Abstract
The direction-selective ganglion cells (DSGCs) and orientation-selective ganglion cells (OSGCs) encode the directional and the orientational information of a moving object, respectively. It is unclear how DSGCs and OSGCs mature in the mouse retina during postnatal development. Here we investigated the development of DSGCs and OSGCs after eye-opening. We show that 1) DSGCs and OSGCs are present at postnatal day 12 (P12), just before eye-opening; 2) the fractions of both DSGCs and OSGCs increase from P12 to P30; 3) the development of DSGCs and OSGCs is subtype dependent; and 4) direction and orientation selectivity are two separate features of retinal ganglion cells (RGCs) in the mouse retina. We classified RGCs into different functional subtypes based on their light response properties. Compared with P12, the direction and orientation selectivity of ON-OFF RGCs but not ON RGCs became stronger at P30. The tuning width of DSGCs for both ON and ON-OFF subtypes decreased with age. For OSGCs, we divided them into non-direction-selective (non-DS) OSGCs and direction-selective OSGCs (DS&OSGCs). For DS&OSGCs, we found that there was no correlation between the direction and orientation selectivity, and that the tuning width of both ON and ON-OFF subtypes remained unchanged with age. For non-DS OSGCs, the tuning width of ON but not ON-OFF subtype decreased with development. These findings provide a foundation to reveal the molecular and synaptic mechanisms underlying the development of the direction and orientation selectivity in the retina.
Keywords: retinal ganglion cell, multielectrode array, direction-selective ganglion cell, orientation-selective ganglion cell
different types of retinal ganglion cells (RGCs) are tuned to respond to different features of visual scenes. For example, direction-selective ganglion cells (DSGCs) extract motion information from the visual field, i.e., they respond maximally when a stimulus moves in one direction (preferred), but little or no response when the stimulus moves in the opposite direction (null) (Barlow and Hill 1963; Oyster and Barlow 1967). By contrast, orientation-selective ganglion cells (OSGCs) (also called “axis-selective” RGCs) detect local bars and edges in the visual field and encode their orientations (Levick 1967). In other words, OSGCs selectively respond to the alignment orientation of stimuli as opposed to the direction of its movement.
The response properties of individual DSGCs have been characterized in rabbits (Fried et al. 2002; He and Masland 1997; Lee et al. 2010), and OSGCs in primates (Passaglia et al. 2002), cats (Levick and Thibos 1982), rabbits (Bloomfield 1994; He et al. 1998; Venkataramani and Taylor 2010), and mice (Zhao et al. 2013). Because mouse genetics in particular allow for the examination of functions and circuitry of defined cell types, studies in mice have started to reveal the synaptic mechanisms underlying the direction selectivity of RGCs (Briggman et al. 2011; Wei et al. 2011). It is still not clear whether and how the physiological properties of DSGCs and OSGCs mature during postnatal development. Previous studies reported that DSGCs are present around the time of eye opening in rabbits (Chan and Chiao 2008) and mice (Chen et al. 2009; Elstrott et al. 2008), suggesting that visual experience and patterned activity were not required to produce direction selectivity. However, the directional preference of DSGCs can be altered by adaptive moving stimulation (Rivlin-Etzion et al. 2012), indicating a possible activity-dependent plasticity for DSGCs. In addition, the tuning width of ON-OFF DSGCs decreased and the distribution of the preferred directions became more symmetric after eye opening (Elstrott et al. 2008), suggesting that some properties of DSGCs continue to mature with age. At the same time, little is known of the development of OSGCs in the mouse retina, especially whether the development of orientation selectivity correlates with the development of direction selectivity. In this paper, we examined the developmental changes of the populations of DSGCs and OSGCs in the mouse retina.
METHODS
Animals.
C57BL/6 wild-type mice of either sex reared in 12:12-h light-darkness were used in this study. All animal procedures conformed to the guidelines on the Use of Animals in Neuroscience Research from the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at Yale University and University of Utah.
Multielectrode array recordings.
Mouse retinas were dissected and then recorded using a 60-channel multielectrode array (MEA1060, Multichannel Systems, Reutlingen, Germany) (Cantrell et al. 2010; Feng et al. 2013; Tian and Copenhagen 2003; Zhao et al. 2013). Visual stimuli were generated by the VersionWorks (Vision Research Graphics, Durham, NH). The full-field flash stimulus consisted of 2 s of light ON, followed by 8 s of light OFF, repeating 50 times. Moving bar stimulus was used to evaluate the direction and orientation selectivity of RGCs. The moving bar was a white rectangle moving on a black background (600 μm × 4,000 μm, 1,000 μm/s at 30° intervals) repeating 50 times with pseudorandom sequences of 12 directions. The light-evoked action potentials were recorded, and the electrical signals were filtered between 100 Hz and 3 kHz and sampled at 25 kHz.
Spike waveforms recorded by each electrode were sorted into single units in Offline Sorter (Plexon, Dallas, TX) (Koehler et al. 2011; Nirenberg et al. 2001; Tian and Copenhagen 2003). Briefly, a threshold of 68 μV was used to detect action potentials, the first and second principal components calculated, and clusters formed with action potentials having similar principal components (Elstrott et al. 2008; Tian and Copenhagen 2003). We selected clusters that could be clearly isolated from other spread-out activities for further analysis. From each isolated cluster, we defined a template by selecting a small fraction of spikes (∼10–20% of the cluster) located in the center of the cluster. We also adjusted the value of fit tolerance manually to avoid overlaps among different clusters. Using this template and fit tolerance, we sorted the action potentials from individual clusters (Tian and Copenhagen 2003). For each sorted unit, an autocorrelation function was applied to eliminate potential contaminations from other cells or noise (Elstrott et al. 2008; Nirenberg et al. 2001). Because neurons have a refractory period of ∼1 ms, spikes in the first 1-ms bin of the autocorrelogram may represent electronic noise or spikes from other cells (Nirenberg et al. 2001). Our data showed that 95% of total units contained <2% of spikes within this 1-ms window (median of distribution: 0.3; data not shown). Because these spikes were known to be contaminants, they were removed from further analysis. In addition, we manually removed spikes with irregular waveforms, which were generally created by overlapping of two or more spikes in a short time period. Each step of the offline-sorting processes was cross-examined and confirmed by another independent observer. Finally, the timestamps of spikes from each unit were exported to MATLAB (Mathworks, Natick, MA), where custom analyses were applied.
The response dominance index.
Peristimulus time histograms (PSTHs) of individual units with a 10-ms bin width were generated from cells' responses to both full field-flash and moving bars. The response dominance index (RDI) was determined with the scalar value:
where RON and ROFF were the maximum ON and OFF responses, respectively (Cantrell et al. 2010; Tian and Copenhagen 2003). For full field flash stimuli, the RON and ROFF were defined as the peak frequency during first 0.5 s of ON and OFF portions of the stimulus. For moving bar stimuli, the ON and OFF responses represented the responses when the bar moving into and out of the cell's receptive field (RF). For each direction, the ON and OFF responses were first determined as the clusters in the PSTHs, and then the peak frequencies were calculated from the ON and OFF clusters. The RON and/or ROFF were defined as the maximum value of the peak frequencies from the 12 directions for each cell.
For both stimuli, the value of the RDI ranges from −1 to 1. We classified cells into three functional subgroups: cells with RDI smaller than −0.6 were defined as OFF-dominating RGCs; cells with the RDI larger than 0.6 as ON-dominating RGCs; and cells with the RDI between −0.6 and 0.6 as ON-OFF RGCs (Cantrell et al. 2010). Cells that exhibited different response properties (<10% of all cells recorded) were analyzed manually if the cell had clear clusters of ON and/or OFF responses in the PSTHs to the moving bar.
Direction selectivity index and orientation selectivity index.
Direction selectivity index (DSI) was calculated as:
where Rpref is the peak response at the preferred direction, defined as the stimulus direction with the maximum response, and Rnull is the peak response at the opposite direction. Cells with a DSI near 1 had response only at the preferred direction, but no response at null direction, and cells with a DSI near 0 had responses with same strength at both preferred and null directions. For ON-OFF RGCs, the maximum value of DSI either from ON or OFF responses was selected as the DSI for the cell.
Most studies had used DSI cutoff of 0.33, i.e., when the Rpref was twofold larger than the Rnull, the cell was classified as a DSGC (Rivlin-Etzion et al. 2012; Sun et al. 2011; Zhao et al. 2013), but see Rivlin-Etzion et al. (2011), which used DSI cutoff value of 0.4 and Elstrott et al. (2008) used 0.6. In this study, we mainly used 0.33 as the DSI cutoff and also tested other cutoff values.
Orientation selectivity index (OSI) was defined as:
where Rpref is the peak response at the preferred orientation, and Rorth is the mean response at the two directions orthogonal to the preferred one. Similarly to DSGCs, cells with an OSI near 1 have response only at preferred orientation, and cells with an OSI near 0 have responses with same strength at both preferred and orthogonal orientations. We again used 0.33 as the cutoff to separate cells into OSGCs and non-OSGCs.
The responses at 12 directions were fitted with a bimodal Gaussian, as follows:
where R is the response, θ is the direction, R0 is the baseline, θpref is the preferred direction, σ is the SD, and A1 and A2 are the peak amplitudes (Zhao et al. 2013). Fitted tuning curves with R2 larger than 0.3 were used for further analysis. The peak of the fitted curve around the preferred direction was selected, and then the tuning width was defined as the full-width of the half-maximum of the peak.
A Student t-test was used to examine the difference between paired samples and a Kolmogorov-Smirnov (K-S) test to compare the distributions of continuous-valued variables (such as the DSI and the OSI). A two-sample χ2 test was used to compare distributions of categorical variables (such as percentage ON, OFF, and ON-OFF RGCs). Data are expressed as the means ± SE (standard error of mean). The R2 value was calculated as a measure of the goodness of fit in linear regression. Statistical analyses and graphing were done in Prism (GraphPad Software, La Jolla, CA).
RESULTS
Characterization of RGC direction selectivity using the moving bar stimuli.
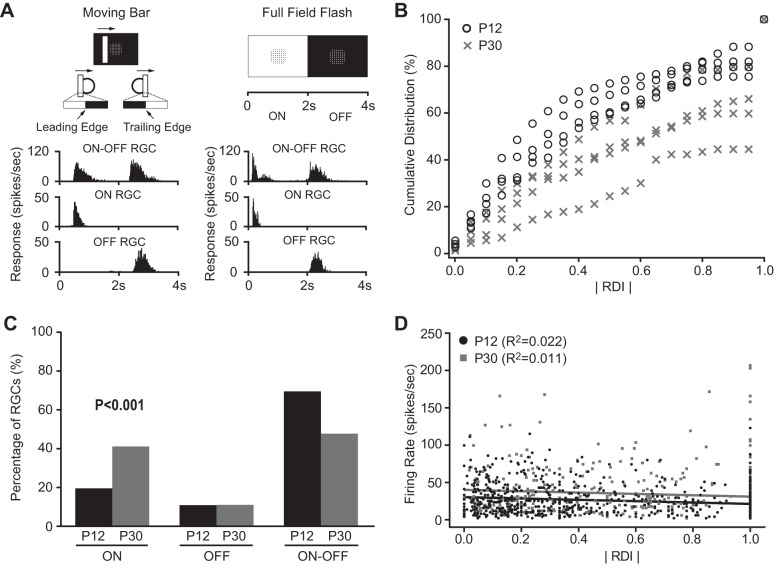
Retinas were dissected and RGC responses to the moving bar stimuli were recorded at postnatal day 12 (P12) (n = 615 cells) and P30 (n = 425 cells, Fig. 1A). The ON- and/or OFF-dominated responses of each cell were characterized by RDI (see methods for details). We also confirmed a cell's ON and OFF responses using the full-filed flash stimulus (Fig. 1A) and found that around 90% of cells had the same ON and OFF responses to both the moving bar and the full-field flash stimuli (data not shown).
Fig. 1.
Characterization of direction-selective ganglion cells (DSGCs) by a 60-channel multielectrode array (MEA) system. A: retinal ganglion cells (RGCs) were classified into ON, OFF, and ON-OFF subtypes based on their responses to the moving bar (left) and/or full-field flash (right) stimuli. Left panel shows the white bar moving upon a black background. The circle illustrates a cell's receptive field (RF). Right panel shows the full screen flash stimuli (with MEA electrodes positioned at the center). B: the cumulative distribution of absolute values of response dominance index (RDI) of all recorded RGCs of mice at postnatal day 12 (P12) (n = 4) and P30 (n = 4). ON-OFF RGCs had RDI close to 0, while ON RGCs and OFF RGCs were close to 1 or −1. Bin width: 0.05. C: more ON RGCs and fewer ON-OFF RGCs were found at P30 compared with P12 (P < 0.001 in χ2 test). D: plot of absolute RDI vs. firing rate of RGCs at P12 and P30. Solid lines represent the linear regressions. The value of R2 ranges from 0 to 1, with 0 representing no linear relationship and 1 perfect linear relationship between the two parameters.
We compared the absolute value of RDIs for all cells recorded at P12 and P30. The mean absolute value of RDI was 1.5-fold higher at P30 (0.62 ± 0.02) than P12 (0.42 ± 0.01; P < 0.001 in Student's t-test), indicating the maturational transition from ON-OFF to ON- or OFF-dominated RGCs. Because RGC response properties may vary from experiment to experiment (Chichilnisky and Kalmar 2002), we examined whether the age-dependent change of RDI was due to the recording variation in different experiments. We compared the RDIs of RGCs from individual retinas at P12 and P30 (n = 4 in each group, Fig. 1B). Although there were some variations within the same age group, the distribution of the absolute value of RDIs from the two age groups has minimum overlap, and the difference of the absolute value of RDIs from the two groups was significant (P < 0.001 in two-way ANOVA test, Fig. 1B). Our data suggest that the change of RDIs with age was not due to the variation of individual experiments.
We classified RGCs into ON, OFF, and ON-OFF, three functional subgroups, as described in methods. We confirmed that the percentage of ON RGCs increased from 19.5% at P12 to 41.2% at P30, and ON-OFF RGCs decreased from 69.6% at P12 to 47.8% at P30 (P < 0.001 in χ2 test, Fig. 1C and Table 1). These results are consistent with our laboratory's previous findings on the maturational transition from ON-OFF to ON or OFF RGCs after eye-opening (Cantrell et al. 2010; Tian and Copenhagen 2003).
Table 1.
Summary of the numbers of RGCs at P12 and P30
| All RGCs |
DSGCs (DSI ≥ 0.33) |
OSGCs (OSI ≥ 0.33) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Retinas | No. of Cells | ON | OFF | ON-OFF | Total | ON | OFF | ON-OFF | Total | ON | OFF | ON-OFF | |
| P12 | 4 | 615 | |||||||||||
| n | 120 | 67 | 428 | 171 | 22 | 13 | 136 | 180 | 25 | 18 | 137 | ||
| % | 19.5 | 10.9 | 69.6 | 27.8 | 3.6 | 2.1 | 22.1 | 29.3 | 4.1 | 2.9 | 22.3 | ||
| P30 | 4 | 425 | |||||||||||
| n | 175 | 47 | 203 | 181 | 44 | 12 | 125 | 203 | 60 | 19 | 124 | ||
| % | 41.2 | 11.0 | 47.8 | 42.6 | 10.4 | 2.8 | 29.4 | 47.8 | 14.1 | 4.5 | 29.2 | ||
n, No. of retinal ganglion cells (RGCs). DSGC, direction-selective ganglion cells; DSI, direction selectivity index; OSGC, orientation-selective ganglion cells; OSI, orientation selectivity index; P12 and P30, postnatal days 12 and 30, respectively.
We next determined whether the change of cell-type percentage was due to the change of a cell's light response strength with age. We calculated the mean peak firing rate to full field flash stimuli and found it increased from P12 (26.5 ± 0.8 spikes/s, n = 615) to P30 (34.5 ± 1.5 spikes/s, n = 425; P < 0.001 in Student's t-test). We next examined the correlations between the RDI and the peak firing rate at P12 and P30. As shown in Fig. 1D, there was no linear correlation between the absolute value of RDI and the peak firing rate (R2 = 0.01 at P12 and R2 = 0.02 at P30). Together our data suggest that the cell-type percentage was not affected by cells' light response strength.
The direction selectivity of RGCs enhanced with age.
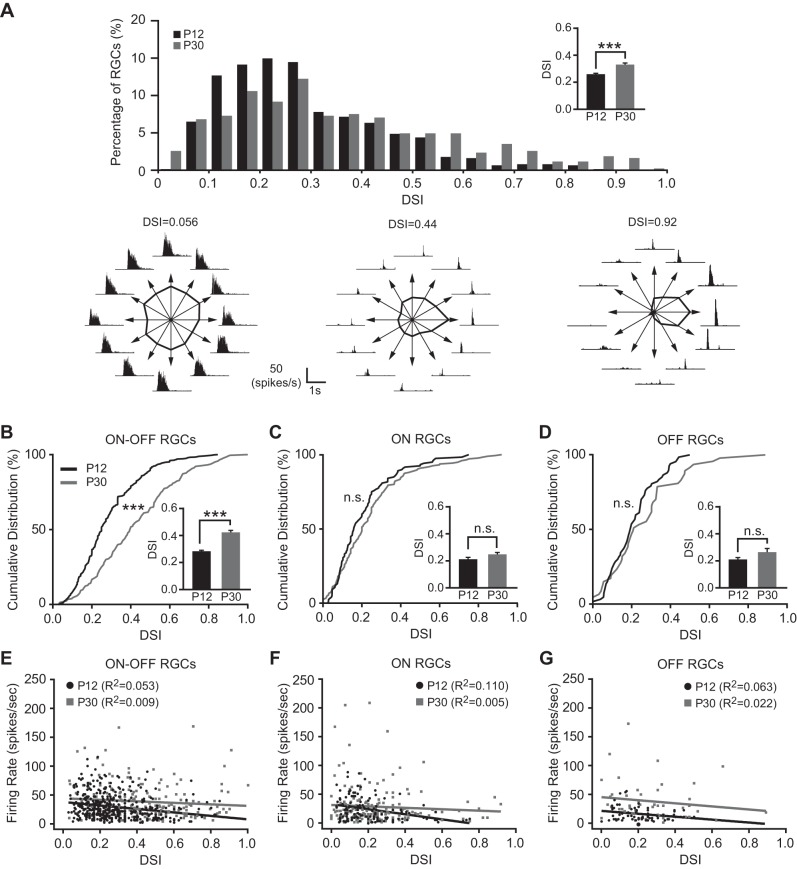
We calculated the DSI for individual RGCs using the response magnitude at preferred and null directions (see methods for details; Fig. 2A). Robust direction-selective (DS) responses were detected at P12 (Fig. 2A), as shown in previous studies (Chen et al. 2009; Elstrott et al. 2008). At 2 wk after eye-opening (P30), the distribution of DSI shifted to the right compared with P12 (Fig. 2A), indicating that more RGCs with strong direction selectivity were detected at P30. The mean DSI at P30 was 0.33 ± 0.01 (n = 425), which was significantly higher than the mean DSI at P12 (0.26 ± 0.01, n = 615; P < 0.001 in Student's t-test; Fig. 2A).
Fig. 2.
The direction selectivity of RGCs enhanced with age. A, top: distributions of direction selectivity index (DSI) from all RGCs at P12 and P30. Bottom: representative recordings of RGCs with different DSIs. The tuning curve was calculated based on the peak spike frequency for 12 directions and plotted as a function of directions of the moving object (see methods). B–D: cumulative distributions of DSI from ON-OFF (B), ON (C), and OFF (D) RGCs at P12 and P30. Insets: mean DSIs of ON-OFF, ON, and OFF RGCs at P12 and P30. E–G: plots of DSI vs. firing rate of ON-OFF (E), ON (F), and OFF (G) RGCs at P12 and P30. Solid lines represent the linear regressions. ***P < 0.001; n.s., not significant (P > 0.05) in Kolmogorov-Smirnov (K-S) test and Student's t-test.
We classified RGCs into ON, OFF, and ON-OFF subtypes and calculated the mean DSI of each functional subtype of RGCs at P12 and P30. The mean DSI of ON-OFF RGCs increased from P12 (0.28 ± 0.01, n = 428) to P30 (0.42 ± 0.02, n = 203; P < 0.001 in Student's t-test and K-S test; Fig. 2B). In contrast, the mean DSI for ON RGCs were not changed from P12 (0.21 ± 0.01, n = 120) to P30 (0.24 ± 0.01, n = 175; P > 0.05 in Student's t-test and K-S test; Fig. 2C); so was the OFF RGCs (P12: 0.21 ± 0.01, n = 67; P30: 0.26 ± 0.03, n = 47; P > 0.05 in Student's t-test and K-S test; Fig. 2D). We also confirmed that the enhanced direction selectivity with age was not due to the increase of peak firing rate. We performed a linear regression between the DSI and the peak firing rate of P12 and P30 RGCs and found that there was no correlation between the DSI and the peak firing rate for all three subtype RGCs (R2 < 0.2, Fig. 2, E–G).
Fraction of DSGCs increases with age.
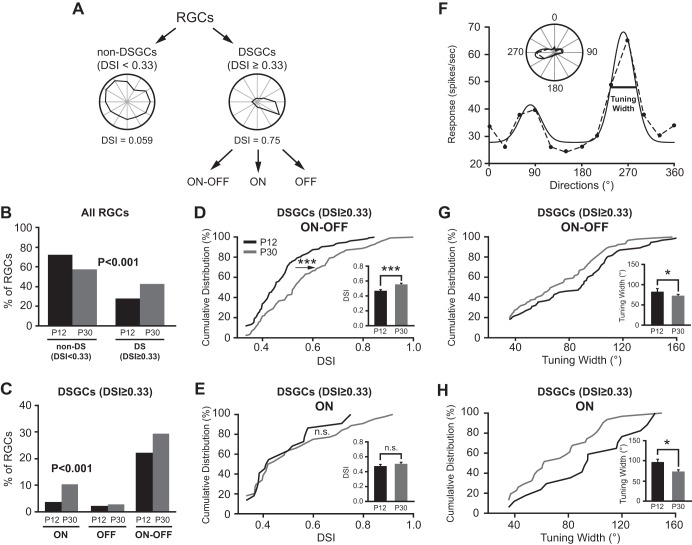
We classified RGCs into DSGCs and non-DSGCs (Fig. 3A). When the DSI exceeds 0.33, the 2:1 ratio for the preferred over null direction responses, we classified the cell as a DSGC. When the DSI is smaller than 0.33, we classified it as a non-DSGC. We find more DSGCs at P30 (42.6%) compared with P12 (27.8%; P < 0.001 in χ2 test; Fig. 3B and Table 1). We also used a stricter criterion with DSI ≥ 0.5, a 3:1 ratio for the preferred over null direction responses, and further confirmed that the percentage of DSGCs increased with age (8.9% at P12 to 22.4% at P30, P < 0.001 in χ2 test).
Fig. 3.
Development of DSGCs is subtype dependent. A: the schematic diagram of RGC classification. B: the percentage of DSGCs increased while non-DSGCs decreased from P12 to P30. P < 0.001 in χ2 test. C: the percentages of both ON and ON-OFF DSGCs increased from P12 to P30. P < 0.001 in χ2 test. D and E: cumulative distributions of DSI for ON-OFF (D) and ON (E) DSGCs. Insets: mean DSIs of ON-OFF (D) and ON (E) DSGCs at P12 and P30. F: tuning width was calculated as the full-width of the half-maximum of the fitted tuning curve (solid line, see methods for details). G and H: the tuning width decreased with age for both ON-OFF (G) and ON (H) DSGCs. Insets: mean tuning width of ON-OFF and ON DSGCs at P12 and P30. *P < 0.05; ***P < 0.001; n.s. (P > 0.05) in K-S test and Student's t-test.
DSGCs were also classified into ON, OFF, and ON-OFF three subtypes (Fig. 3A). The fractions of ON and ON-OFF DSGCs increased from P12 to P30 (ON: 3.6% to 10.4%; ON-OFF: 22.1% to 29.4%), while OFF DSGCs were not changed (2.1% vs. 2.8%, Fig. 3C and Table 1). We excluded the OFF DSGCs from further analysis because of the small sampling numbers.
The development of direction selectivity is subtype dependent.
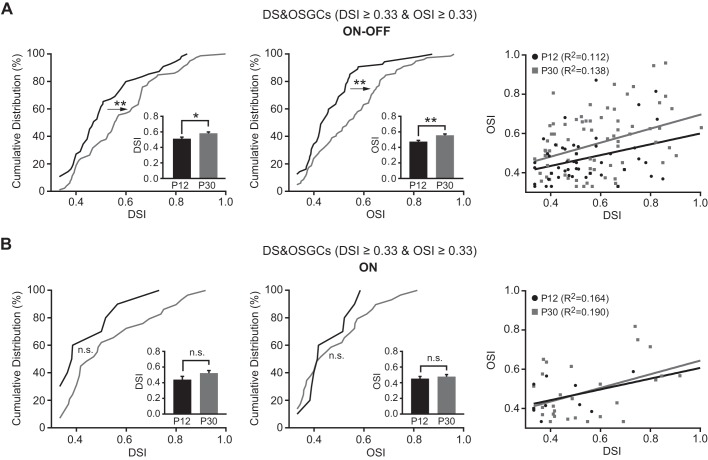
For ON-OFF DSGCs, the cumulative distribution of DSI at P30 shifted to the right compared with P12 (Fig. 3D, P < 0.001 in K-S test), and the mean DSI increased from 0.47 ± 0.01 at P12 (n = 136) to 0.56 ± 0.01 at P30 (Fig. 3D, inset; n = 125; P < 0.001 in Student's t-test). These results indicated that ON-OFF RGCs exhibited stronger direction selectivity at P30 than P12. By contrast, the cumulative distribution of DSI and the mean DSI of ON DSGCs (Fig. 3E) showed no significant difference between P12 and P30 (P30: 0.51 ± 0.03, n = 44; P12: 0.48 ± 0.03, n = 22; P = 0.85 in K-S test, and P = 0.46 in Student's t-test).
The tuning widths of DSGCs were calculated at P12 and P30 (Fig. 3, F–H). At P12, the mean tuning width for ON-OFF DSGCs was 82.0 ± 3.8° (n = 93) and the ON DSGCs was 95.0 ± 8.8° (n = 17). At P30, the mean tuning width decreased for both ON-OFF (71.5 ± 3.3°, n = 91) and ON DSGCs (71.8 ± 5.5°, n = 31; P < 0.05 in Student's t-test; Fig. 3, G and H). Our results show that the fractions of both ON-OFF and ON DSGCs increased and their tuning widths decreased, and that the direction selectivity of ON-OFF but not ON DSGCs was enhanced with age.
Fraction of OSGCs increases with age.
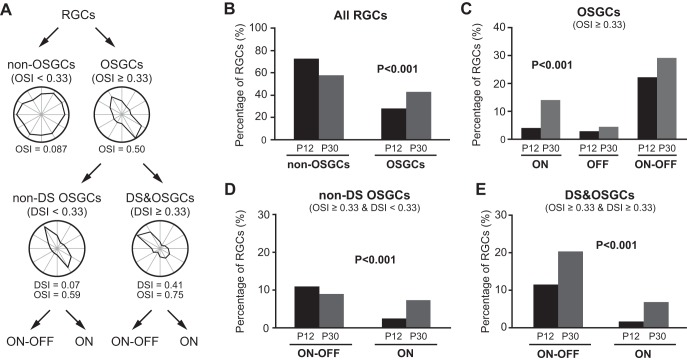
We next examined the OSGCs at P12 and P30. The OSI for each individual cell was calculated (see methods for details), and cells were classified into OSGCs (OSI ≥ 0.33) and non-OSGCs (OSI < 0.33, Fig. 4, A and B). The percentage of OSGCs increased from 29.3% at P12 to 47.8% at P30 (P < 0.001 in χ2 test; Fig. 4B and Table 1). We also applied the cutoff value of 0.5 and found similar increase (7.6% at P12 to 22.6% at P30, P < 0.001 in χ2 test). This increase is due to the increase of the percentages of both ON and ON-OFF OSGCs (ON: 4.1% to 14.1%; ON-OFF: 22.3% to 29.2%, P < 0.001 in χ2 test; Fig. 4C and Table 1).
Fig. 4.
The fraction of orientation-selective ganglion cells (OSGCs) increases with age. A: the schematic diagram of RGC classification. B: the percentage of OSGCs increased while non-OSGCs decreased from P12 to P30. P < 0.001 in χ2 test. C: the percentages of both ON and ON-OFF OSGCs increased from P12 to P30. P < 0.001 in χ2 test. D: the increase of non-direction-selective (non-DS) OSGCs was mainly due to the increase of ON cells. P < 0.001 in χ2 test. E: the increase of DS&OSGCs (direction-selective OSGCs) was due to the increases of both ON-OFF and ON cells. P < 0.001 in χ2 test.
Since some OSGCs also showed direction selectivity, the above increase might reflect the increase of DSGCs as well. We thus divided OSGCs into two subtypes based on their DSI value: DS&OSGCs (with both OSI and DSI ≥ 0.33) and non-DS OSGCs (DSI < 0.33 and OSI ≥ 0.33, Fig. 4A). In other words, DS&OSGCs exhibited the characteristic of both orientation and direction selectivity, and non-DS OSGCs exhibited strong orientation but not direction selectivity. We found that the fractions of both non-DS OSGCs and DS&OSGCs were increased from P12 to P30 (non-DS OSGCs: 15.8% to 19.5%; DS&OSGCs: 13.5% to 28.2%; Fig. 4, D and E). Furthermore, the increase of non-DS OSGCs (Fig. 4D) was mainly due to the increase of ON cells (2.4% at P12 to 7.3% at P30). By contrast, the increase of DS&OSGCs (Fig. 4E) was due to the increases of both ON-OFF (11.4% at P12 to 20.2% at P30) and ON cells (1.6% at P12 to 6.8% at P30).
The development of OSGCs exhibits different patterns compared with the DSGCs.
The DS&OSGCs (with both OSI and DSI ≥ 0.33) have both strong direction and orientation selectivity, raising the possibility that the DS and OS properties might be regulated by the same mechanism in these cells. We thus examined the developmental profiles of the direction and orientation selectivity of DS&OSGCs. For ON-OFF cells, the mean DSI was 0.51 ± 0.02 (n = 55), and the mean OSI was 0.47 ± 0.02 (n = 55) at P12. They both increased with age (DSI at P30: 0.58 ± 0.02, n = 79, P < 0.05 in Student's t-test and P < 0.01 in K-S test; OSI at P30: 0.55 ± 0.02, n = 79; P < 0.01 in Student's t-test and K-S test; Fig. 5A). In contrast, the mean DSI and mean OSI of the ON cells did not change significantly (P > 0.05 in Student's t-test and K-S test; Fig. 5B). The mean tuning widths of both ON-OFF and ON DS&OSGCs were similar between the two age groups (ON-OFF: 71.6 ± 4.1°, n = 53 at P12 and 64.2 ± 3.2°, n = 71 at P30; ON: 78.0 ± 7.6°, n = 10 at P12 and 66.7 ± 5.7°, n = 23 at P30; P > 0.05 in Student's t-test).
Fig. 5.
For DS&OSGCs, the direction and orientation selectivity of ON-OFF cells were enhanced, while ON cells were unchanged. A and B, left: cumulative distributions of DSI for ON-OFF (A) and ON (B) DS&OSGCs at P12 and P30. Middle: cumulative distributions of OSI for ON-OFF and ON DS&OSGCs. Insets: mean OSIs and tuning widths of ON-OFF and ON DS&OSGCs. Right: plots of DSI vs. OSI of ON-OFF and ON DS&OSGCs show that their direction and orientation selectivity are not correlated. Solid lines represent the linear regressions. *P < 0.05; **P < 0.01; n.s. (P > 0.05) in K-S test and Student's t-test.
We next tested whether the direction selectivity correlated with the orientation selectivity for the DS&OSGCs. We performed a linear regression between DSI and OSI at P12 and P30. As shown in Fig. 5, A and B (right panels), the correlation between DSI and OSI was very week (R2 < 0.2). The slopes of the fitted lines at P12 and P30 were identical for both ON-OFF and ON DS&OSGCs. Together these results suggest that the direction and orientation selectivity are two distinct properties of RGCs.
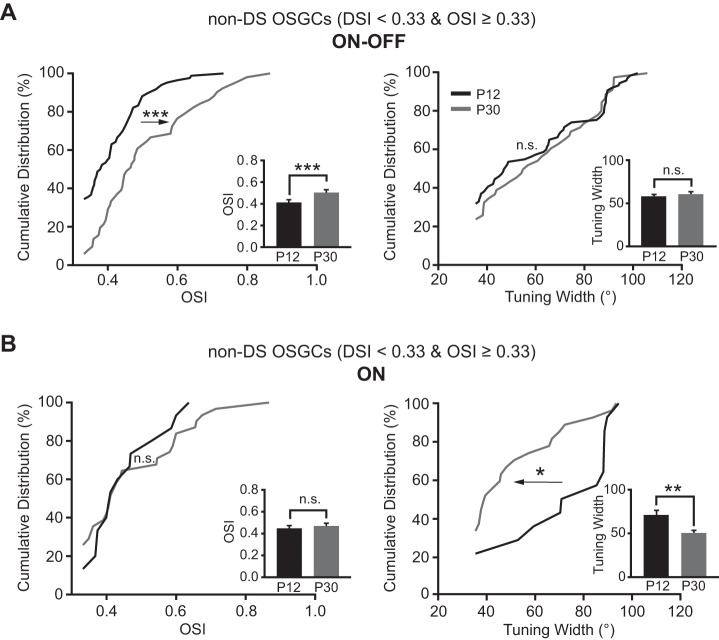
We also examined whether the OSI of non-DS OSGCs (DSI < 0.33 and OSI ≥ 0.33) increased with age. For ON-OFF RGCs, the mean OSI of non-DS OSGCs was 0.51 ± 0.02 at P30 (n = 51), larger than that of P12 (0.41 ± 0.01, n = 84; P < 0.001 in Student's t-test and K-S test; Fig. 6A, left panel). Their tuning width exhibited no difference between P12 (57.7 ± 2.6°, n = 78) and P30 (60.2 ± 3.3°, n = 46; P = 0.5 in Student's t-test and P = 0.7 in K-S test; Fig. 6A, right panel). By contrast, for ON RGCs, the mean OSI of non-DS OSGCs showed no significant change between P12 (0.45 ± 0.03, n = 15) and P30 (0.47 ± 0.03, n = 31; P = 0.6 in Student's t-test and P = 0.7 in K-S test; Fig. 6B, left panel). Their mean tuning width decreased from 70.3 ± 6.0° at P12 (n = 14) to 49.8 ± 3.6° at P30 (n = 27; P < 0.01 in Student's t-test and P < 0.05 in K-S test; Fig. 6B, right panel).
Fig. 6.
Development of non-DS OSGCs is also subtype dependent. A and B, left: the OSI of ON-OFF (A) but not ON (B) non-DS OSGCs significantly increased from P12 to P30. Right: the tuning width of ON non-DS OSGCs decreased, while ON-OFF cells were unchanged. Insets: mean OSIs and tuning widths of ON-OFF and ON non-DS OSGCs. *P < 0.05; **P < 0.01; ***P < 0.001; n.s. (P > 0.05) in K-S test and Student's t-test.
Taken together, our data showed that nearly one-third of RGCs were highly tuned for the direction or orientation of motion stimulus at the time of eye-opening. The percentages of DSGCs and OSGCs continued to increase with age. The direction and orientation selectivity was enhanced for ON-OFF, but not ON RGCs with development. For DSGCs, the tuning width of both ON-OFF and ON subtypes decreased with age. For non-DS OSGCs, the tuning width decreased for ON but not ON-OFF subtype. Moreover, there was no correlation between the DSI and the OSI for DS&OSGCs. In other words, DSGCs and OSGCs mature differently in a subtype-dependent manner.
DISCUSSION
Classification of DSGCs.
Three functional subgroups of DSGCs have been characterized: 1) ON DSGCs respond to global motion in one of three directions: upward, downward, or anterior (Oyster 1968; Oyster and Barlow 1967); 2) OFF DSGCs respond to upward motion (Kim et al. 2008); and 3) ON-OFF DSGCs respond preferentially to image motion in one of the four cardinal ocular directions: ventral, dorsal, temporal and nasal (Briggman et al. 2011; Elstrott et al. 2008; Oyster 1968; Oyster and Barlow 1967). In the rabbit retina, ON-OFF DSGCs account for about 21% of the total RGCs (Oyster 1968). In the mouse retina, different studies revealed quite a wide range of DSGCs. A recent study combined the two-photon calcium imaging with the serial-section electron microscopy and suggested about 10% DSGCs in the mouse retina (Briggman et al. 2011). One group of ON-OFF DSGCs labeled by 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) is counted for 16% of total RGCs (Sun et al. 2002), while another study suggested 21% (Xu and Tian 2007). Huberman and colleagues (2009) suggested the total ON-OFF DSGCs is about 20–36%. Here we found about 29% of total RGCs are ON-OFF DSGCs (Fig. 3C and Table 1), which falls into the range in the above study. ON DSGCs are counted for 5% of the total RGCs in the rabbit retina (Oyster 1968) and 3–7% in the mouse retina (Sun et al. 2002; Xu and Tian 2007). Here we show ON DSGCs was ∼10% at P30 (Fig. 3C and Table 1). The differences observed in these studies could be due to that 1) different visual stimulation was applied to identify DSGCs (see discussion below); and 2) different techniques may have different sensitivity to detect certain population of RGCs; 3) RGC dendritic pattern and molecular markers may not completely correlate with its light response properties (Rivlin-Etzion et al. 2011, 2012).
Development of DSGCs and OSGCs.
One study using drifting gratings showed that the distribution of the tuning width for ON-OFF DSGCs at P14 had a broader value range compared with the adult retinas (Elstrott et al. 2008). However, drifting gratings could generate adapted responses and even could reverse the directional preference (Rivlin-Etzion et al. 2012). Another study using the moving bar stimulus showed that the degree of direction selectivity of early postnatal DSGCs (both ON-OFF and ON DSGCs) was almost identical to that in adult retinas (Chen et al. 2009). When we applied the DSI cutoff value of 0.6 (a 4:1 ratio for the preferred over null direction responses) to identify DSGCs, we found that the mean DSIs and the tuning widths of DSGCs had no difference at P12 and P30 (data not shown). Our results are consistent with the notion that the fraction of highly DS RGCs exist at the time of eye opening and exhibit similar directional turning properties as in adult retinas. However, when we used the DSI cutoff of 0.33 or 0.5 (a 2:1 or 3:1 ratio for the preferred over null direction responses), we find that both the fraction and the average DSI of DSGCs are higher at P30 than that at P12 (Fig. 3). These findings suggest that some DSGCs may continue to develop after eye-opening.
Recently it has been shown that OSGCs are abundant in the adult mouse retina (Zhao et al. 2013). Similarly to the DSGCs, both ON-OFF and ON OSGCs were detected at P12, and the fraction of OSGCs increased from P12 to P30 (Fig. 4). However, OSGCs exhibit different developing patterns compared with DSGCs (Figs. 5 and 6). Studies in the rabbit retina found that orientation bias did not contribute to the generation of direction selectivity (He et al. 1998). We also found no correlation between direction and orientation selectivity in the mouse retina (Fig. 5). These findings support the notion that direction and orientation selectivity are two distinct features of RGCs.
Different mechanisms for generating direction and orientation selectivity have been suggested. The direction selectivity was generated by asymmetric inhibitory and excitatory inputs and temporal offset between inhibitory and excitatory inputs (Vaney et al. 2012; Wei and Feller 2011). Other studies suggest that an elongated RF could simply contribute the orientation bias (Levick and Thibos 1982). Alternatively, orientation bias could result from electrical coupling with neighboring RGCs of the same subtype (Volgyi et al. 2009). The coupled cells may respond more to the orientation that cross their RFs. Some studies also showed that the orientation selectivity is mediated by presynaptic inhibition (Bloomfield 1994; Venkataramani and Taylor 2010). Much remains to be investigated of the developmental mechanisms of DSGCs and especially OSGCs.
Development of the direction and orientation selectivity in the visual system.
Orientation selectivity in cortical neurons was suggested to be built from untuned thalamic inputs (Ferster and Miller 2000; Hubel and Wiesel 1962; Shapley et al. 2003; Sompolinsky and Shapley 1997). However, recent studies in mice showed that the orientation-selective cells are abundant in dorsal lateral geniculate nucleus (dLGN) (Marshel et al. 2012; Piscopo et al. 2013; Scholl et al. 2013; Zhao et al. 2013), and the orientation selectivity in dLGN does not require cortical feedback (Scholl et al. 2013; Zhao et al. 2013). These findings suggest that the orientation selectivity of some dLGN neurons might be transmitted from the retina (Zhao et al. 2013). In fact, there is a disynaptic circuit linking DSGCs with the superficial layers of the primary visual cortex (V1), through a specialized subdivision of the dLGN (Cruz-Martin et al. 2014), further supporting the notion that the direction and orientation selectivity of some V1 neurons may be influenced by the activities of RGCs.
In addition, studies revealed some similarities between the development of direction selectivity of the cortical neurons and the retinal neurons in mice (Elstrott et al. 2008; Rochefort et al. 2011). The direction selectivity of V1 neurons was first detected at the time of eye-opening and emerged independently of visual experience (Rochefort et al. 2011), similarly as in the retina (Elstrott et al. 2008). Furthermore, after eye-opening, the percentages of motion-sensitive and orientation-selective neurons in V1 increased (Rochefort et al. 2011), also similar, as we observed in the retina. More work is needed to examine how the direction and orientation selectivity in the retina emerges and relays to the cortical neurons during postnatal development.
In summary, our data demonstrate that both DS and OS circuits continue to develop after eye opening in the mouse retina. The development of direction and orientation selectivity is subtype dependent with different patterns. Our study provides the foundation to investigate the circuit functions that underlie the development of the direction and orientation selectivity in RGCs, and to understand the visual information processing in the visual system.
GRANTS
The work contained in this paper has been supported by the William & Mary Greve Special Scholar Award from the Research to Prevent Blindness (X. Liu), Northwestern Memorial Foundation/Brinson Foundation (X. Liu), the Illinois Society for the Prevention of Blindness (H. Chen), and National Eye Institute Grants R01-EY-012345 (N. Tian), 5P30-EY-014800 (N. Tian) and R01-EY-019034 (X. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.C. and N.T. conception and design of research; H.C. performed experiments; H.C., X.L., and N.T. analyzed data; H.C., X.L., and N.T. interpreted results of experiments; H.C., X.L., and N.T. prepared figures; H.C., X.L., and N.T. drafted manuscript; H.C., X.L., and N.T. edited and revised manuscript; H.C., X.L., and N.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jianhua Cang, Dr. John B. Troy, Brent K. Young, and Kevin Huang for critical reading.
REFERENCES
- Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science 139: 412–414, 1963. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA. Orientation-sensitive amacrine and ganglion cells in the rabbit retina. J Neurophysiol 71: 1672–1691, 1994. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471: 183–188, 2011. [DOI] [PubMed] [Google Scholar]
- Cantrell DR, Cang J, Troy JB, Liu X. Non-centered spike-triggered covariance analysis reveals neurotrophin-3 as a developmental regulator of receptive field properties of ON-OFF retinal ganglion cells. PLoS Comput Biol 6: e1000967, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Chiao CC. Effect of visual experience on the maturation of ON-OFF direction selective ganglion cells in the rabbit retina. Vision Res 48: 2466–2475, 2008. [DOI] [PubMed] [Google Scholar]
- Chen M, Weng S, Deng Q, Xu Z, He S. Physiological properties of direction-selective ganglion cells in early postnatal and adult mouse retina. J Physiol 587: 819–828, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci 22: 2737–2747, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martin A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507: 358–361, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron 58: 499–506, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zhao Y, Yoshida M, Chen H, Yang JF, Kim TS, Cang J, Troy JB, Liu X. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Invest Ophthalmol Vis Sci 54: 1106–1117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23: 441–471, 2000. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420: 411–414, 2002. [DOI] [PubMed] [Google Scholar]
- He S, Levick WR, Vaney DI. Distinguishing direction selectivity from orientation selectivity in the rabbit retina. Vis Neurosci 15: 439–447, 1998. [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature 389: 378–382, 1997. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol 160: 106–154, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62: 327–334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature 452: 478–482, 2008. [DOI] [PubMed] [Google Scholar]
- Koehler CL, Akimov NP, Renteria RC. Receptive field center size decreases and firing properties mature in ON and OFF retinal ganglion cells after eye opening in the mouse. J Neurophysiol 106: 895–904, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68: 1159–1172, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick WR. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol 188: 285–307, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick WR, Thibos LN. Analysis of orientation bias in cat retina. J Physiol 329: 243–261, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76: 713–720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg S, Carcieri SM, Jacobs AL, Latham PE. Retinal ganglion cells act largely as independent encoders. Nature 411: 698–701, 2001. [DOI] [PubMed] [Google Scholar]
- Oyster CW. The analysis of image motion by the rabbit retina. J Physiol 199: 613–635, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science 155: 841–842, 1967. [DOI] [PubMed] [Google Scholar]
- Passaglia CL, Troy JB, Ruttiger L, Lee BB. Orientation sensitivity of ganglion cells in primate retina. Vision Res 42: 683–694, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci 33: 4642–4656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Wei W, Feller MB. Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron 76: 518–525, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci 31: 8760–8769, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron 71: 425–432, 2011. [DOI] [PubMed] [Google Scholar]
- Scholl B, Tan AY, Corey J, Priebe NJ. Emergence of orientation selectivity in the mammalian visual pathway. J Neurosci 33: 10616–10624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron 38: 689–699, 2003. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Shapley R. New perspectives on the mechanisms for orientation selectivity. Curr Opin Neurobiol 7: 514–522, 1997. [DOI] [PubMed] [Google Scholar]
- Sun L, Han X, He S. Direction-selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLos One 6: e19477, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol 451: 115–126, 2002. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 39: 85–96, 2003. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci 13: 194–208, 2012. [DOI] [PubMed] [Google Scholar]
- Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci 30: 15664–15676, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol 512: 664–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Feller MB. Organization and development of direction-selective circuits in the retina. Trends Neurosci 34: 638–645, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469: 402–406, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Tian N. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening. J Comp Neurol 503: 244–259, 2007. [DOI] [PubMed] [Google Scholar]
- Zhao X, Chen H, Liu X, Cang J. Orientation-selective responses in the mouse lateral geniculate nucleus. J Neurosci 33: 12751–12763, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]