are modified with experience.
Although a learned eyelid response is the target of trace eyeblink conditioning, it is possible that other muscles may show innate or quickly learned responses to a given CS. Although this seems an unlikely scenario to explain persistent responses across species, modalities, and tasks, it is possible given the clear motor-response feedback to the mPFC shown in at least one previous study (Siegel and Mauk 2013). It should be noted that this is a potential issue for all studies focused on persistent neural responses. Similarly, the brain areas that modulate the alerted state of an animal following CS presentation could provide feedback to the mPFC that might result in apparent persistent responses, in some or all cases. Evidence against such a possibility includes in vitro work in which cells can respond persistently to discrete stimulation (e.g., Dembrow et al. 2010). Nevertheless, it remains a viable hypothesis that has yet to be adequately addressed.
Hypothesis 2: modification of putative network and/or cellular mechanisms that drive persistent responses in the mPFC between ACQ1 and ACQ2.
One possibility is the observed decrease in the magnitude of spike output during the course of a session is due to network or cellular mechanisms responsible for the modulation of excitation levels. Examples of such mechanisms include changes in the response of inhibitory cells to stimuli-associated inputs or within reverberatory networks (Wang et al. 2004), alterations in the dendritic excitability of persistent cells, and/or decreased synaptic efficacy or background activity (McCormick et al. 2003; Wang 2001). Because the modification of CS-evoked responses with repeated trial presentations between ACQ1 and ACQ2 was specific to persistent cells, candidate mechanisms to counteract or block these putative changes should be capable of operating on a cell- or synapse-specific basis, assuming that persistent and phasic cells receive tone input from a common source. Further studies are necessary to determine which mechanisms may play a role in this change and whether the observed change plays a role in learning. For example, in vitro experiments on mPFC slices taken from animals after 1 day of training can be used to investigate the multiple mechanistic possibilities. If the mechanism can be blocked or induced, then such manipulations in vivo should be able to enhance (if blocked on ACQ1) or retard learning (if induced each day thereafter).
Hypothesis 3: neuromodulation of persistent responses in the mPFC.
It is currently unknown whether the persistent mPFC neurons recorded during trace conditioning indeed project to the pons, but previous work suggests that it is likely (Dembrow et al. 2010; Kalmbach et al. 2013; Moya et al. 2014). In vitro work has shown that pyramidal tract projecting mPFC cells displayed persistent spike activity to phasic stimuli with activation of cholinergic or metabotropic glutamate receptors, whereas other mPFC cells were less likely to show persistent spike responses (Dembrow et al. 2010; Kalmbach et al. 2013). Therefore, a second hypothesis that could explain the increased robustness of persistent responses between training days is a change in the presence or the effects of modulatory tone over the course of sessions between ACQ1 and ACQ2 (and likely thereafter). In vitro work examining the effects of various neuromodulator agonists or antagonists between naive animals and after 1 day of training would be one way to begin to address this possibility. Viable candidates should differentially affect persistent and phasic cell types, which can then be tested in vivo by either stimulation or pharmacological inactivation of neuromodulatory centers while mPFC cells are recorded during the initial days of training.
Hypothesis 4: a specific role for the hippocampus in the acquisition of trace CRs.
The precise functional relationship between the transitory role of hippocampus and the ongoing role of the mPFC in trace conditioning has been enigmatic. The hippocampus shows increased excitability in trace-conditioned animals that is observed for several days before returning to naive levels (Moyer et al. 1996). It is possible that the change in persistent spike responses in the mPFC between training days could be attributed to long-term potentiation (LTP)-like changes in response to hippocampal inputs that occur in association with other tone inputs to the mPFC, most likely via the entorhinal cortex (Insausti et al. 1997; Morrissey et al. 2012). In rats, infusion of NMDA antagonist into the mPFC impaired acquisition of trace CRs (Takehara-Nishiuchi et al. 2005). The increased spike output observed in the current study during the first 200 ms after tone presentation between ACQ1 and ACQ2 could be the result of increased synaptic responses in mPFC cells to hippocampal and/or auditory input (Fig. 8A, top). Hippocampal lesion data further suggest that in the absence of this putative supportive role, the mPFC is unable to effectively induce cerebellar learning within a reasonable time frame (Kim et al. 1995; Kotani et al. 2003; Moyer et al. 1990; Solomon et al. 1986; Takehara et al. 2003). One prediction of this hypothesis is that the magnitude of persistent mPFC responses in hippocampus-lesioned animals would continue to decrease during the second half of sessions on subsequent training days.
The data reported in this study address one of the most pressing questions regarding persistent neural responses: whether or not such responses are acquired as a result of training or experience. Although experiencing at least one paired trial was not a prerequisite for persistent responses, such responses were modulated by experience early in learning. The current study provides the necessary framework from which to pose specific experimental questions regarding how and why persistent neural activity is modulated to support learning and memory.
GRANTS
This work was supported by National Institute of Mental Health Grants MH74006 (to M. D. Mauk) and MH46904 (to M. D. Mauk and D. Johnston) and by the McKnight Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
J.J.S. conception and design of research; J.J.S. performed experiments; J.J.S. analyzed data; J.J.S. interpreted results of experiments; J.J.S. prepared figures; J.J.S. drafted manuscript; J.J.S. edited and revised manuscript; J.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
I am indebted to L. K. Cormack for invaluable guidance regarding the application and use of bootstrapping procedures and to M. D. Mauk, R. A. Chitwood, N. Desai, W. Li, and B. Houck for essential feedback on the manuscript.
REFERENCES
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex 11: 441–451, 2001. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yang L, Xu Y, Wu GY, Yao J, Zhang J, Zhu Z, Hu ZA, Sui J, Hu B. Prefrontal control of cerebellum-dependent associative motor learning. Cerebellum 13: 64–78, 2014. [DOI] [PubMed] [Google Scholar]
- Chang C, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One 5: e11971, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci 30: 16922–16937, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilgen J, Tejeda HA, O'Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol 110: 221–229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen E, Tahvildari B, Egorov A, Hasselmo M, Alonso A. Mechanism of graded persistent cellular activity of entorhinal cortex layer V neurons. Neuron 49: 735–746, 2006. [DOI] [PubMed] [Google Scholar]
- Fritz JB, David SV, Radtke-Schuller S, Yin P, Shamma SA. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nat Neurosci 13: 1011–1019, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol 36: 61–78, 1973. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci 119: 1496–1510, 2005. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, Diba K. Prefrontal activity links nonoverlapping events in memory. J Neurosci 33: 10910–10914, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 14: 477–485, 1995. [DOI] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Prog Psychobiol Physiol Psychol 10: 197–275, 1983. [Google Scholar]
- Hattori S, Yoon T, Disterhoft JF, Weiss C. Functional reorganization of a prefrontal cortical network mediating consolidation of trace eyeblink conditioning. J Neurosci 34: 1432–1445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80: 335–342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212: 149–179, 2007. [DOI] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus 7: 146–183, 1997. [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Chitwood RA, Dembrow NC, Johnston D. Dendritic generation of mGluR-mediated slow afterdepolarization in layer 5 neurons of prefrontal cortex. J Neurosci 33: 13518–13532, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyeblink conditioning. Learn Mem: 86–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyeblink conditioning. J Neurophysiol 104: 627–640, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109: 195–203, 1995. [DOI] [PubMed] [Google Scholar]
- Kotani S, Kawahara S, Kirino Y. Trace eyeblink conditioning in decerebrate guinea pigs. Eur J Neurosci 17: 1445–1454, 2003. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem 69: 147–162, 1998. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol 34: 337–347, 1971. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex 13: 1219–1231, 2003. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and Pavlovian conditioning: trace versus delay conditioning. Behav Neurosci 116: 37–47, 2002. [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey MD, Maal-Bared G, Brady S, Takehara-Nishiuchi K. Functional dissociation within the entorhinal cortex for memory retrieval of an association between temporally discontiguous stimuli. J Neurosci 32: 5356–5361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya MV, Siegel JJ, McCord ED, Kalmbach BE, Dembrow N, Johnston D, Chitwood RA. Species-specific differences in the medial prefrontal projections to the pons between rat and rabbit. J Comp Neurol 522: 3052–3074, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104: 243–252, 1990. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16: 5536–5546, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 115: 1029–1038, 2001. [DOI] [PubMed] [Google Scholar]
- Procyk E, Goldman-Rakic PS. Modulation of dorsolateral prefrontal delay activity during self-organized behavior. J Neurosci 26: 11313–11323, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyeblink conditioning. J Neurophysiol 107: 50–64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Mauk MD. Persistent activity in prefrontal cortex during trace eyeblink conditioning: dissociating responses that reflect cerebellar output from those that do not. J Neurosci 33: 15272–15284, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Neunuebel JP, Knierim JJ. Dominance of the proximal coordinate frame in determining the locations of hippocampal place cell activity during navigation. J Neurophysiol 99: 60–76, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 100: 729–744, 1986. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23: 9897–9905, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, Kawahara S, Kirino Y. NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn Mem 12: 606–614, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963, 2008. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Dégenètais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci 20: 514–524, 2004. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O'Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse 59: 412–417, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Persistent neural activity: experiments and theory. Cereb Cortex 13: 1123, 2003. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24: 455–463, 2001. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegnér J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience 145: 288–302, 2007. [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behav Neurosci 125: 318–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]