Abstract
Intrathecal administration of the neurotoxin bombesin-saporin reduces or abolishes pruritogen-evoked scratching behavior. We investigated whether spinal neurons that respond to intradermal (ID) injection of pruritogens also respond to spinal superfusion of bombesin and vice versa. Single-unit recordings were made from superficial lumbar spinal dorsal horn neurons in anesthetized mice. We identified neurons with three search strategies: 1) ID injection of the nonhistaminergic itch mediator chloroquine, 2) spinal superfusion of bombesin, and 3) noxious pinch. All units were tested with an array of itch mediators (chloroquine, histamine, SLIGRL, BAM8-22), algogens [capsaicin, allyl isothiocyanate (AITC)], and physical stimuli (brush, pinch, noxious heat, cooling) applied to the hindlimb receptive field. The vast majority of chloroquine-responsive units also responded to bombesin. Of 26 chloroquine-sensitive units tested, most responded to SLIGRL, half responded to histamine and/or BAM8-22, and most responded to capsaicin and/or AITC as well as noxious thermal and mechanical stimuli. Of 29 bombesin-responsive units, a large majority also responded to other itch mediators as well as AITC, capsaicin, and noxious thermal and mechanical stimuli. Responses to successive applications of bombesin exhibited tachyphylaxis. In contrast, of 36 units responsive to noxious pinch, the majority (67%) did not respond to ID chloroquine or spinal bombesin. It is suggested that chloroquine- and bombesin-sensitive spinal neurons signal itch from the skin.
Keywords: bombesin, gastrin-releasing peptide, mice, superficial dorsal horn, Mrgpr, TRPA1, TRPV1
itch is an all-too common symptom of many skin and systemic diseases that negatively impacts the quality of life. Chronic itch associated with atopic dermatitis has been estimated to exact socioeconomic costs exceeding $3 billion annually in the US (NIAMS 2009). Most cases of chronic itch are poorly treated by antihistamines, and a better understanding of the underlying neural mechanisms of itch is required to develop more effective evidence-based treatments.
Recent studies implicate gastrin-releasing peptide (GRP) as a neuropeptide transmitter released from intraspinal terminals of primary afferent pruriceptors (Liu et al. 2014; Sun et al. 2009; Sun and Chen 2007), or from excitatory spinal interneurons (Mishra and Hoon 2013), to excite superficial spinal dorsal horn neurons expressing the GRP receptor (GRPR) that in turn signal itch to higher levels of the nervous system. GRP is a mammalian homolog of bombesin, a 14-amino acid peptide from frog skin that acts at the three known bombesin receptors, one of which is GRPR (also known as BB2) (Jensen et al. 2008). Intradermal (ID) (Andoh et al. 2011) and intrathecal (IT) injection of GRP (Mishra and Hoon 2013; Sukhtankar and Ko 2013; Sun et al. 2009) elicited itch-related scratching behavior in mice and excited a small subset of rat dorsal horn neurons (Koga et al. 2011). Importantly, destruction of superficial dorsal horn neurons by IT delivery of the neurotoxin bombesin-saporin reduced or abolished pruritogen-evoked scratching behavior (Liu et al. 2014; Mishra and Hoon 2013; Sun et al. 2009), implicating GRPR-expressing spinal neurons in signaling itch.
A subset of neurons in the murine superficial dorsal horn respond to ID injection of histamine and a variety of nonhistaminergic pruritogens such as serotonin and SLIGRL (reviewed in Akiyama and Carstens 2013). Recent studies have implicated three of the family of Mas-related G protein-coupled receptors (Mrgprs) (Dong et al. 2001), namely, MrgprA3, C11, and D, in peripheral itch transduction (Liu et al. 2009, 2011, 2012; Wilson et al. 2011). MrgprA3 is activated by the antimalarial drug chloroquine, which often elicits itch in patients (Mnyika and Kihamia 1991) and scratching behavior in rodents (Akiyama et al. 2012a, 2012b; Inan and Cowan 2004; Liu et al. 2009; Wilson et al. 2011). Chloroquine excites superficial dorsal horn neurons, consistent with their proposed role in mediating itch (Akiyama et al. 2012b, 2014). Because GRPR-expressing spinal neurons have been implicated in signaling itch, the first aim of the present study was to investigate whether spinal neurons responsive to cutaneously delivered pruritogens also respond to intraspinal delivery of bombesin. Conversely, we also tested whether bombesin-sensitive spinal neurons respond to cutaneous delivery of pruritogens. A second aim was to assess the relative proportions of pruritogen-sensitive and -insensitive nociceptive neurons in the superficial dorsal horn. To this end, we investigated whether neurons identified by their response to a noxious pinch search stimulus additionally respond to pruritogens.
METHODS
The procedures used in this study were approved by the UC Davis Animal Care and Use Committee. Adult male C57BL/6 mice (20–32 g) were anesthetized with pentobarbital sodium (60 mg/kg ip) and prepared for single-unit recording from the lumbosacral spinal cord as described previously (Akiyama et al. 2014). Briefly, a laminectomy exposed the lumbar spinal cord for introduction of a tungsten microelectrode to record extracellular action potentials. With a gravity-driven perfusion system, the spinal cord was continually superfused with artificial cerebrospinal fluid (ACSF) consisting of (in mM) 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose, equilibrated with 95% O2-5% CO2 at 37°C. Unit activity was amplified and digitally displayed with PowerLab (AD Instruments, Colorado Springs, CO) and Spike2 (CED, Cambridge, UK) software. In the vast majority of experiments only one unit was recorded in a given animal. On rare occasions, a second unit was recorded on the opposite side of the spinal cord.
Units were isolated with one of three separate search strategies as described below.
To identify units responsive to ID chloroquine, a small ID microinjection of chloroquine (∼0.25 μl, 100 μg/μl) was made in the hindpaw and the microelectrode was inserted into the superficial dorsal horn to isolate an action potential exhibiting ongoing firing. After activity waned, a second ID microinjection (1-μl volume) of chloroquine was made. Units exhibiting a substantial (>30%) increase in the number of action potentials recorded over a 60-s period were considered to be responsive and were tested further. As noted further below, units were subsequently tested with vehicle. Only those units whose response to chloroquine was >30% greater than their response to vehicle were included in the final data set. Additional testing included the following. The cutaneous receptive field was mapped with graded von Frey monofilaments, cotton, brush and pinch stimuli, and noxious heat (to 54°C) and cooling (to 4°C) delivered by computer-controlled Peltier thermode. Units were categorized as either wide dynamic range (WDR) type if they exhibited graded, increasing responses to innocuous vs. noxious mechanical stimuli or nociceptive specific [high threshold (HT)] if they were only activated by noxious levels of mechanical and/or thermal stimulation. Units were then tested with spinal superfusion of bombesin as described below. After that, units were additionally tested with the following chemical stimuli: ID histamine (50 μg/1 μl), ID BAM8-22 (100 μg/1 μl; MrgprC11 agonist), ID SLIGRL-NH2 (50 μg/1 μl; PAR-2/MrgprC11 agonist), topical allyl isothiocyanate (AITC; 75% in 2 μl; TRPA1 agonist), ID capsaicin (30 μg/1 μL; TRPV1 agonist), and vehicles (ID saline, ID 7% Tween 80, topical mineral oil).
To identify bombesin-responsive units, the ACSF superfusion solution was replaced with ACSF containing bombesin (40 μg/ml; 10 ml/min) that was delivered to the spinal cord for 1 min, followed by application of ACSF alone. The microelectrode was positioned to isolate an action potential exhibiting ongoing firing. After activity waned, ACSF containing a higher concentration of bombesin (200 μg/ml) was superfused over the spinal cord for 3 min. This concentration was determined in pilot experiments in which we tested a range of bombesin concentrations (20–200 μg/ml) based on the concentration range of bombesin that elicited scratching behavior after IT injection (Gmerek et al. 1983). Units exhibiting a >30% increase in firing were studied further. They were first tested for responsiveness to ID chloroquine, followed by mechanical, thermal, and chemical stimuli as described above.
To identify nociceptive neurons, the recording microelectrode was positioned to isolate single units responding to brief noxious pinch stimuli delivered to the hindpaw. Units were then tested for responsiveness to bombesin superfusion, ID chloroquine, and additional stimuli as described above.
In some experiments, responses to successive ID injections of chloroquine, or spinal superfusions of bombesin, were recorded to test for tachyphylaxis.
For each group of neurons, the mean firing rate was averaged over 1-min periods before and immediately after each stimulus and compared by paired t-tests or one-way analysis of variance (ANOVA) followed by post hoc Bonferroni tests, with P < 0.05 set as significant. At the conclusion of the experiment, an electrolytic lesion was made at the recording site and the spinal cord was postfixed in 10% buffered formalin. Spinal cord sections were cut and examined under the microscope to identify lesion sites.
RESULTS
Chloroquine search.
Collectively, 154 dorsal horn units responsive to ID chloroquine were identified, with 44% being WDR type and the remainder (56%) HT. All units had cutaneous receptive fields on the ipsilateral hindpaw.
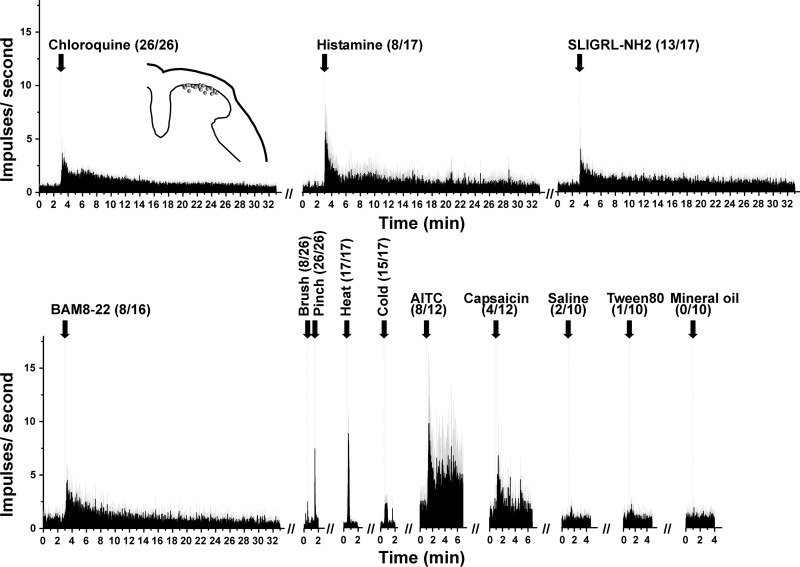
In the initial series of experiments, we tested 26 units selected with the chloroquine search stimulus for their responses to additional pruritogens and algogens; bombesin was not tested in these experiments. The data are summarized in Fig. 1, which shows averaged peristimulus time histograms (PSTHs) of responses to the various stimuli tested. A minority (47%) additionally responded to histamine, while the majority (76%) responded to SLIGRL and 50% responded to BAM8-22. All units responded to noxious pinch; 31% of these responded at a lower frequency to innocuous brushing (WDR type). All units tested responded to noxious heat, and most (88%) also responded to skin cooling. The majority (67%) responded to AITC, while fewer (33%) responded to capsaicin. Unit recording sites were histologically localized to the superficial dorsal horn (Fig. 1, inset) at a mean depth of 39.8 ± 10.6 (SE) μm below the surface of the spinal cord.
Fig. 1.
Summary of responses of 26 chloroquine-sensitive units to additional stimuli. Shown are averaged peristimulus time histograms (PSTHs; bins: 1 s) of responses to stimuli delivered at arrows aligned with dashed vertical lines. Numbers in parentheses show no. of units responding/no. tested. Gray error bars show SE. Inset, histologically recovered recording sites (dots) plotted on a drawing of midlumbar spinal cord. AITC, allyl isothiocyanate.
Seven chloroquine-responsive units were retested with the same dose of chloroquine delivered at the same site 60 min after the first chloroquine injection. The second chloroquine-evoked response was not significantly different compared with the first [baseline pre-chloroquine: 45 ± 24.6 (SE) impulses/60 s, 1st chloroquine-evoked response 108.7 ± 23.1 impulses/60 s, baseline pre-2nd chloroquine: 49 ± 25.7 impulses/60 s, 2nd chloroquine: 71.3 ± 24.4 impulses/60 s; P > 0.05 comparing 1st and 2nd chloroquine-evoked responses, paired t-test].
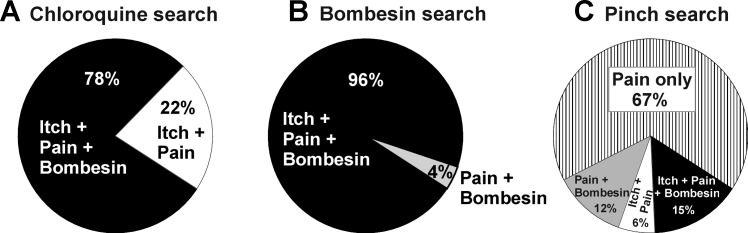
In subsequent experiments, a total of 128 units isolated with a chloroquine search stimulus were tested with spinal superfusion of bombesin, and 100 (78%) responded (Fig. 2A). For many of these units, additional data were collected for a separate study of the effects of spinal antagonists on ID chloroquine-evoked neuronal firing (Akiyama et al. 2014).
Fig. 2.
Incidence of responsiveness of units to chloroquine (“itch”), noxious pinch (“pain”), and spinal superfusion of bombesin. A: units selected with chloroquine search stimulus. All units also responded to pinch, and 78% responded to bombesin. B: units selected by response to spinal superfusion of bombesin. All units additionally responded to pinch, and 96% also responded to intradermal (ID) chloroquine. C: units selected with pinch search stimulus. Of these, 21% also responded to chloroquine and 27% responded to bombesin.
Bombesin search.
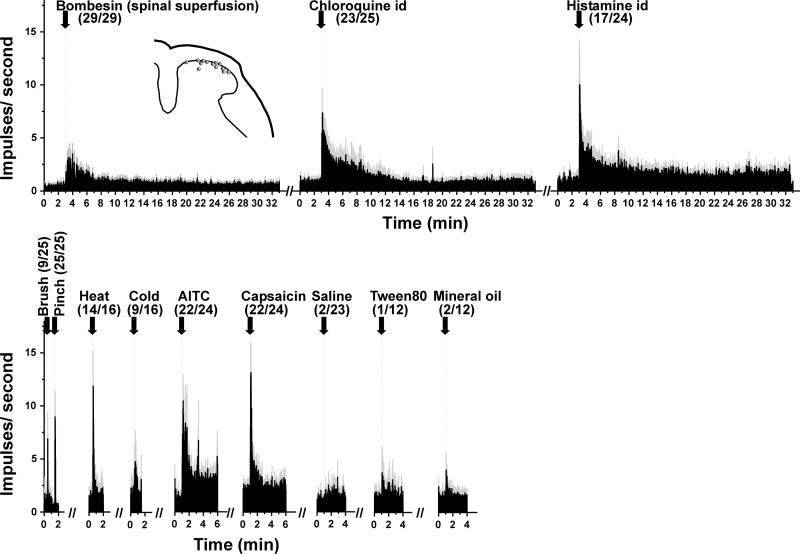
A total of 29 units were identified by their response to spinal superfusion of bombesin. Of these, 36% were WDR type and the remainder HT. Units were localized to the superficial dorsal horn (Fig. 3, inset) at a mean depth of 94.3 ± 11.5 μm below the surface of the spinal cord. Figure 3 shows mean PSTHs of responses of bombesin-sensitive units to other stimuli; the vast majority (92%) also responded to ID chloroquine, and a majority (71%) also responded to ID histamine. In addition, the majority responded to noxious heat (88%), AITC (92%), and capsaicin (92%), while very few exhibited increased firing to vehicles.
Fig. 3.
Responses to additional stimuli of units isolated with a bombesin search strategy (format as in Fig. 1).
Responses to successive applications of bombesin exhibited desensitization. The mean response of nine units to a second spinal superfusion of bombesin was significantly weaker compared with the first response [baseline pre-bombesin: 15.8 ± 6 (SE) impulses/60 s, 1st bombesin-evoked response: 108.8 ± 32.6 impulses/60 s, baseline pre-2nd bombesin: 10.4 ± 6.1 impulses/60 s, 2nd bombesin-evoked response: 60.2 ± 26.1 impulses/60 s; P < 0.05 comparing 1st and 2nd bombesin-evoked responses, paired t-test].
Pinch search.
A total of 36 units were identified with a pinch search stimulus (69% WDR, 31% HT). They were located in the superficial dorsal horn at a mean depth of 87.5 ± 9.8 μm below the surface of the spinal cord. Of these, 21% responded to ID chloroquine and 27% responded to bombesin (of which 15% also responded to chloroquine), while 67% responded to neither chloroquine nor bombesin (Fig. 2C). Units were also tested for thermal responses, with 56% responding to noxious heat and 28% to cooling.
Comparison among groups.
It is of interest to compare the magnitude of responses (peak firing rate) among units identified by chloroquine, bombesin, and pinch search strategies. The mean peak responses to bombesin were not significantly different in the bombesin + pain (i.e., bombesin and pinch sensitive) group compared with the itch + pain + bombesin (i.e., chloroquine, pinch, and bombesin sensitive) group [30.8 ± 13.6 (SE) vs. 4.8 ± 1.4 impulses/s]. There was no significant difference in the mean peak responses to pinch between bombesin-responsive and bombesin-insensitive neurons (pinch: 16.1 ± 1.7 vs. 17.1 ± 5.4 impulses/s). In contrast, the mean peak response to chloroquine was significantly greater in bombesin-sensitive compared with bombesin-insensitive units (12.7 ± 1.2 vs. 9.1 ± 1.2 impulses/s). We also compared responses of bombesin-sensitive units to chloroquine and AITC. Again, there were no significant differences among the mean peak responses to bombesin (11.3 ± 2 impulses/s), chloroquine (13.1 ± 2.7 impulses/s), or AITC (17.5 ± 4.3 impulses/s).
DISCUSSION
In the present study we employed three different search strategies to identify spinal neurons involved in signaling itch and/or pain. To identify putative itch-signaling neurons, we searched for units responsive to ID chloroquine as well as units responsive to spinal superfusion of bombesin. It should be noted that these search strategies were highly biased and selected only chloroquine- or bombesin-sensitive units. Most chloroquine-responsive units also responded to bombesin, and vice versa, and nearly all of these units also responded to the algogens AITC and/or capsaicin, consistent with previous studies (Akiyama and Carstens 2013). We used a pinch search stimulus to identify nociceptive neurons. Two-thirds of the units identified by the pinch search stimulus did not respond to either chloroquine or bombesin, consistent with our previous report that 59% of trigeminal subnucleus caudalis (Vc) units identified by an AITC search strategy did not respond to histamine or other pruritogens (Akiyama et al. 2010). Our data are generally consistent with a recent study in which the unbiased method of antidromic stimulation was used to identify trigeminothalamic projection neurons in rats (Moser and Giesler 2014). In this latter study, 56% of trigeminothalamic units that responded to noxious mechanical stimuli also responded to one or more pruritogens. In another study of antidromically identified spinothalamic tract neurons in monkeys, 20% responded to ID histamine and 13% to cowhage (Davidson et al. 2012). Overall, these studies indicate that ∼33–56% of nociceptive spinal and trigeminal dorsal horn neurons respond to pruritogens.
The summary diagram (Fig. 4) shows considerable overlap of bombesin- and chloroquine-responsive units, with most or all pruritogen-sensitive neurons also responding to algogens. We speculate that the chloroquine- and bombesin-sensitive units signal itch sensation and that pruritogen-insensitive nociceptive neurons signal pain. Consistent with this idea, the mean chloroquine-evoked response was significantly greater in bombesin-sensitive compared with bombesin-insensitive units. Surprisingly, the mean bombesin-evoked response was numerically larger in chloroquine-unresponsive compared with chloroquine-sensitive units identified by pinch. However, it is possible that the chloroquine-unresponsive units might have responded to other pruritogens had they been tested. It should also be noted that only a minority of units identified by pinch additionally responded to either bombesin or chloroquine. Because pruritic and algesic stimuli excite overlapping neuronal populations, itch and pain may occur simultaneously. Discrimination between itch and pain may depend on the relative level of activity in the two subpopulations.
Fig. 4.
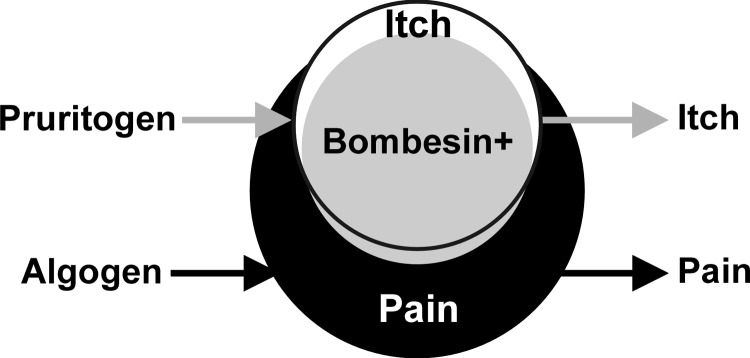
Summary. Venn diagram shows considerable overlap of pruritogen-sensitive (white circle) with algogen-sensitive (black circle) neurons and of bombesin-sensitive (gray circle) with pruritogen-sensitive neurons. We propose that itch is signaled by the pruritogen (and largely bombesin)-sensitive neurons, while pain is signaled by a larger population of pruritogen-insensitive but algogen-sensitive neurons (see text).
Recent studies suggest that itch and pain may be mediated via distinct labeled-line pathways (Han et al. 2013; Ma 2010). However, the evidence to date cannot rule out the possibility that pruritogen- and algogen-responsive spinal neurons signal itch at low and pain at higher firing frequencies, as predicted by the intensity theory. In this case, neurons should respond at a higher firing rate to algogens than pruritogens, as has been reported for nearly all pruritogen-responsive spinal and trigeminal dorsal horn neurons recorded to date (Akiyama et al. 2009a, 2009b, 2010; Davidson et al. 2007, 2012; Klein et al. 2011; Moser and Giesler 2014; Simone et al. 2004). Our present data are consistent with this, in that algogen-evoked responses were consistently larger compared with those evoked by bombesin or chloroquine (Figs. 1 and 3).
We hypothesized that neurons responsive to pruritogens and spinal superfusion of bombesin signal itch. Bombesin excites spinal neurons expressing GRPR, which were previously reported to be important for spinal itch signaling (Akiyama et al. 2013, 2014; Mishra and Hoon 2013; Sun et al. 2009; Sun and Chen 2007). Bombesin acts at G protein-coupled receptors BB1 (Neuromedin B) and BB2 (GRPR) with a five- to sixfold greater preference for the latter (González et al. 2009; Jansen et al. 2008). GRPR and Neuromedin B receptors selectively bind GRP and neuromedin B, respectively. IT injection of the neurotoxin bombesin-saporin selectively ablated GRPR-expressing spinal neurons without affecting neuromedin B receptor-expressing spinal neurons (Mishra and Hoon 2013; Sun et al. 2009). IT administration of bombesin or GRP elicited significant scratching behavior in mice, while neuromedin B was much less effective (Sukhtankar and Ko 2013). Moreover, ablation of spinal GRPR-expressing neurons with bombesin-saporin reduced or abolished pruritogen-evoked scratching behavior (Mishra and Hoon 2013; Sun et al. 2009), in support of our rationale to use bombesin to excite GRPR-expressing spinal neurons postulated to signal itch. However, it was recently reported that the GRPR antagonist RC-3095 did not attenuate scratching behavior elicited by intracerebroventricular administration of bombesin in rats (Su and Ko 2011). Moreover, scratching elicited by IT injection of GRP, but not bombesin, was attenuated by IT pretreatment with RC-3095 in NIH Swiss mice (Sukhtankar and Ko 2013), suggesting that bombesin may act at an additional unidentified receptor in rats and this mouse strain. Despite this caveat, we believe that the concordance of pruritogen and bombesin sensitivity of spinal units is consistent with their proposed role in signaling itch.
In the present study we tested an array of pruritogens including chloroquine, which acts at MrgprA3 (Liu et al. 2009), histamine, which acts at histamine H1 and H4 receptors (Bell et al. 2004), BAM8-22, which acts at MrgprC11 (Sikand et al. 2011; Wilson et al. 2011), and SLIGRL, which acts at MrgprC11 and PAR-2 (Liu et al. 2011), as well as the algogens capsaicin, which acts at TRPV1 (Caterina et al. 1997), and AITC, which acts at TRPA1 (Jordt et al. 2004; Story et al. 2003). Most chloroquine-sensitive units responded to SLIGRL, about half responded to BAM8-22 and/or histamine, and most responded to AITC and/or capsaicin. Likewise, most bombesin-sensitive spinal units also responded to chloroquine, histamine, capsaicin, and AITC. The broadly tuned chemical responsiveness of the spinal units may reflect their afferent pruriceptor input. Microelectrode recordings from identified MrgprA3-expressing dorsal root ganglion (DRG) cells revealed that 78–100% responded to chloroquine, histamine, BAM8-22, capsaicin, and cowhage (Han et al. 2013). Moreover, >90% of MrgprA3-expressing DRG cells coexpressed MrgprC11 and appeared to synaptically contact GRPR-expressing neurons in the superficial dorsal horn (Han et al. 2013), consistent with the present findings. Collectively, these findings indicate that GRPR-expressing spinal neurons receive afferent input from pruriceptors that coexpress a variety of different molecular receptors to account for their broadly tuned chemical sensitivity. Recent evidence supports a role for MrgprA3-expressing sensory afferents in itch sensation. In global TRPV1-knockout mice, TRPV1 was selectively expressed back into MrgprA3-expressing DRG cells. In these animals, ID cheek injection of capsaicin, which normally elicits pain-related forelimb wiping, instead elicited itch-related hindlimb scratching behavior, indicating that MrgprA3-expressing sensory neurons mediate itch regardless of their mode of activation (Han et al. 2013).
A second successive ID injection of chloroquine elicited a mean response in dorsal horn units that was not significantly different from the first response. This is consistent with our report that successive ID injections of chloroquine (40 min apart) elicited equivalent numbers of scratch bouts (Akiyama et al. 2012c). In contrast, spinal neuronal responses to superfusion of bombesin exhibited significant tachyphylaxis. This might be attributed to a long recovery cycle following internalization of the bound GRPR (Liu et al. 2011).
The exact spinal pathway mediating itch is currently under debate. Based on the presence of GRP mRNA and peptide within DRG cells, it has been suggested that GRP is released from the intraspinal terminals of pruriceptors to excite GRPR-expressing second-order spinal neurons in the superficial dorsal horn to signal itch (Liu et al. 2014; Sun et al. 2009). Alternatively, brain natriuretic peptide (BNP) has been implicated as a neuropeptide transmitter released from intraspinal terminals of pruriceptors (Mishra and Hoon 2013). Two groups have reported that neurotoxic destruction of GRPR-expressing spinal neurons with bombesin-saporin reduced or abolished pruritogen-evoked scratching (Mishra and Hoon 2013; Sun et al. 2009). In the latter study (Mishra and Hoon 2013), pruritogen-evoked scratching was also reduced or abolished by neurotoxic ablation of spinal neurons expressing the BNP receptor Npra. However, IT administration of GRP in these animals still elicited normal scratching behavior, indicating that GRPR-expressing spinal neurons are downstream of Npra-expressing neurons. It is conceivable that GRPR-expressing spinal neurons may function as local excitatory interneurons (Fleming et al. 2012; Wang et al. 2013).
Our group has obtained evidence that multiple neurotransmitters signal chloroquine-evoked itch (Akiyama et al. 2014). We showed that combined IT administration of antagonists of GRPR (RC3095), NK-1 (L733060), and the glutamate AMPA receptor (CNQX) virtually abolished chloroquine-evoked spinal neuronal firing, and scratching behavior, while individual or dual antagonist administration was much less effective. Moreover, subpopulations of DRG cells shown by calcium imaging to respond to chloroquine also expressed GRP and/or substance P, and >80% coexpressed the vesicular glutamate transporter VGlut2 (Akiyama et al. 2013, 2014). In contrast, histamine-evoked spinal neuronal activity, and scratching behavior, was abolished by IT administration of CNQX alone, implicating glutamate as the primary spinal neurotransmitter for histaminergic itch (Akiyama et al. 2014). This is consistent with a report that C-fiber input to GRP-responsive spinal neurons in rats was abolished by CNQX (Koga et al. 2011). Our present findings support a role for spinal bombesin-sensitive neurons in the transmission of nonhistaminergic itch, although our approach cannot distinguish whether the pruritogen-sensitive spinal neurons receive direct synaptic input from GRP-containing primary afferent terminals or from second-order excitatory interneurons.
GRANTS
The study was funded by National Institutes of Health Grants AR-057194, AR-063228, and DE-021183.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A., M.T., and E.C. conception and design of research; T.A., M.T., and M.I.C. performed experiments; T.A., M.T., M.I.C., and E.C. analyzed data; T.A., M.T., and E.C. interpreted results of experiments; T.A. and E.C. edited and revised manuscript; T.A., M.T., K.T.M., M.I.C., and E.C. approved final version of manuscript; E.C. prepared figures; E.C. drafted manuscript.
REFERENCES
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience 250: 697–714, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol 104: 2442–2450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol 132: 1886–1891, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol 102: 2176–2183, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Iodi Carstens M, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci 29: 6691–6699, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Carstens MI, Carstens EE. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci 36: 2311–2316, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience 226: 305–312, 2012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, Carstens MI, Carstens E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol 109: 742–748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155: 80–92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T, Kuwazono T, Lee JB, Kuraishi Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides 32: 2098–2103, 2011. [DOI] [PubMed] [Google Scholar]
- Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol 142: 374–380, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol 108: 1711–1723, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632, 2001. [DOI] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain 8: 52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A, Vaught JL. Intrathecal bombesin in rats: effects on behaviour and gastrointestinal transit. Eur J Pharmacol 94: 141–143, 1983. [DOI] [PubMed] [Google Scholar]
- González N, Nakagawa T, Mantey SA, Sancho V, Uehara H, Katsuno T, Jensen RT. Molecular basis for the selectivity of the mammalian bombesin peptide, neuromedin B, for its receptor. J Pharmacol Exp Ther 331: 265–276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16: 174–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Cowan A. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur J Pharmacol 502: 233–237, 2004. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60: 1–42, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. [DOI] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 106: 1078–1088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, Zhuo M. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain 7: 47, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, Chen ZF. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147: 447–458, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by β-alanine. J Neurosci 32: 14532–14537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4: ra45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wan L, Huo FQ, Barry DM, Li H, Zhao ZQ, Chen ZF. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol Pain 10: 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 120: 3773–3778, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 340: 968–971, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyika KS, Kihamia CM. Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg 94: 27–31, 1991. [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr. Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol 111: 1574–1589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAMS. NIAMS Pub No. 09-4272. Available from: http://www.niams.nih.gov/Health_Info/Atopic_Dermatitis/default.asp, 2009. [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31: 7563–7567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- Su PY, Ko MC. The role of central gastrin-releasing peptide and neuromedin B receptors in the modulation of scratching behavior in rats. J Pharmacol Exp Ther 337: 822–829, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Ko MC. Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One 8: e67422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448: 700–703, 2007. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 325: 1531–1534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J, Eberhart D, Urban R, Meda K, Solorzano C, Yamanaka H, Rice D, Basbaum AI. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 78: 312–324, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]