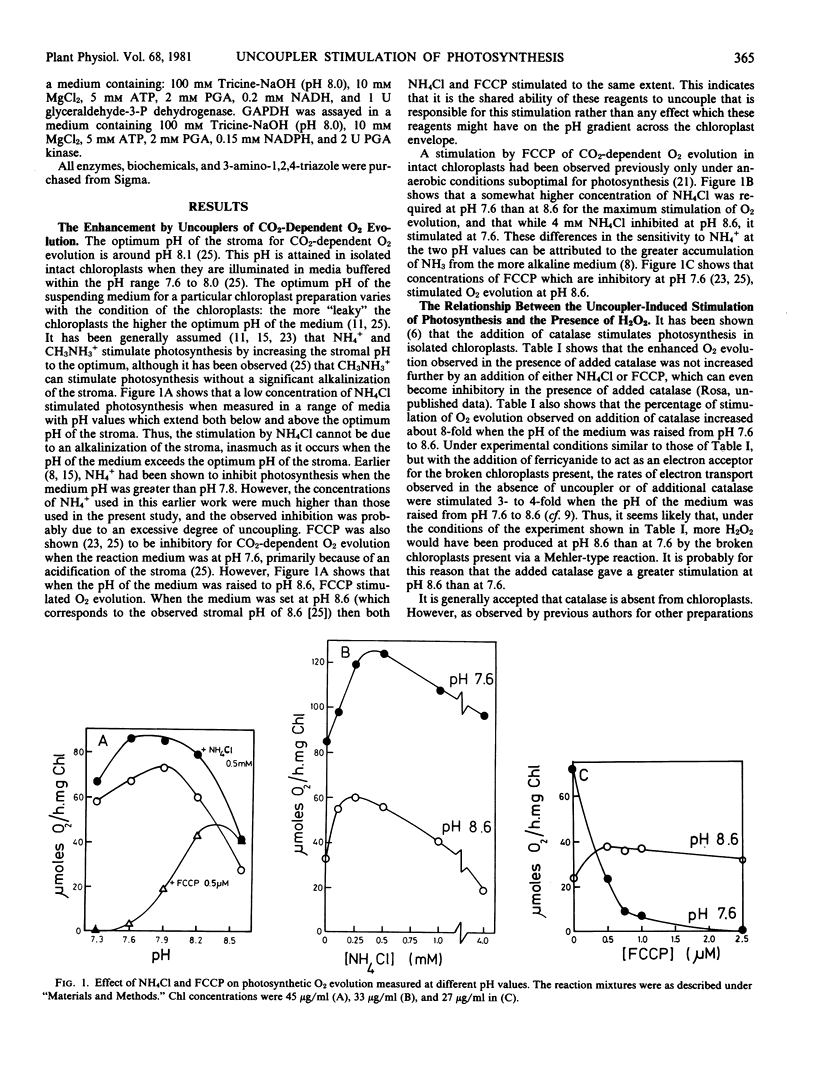
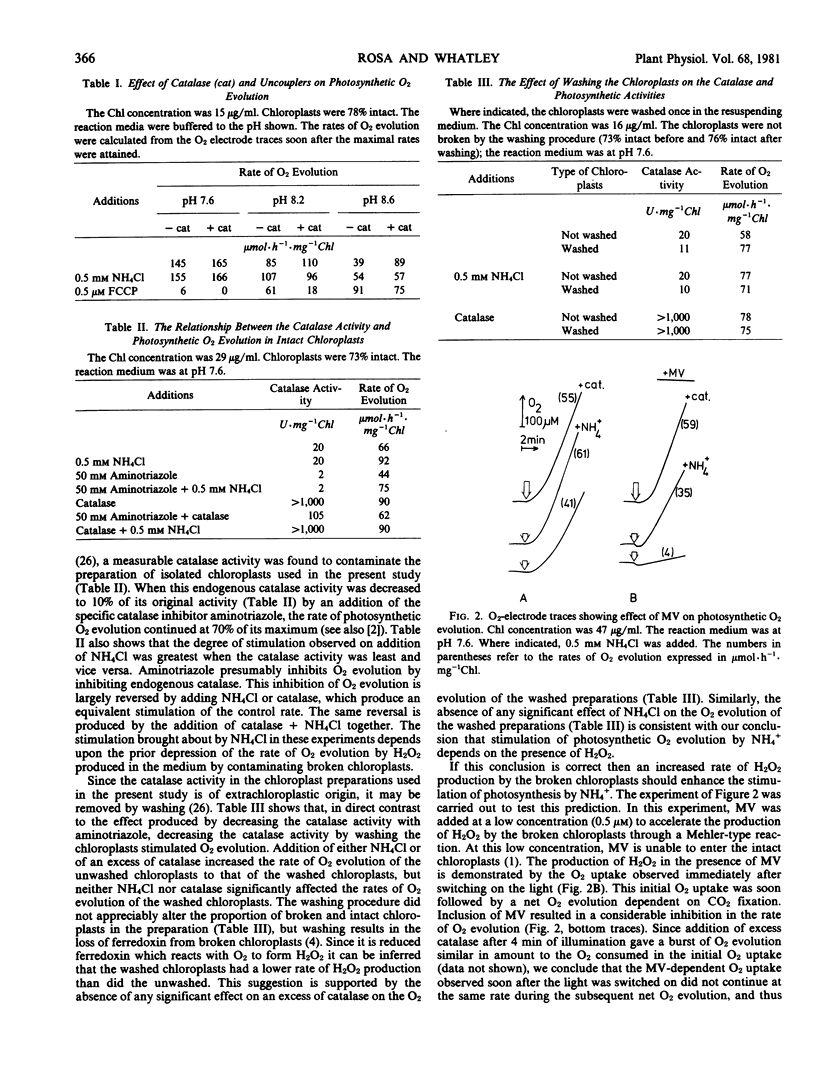
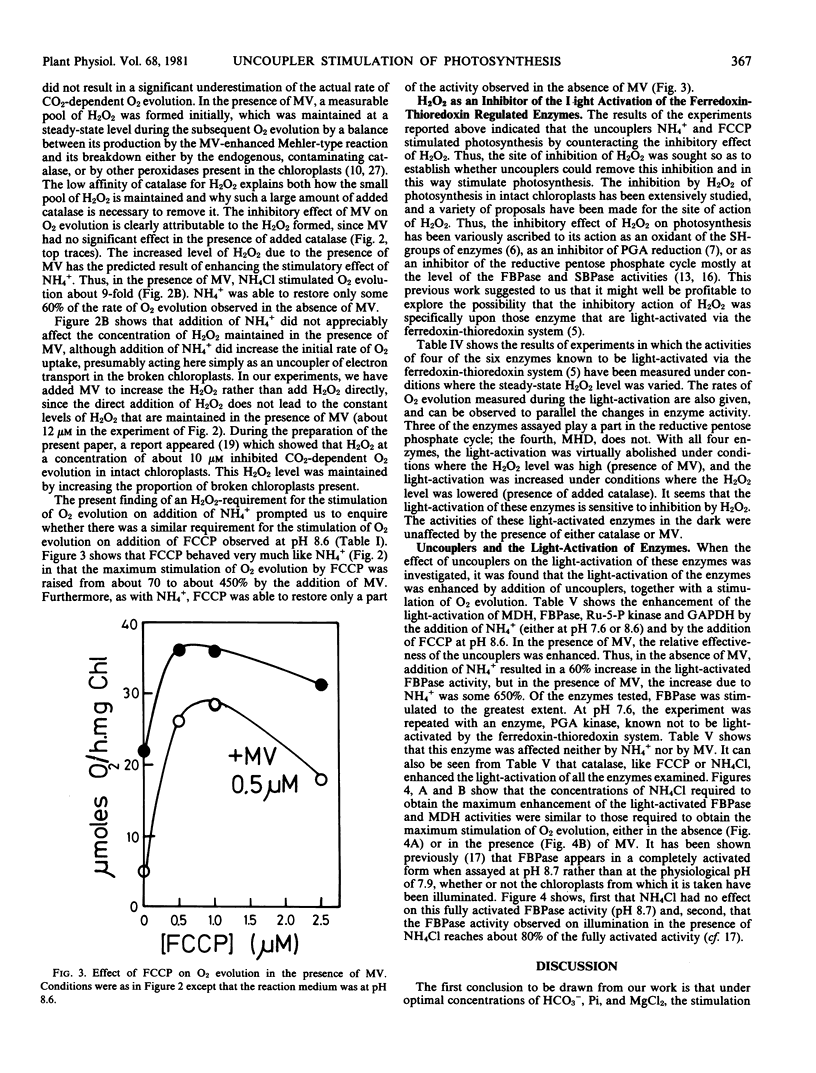
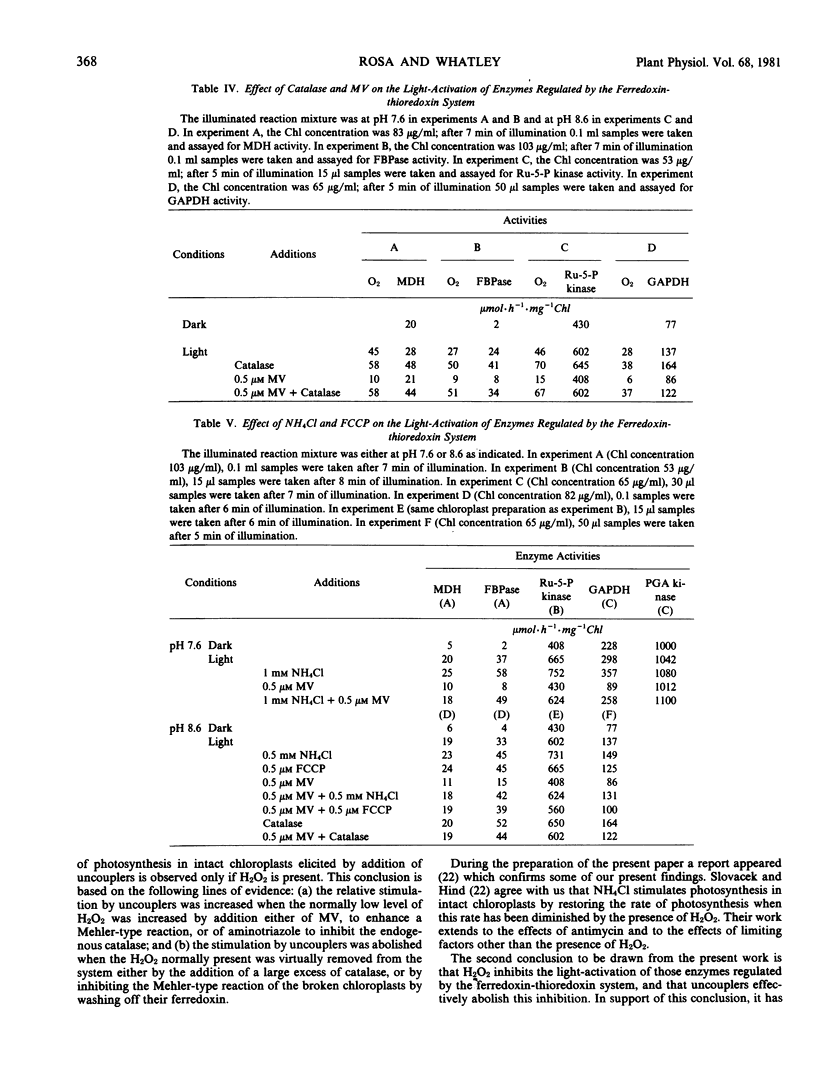
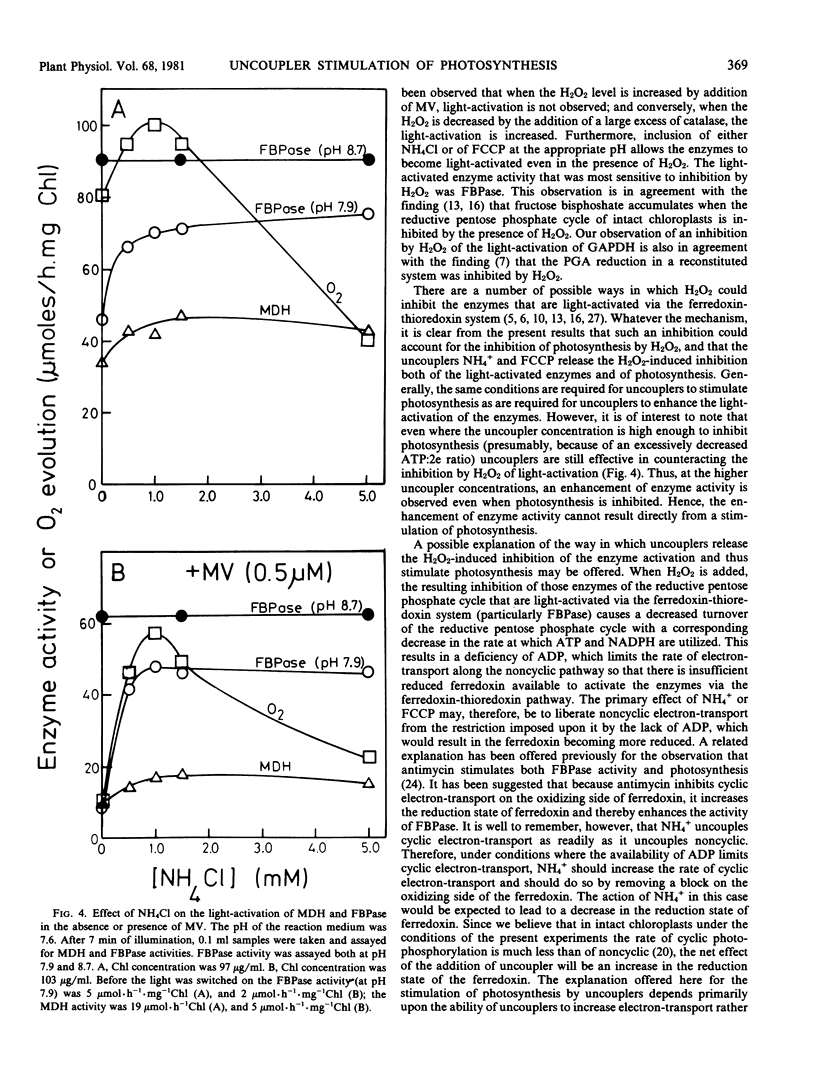
Abstract
Some uncouplers stimulate CO2-dependent O2 evolution by intact spinach chloroplasts at pH 8.6. This effect is not due to alkalinization of the stroma. The stimulation is observed only when photosynthesis has been partly inhibited by the presence of H2O2, generated in a Mehler-type reaction by the broken chloroplasts which always contaminate the intact chloroplast preparations. The addition of methyl viologen increases the Mehler-type reaction and results in greater inhibition of photosynthesis. The addition of excess catalase stimulates photosynthesis by preventing accumulation of H2O2. The uncouplers stimulate photosynthesis primarily by enhancing the light-activation of enzymes that are regulated by the ferredoxin-thioredoxin system, and this effect results from the influence of the uncouplers on the redox poising of the ferredoxin in the intact chloroplasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F., Whatley F. R. Effects of inhibitors of catalase on photosynthesis and on catalase activity in unwashed preparations of intact chloroplasts. Plant Physiol. 1978 Jun;61(6):957–960. doi: 10.1104/pp.61.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965 Sep 24;149(3691):1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Forti G., Gerola P. Inhibition of photosynthesis by azide and cyanide and the role of oxygen in photosynthesis. Plant Physiol. 1977 May;59(5):859–862. doi: 10.1104/pp.59.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Leech R. M. The stimulation of CO2-supported O2 evolution in intact spinach chloroplasts by ammonium ion. Arch Biochem Biophys. 1978 Sep;190(1):221–226. doi: 10.1016/0003-9861(78)90271-0. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Effect of pH on chloroplast photosynthesis. Inhibition of O2 evolution by inorganic phosphate and magnesium. Biochim Biophys Acta. 1979 Jan 11;545(1):131–140. doi: 10.1016/0005-2728(79)90120-8. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Smith M. G., Gibbs M. Influence of Hydrogen Peroxide upon Carbon Dioxide Photoassimilation in the Spinach Chloroplast: I. HYDROGEN PEROXIDE GENERATED BY BROKEN CHLOROPLASTS IN AN "INTACT" CHLOROPLAST PREPARATION IS A CAUSAL AGENT OF THE WARBURG EFFECT. Plant Physiol. 1980 Apr;65(4):755–759. doi: 10.1104/pp.65.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa L. The ATP/2e ratio during photosynthesis in intact spinach chloroplasts. Biochem Biophys Res Commun. 1979 May 14;88(1):154–162. doi: 10.1016/0006-291x(79)91710-8. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Energetic factors affecting carbon dioxide fixation in isolated chloroplasts. Plant Physiol. 1980 Mar;65(3):526–532. doi: 10.1104/pp.65.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Influence of antimycin a and uncouplers on anaerobic photosynthesis in isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):538–542. doi: 10.1104/pp.60.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillberg J. E., Giersch C., Heber U. CO2 reduction by intact chloroplasts under a diminished proton gradient. Biochim Biophys Acta. 1977 Jul 7;461(1):31–47. doi: 10.1016/0005-2728(77)90067-6. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R., Fitzgerald M. P. Photosynthesis in a reconstituted chloroplast system from spinach. Some factors affecting CO2-dependent oxygen evolution with fructose-1,6-bisphosphate as substrate. Biochim Biophys Acta. 1976 Jul 9;440(1):147–162. doi: 10.1016/0005-2728(76)90120-1. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


