Abstract
Impulsivity, the predisposition to act without regard for negative consequences, is a characteristic of several psychiatric disorders and is thought to result in part from genetic variation in the untranslated region of the dopamine transporter (DAT) gene. As the exact link between genetic mutations and impulsivity has not been established, we used oculomotor behavior to characterize rhesus monkeys as impulsive or calm and genetic/epigenetic analysis and positron emission tomography (PET) to correlate phenotype to DAT genotype, DAT gene methylation, and DAT availability. We found three single nucleotide polymorphisms (SNPs) in the 3′-UTR of the DAT gene, one of which provided a potential site for methylation in the impulsive group. Bisulfite analysis showed that the DNA of the impulsive but not the calm subjects was methylated at one SNP. Because genetic/epigenetic modifications could lead to differences in protein expression, we measured DAT availability using [18F]2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)-nortropane ([18F]FECNT) PET and found higher DAT availability in the internal globus pallidus, an output nucleus of the basal ganglia, of the impulsive group. Higher DAT availability lowers dopamine levels, potentially altering neuronal circuits involved in the initiation of action, thus contributing to the impulsive phenotype. The association between increased methylation in the DAT gene and greater DAT availability suggests that mutations to the regulatory portion of the DAT gene lead to a susceptibility to epigenetic modification resulting in a discrete behavioral phenotype.
Keywords: monkey, impulsivity, dopamine transporter, epigenetics, methylation
the propensity to act quickly and without regard for negative consequences is generally referred to as impulsivity. Understanding the neural basis for this type of behavior is of much interest because in many instances such behavior does not seem to be advantageous and, importantly, its incidence in humans correlates with numerous psychiatric disorders (Chamberlain et al. 2005; Moeller et al. 2001).
Impulsivity is thought to result in part from genetic variation in the encoding of the dopamine transporter (DAT; Cook et al. 1995; Gill et al. 1997). The human DAT gene (SLC6A3) contains a variable number of tandem repeats (VNTR) polymorphism in the 3′-untranslated region (UTR), ranging from 3 to 11 repeats of a 40-base pair sequence (Vandenbergh et al. 1992). The presence of the 10-repeat allele (10R) has been correlated with the occurrence of attention deficit hyperactivity disorder (ADHD; Cook et al. 1995; Gill et al. 1997; Waldman et al. 1998; Daly et al. 1999; Winsberg and Comings 1999), of which impulsivity is a cardinal symptom. In addition, greater DAT availability in the striatum has been associated with ADHD (Dougherty et al. 1999; Dresel et al. 2000; Krause et al. 2000; Larish et al. 2006), impulsivity (Costa et al. 2013), and the 10R (Heinz et al. 2000) and 9R (Jacobsen et al. 2000; Spencer et al. 2013) alleles of the DAT VNTR.
DAT is blocked by methylphenidate, a primary treatment for ADHD that in low doses reduces impulsivity in humans (Greenhill 2001; Solanto 1998, 2002) and animals (Rajala et al. 2012). While these correlations suggest that alterations in the DAT gene or its expression mediate impulsive behavior, a direct link has not been established (Spencer et al. 2013; Miller and Madras 2002; Miller et al. 2001). For instance, the 10R allele, which is associated with ADHD, is more common in the general population than is the incidence of ADHD (Mitchell et al. 2000).
Rhesus monkeys, the animal model closest to humans available for studies of nervous system function, have an homologous gene encoding DAT with a fixed number of tandem repeats (FNTRs) in the 3′-UTR. A single nucleotide polymorphism (SNP) in the 3′-UTR was found to be suggestive but not predictive of hyperactivity (Miller et al. 2001). This finding, combined with similarities of the Rhesus monkey to the human in structure and function of the frontal part of the brain (Wise 2008), dopamine (DA) neurotransmission (Berger et al. 1991), and positive response to methylphenidate (Rajala et al. 2012), makes it an ideal system for the study of the mechanisms underlying impulsivity. Accordingly, we used oculomotor behavior to characterize rhesus monkeys as impulsive or calm, and genetic/epigenetic analysis and positron emission tomography (PET) to correlate phenotype to DAT genotype, DAT gene methylation, and DAT availability.
The results show that the differences in the 3′-UTR of the DAT gene could render some subjects more susceptible to epigenetic modification, which can alter gene expression and, consequently, protein levels. Higher DAT availability lowers DA levels (Heinz et al. 1999), potentially altering the response properties of neuronal circuits involved in the initiation of action, thus contributing to the impulsive phenotype.
MATERIALS AND METHODS
Subjects.
Four 9–12 Kg male, adult (7–14 yr) Rhesus monkeys (Macaca mulatta) purchased from the Wisconsin National Primate Research Center were studied. These monkeys were not related. The four subjects had participated in earlier studies of the effects of methylphenidate on cognitive functions, which took place at least 18 mo before the present study; some of those data have been published (Rajala et al. 2012). Subject selection was based solely on availability and intact eye movement recording implants. The animals were housed individually in two rooms with other monkeys of the laboratory colony in the same hallway of the same facility, permitting rich visual, olfactory, and auditory interactions.
Surgical procedures were carried out under aseptic conditions while the animals were under general anesthesia. All efforts were made to minimize suffering of the subjects. To ameliorate pain, the monkeys received analgesics for 4–7 days after surgery. The animals were not killed after the experiment. The preparation and surgical details have been published (Populin 2006). All experimental procedures were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC) and were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental setup, eye movement recordings, experimental task, and eye movement analysis.
The experiments were done in a dimly illuminated sound-proof chamber (Acoustic Systems, Austin, TX). Eye position was measured with the scleral search coil technique (Robinson 1963; CNC Engineering, Seattle, WA). The signals were digitized (Tucker Davis Technologies, Alachua, FL) and stored for offline analysis.
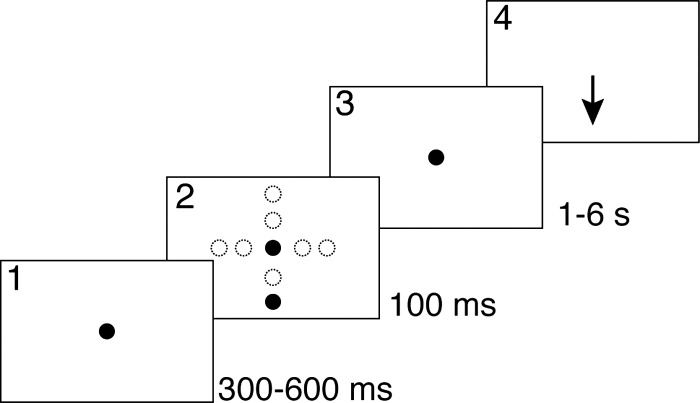
A memory-guided saccade task with targets at two eccentricities (10 and 20°) and six delays (1–6 s in 1-s steps), presented in random order, was used (Fig. 1). Subjects were presented with a red light emitting diode (LED) straight ahead. A variable time (300–600 ms) after acquiring the fixation LED, a 100-ms target was presented from one of the eight target locations. This was followed by a randomly selected delay period, at the end of which the fixation LED was turned off thereby signaling the subject to make an eye movement to the remembered location of the target. Water was delivered after the completion of successful trials, which required meeting both temporal and spatial criteria. Temporal criteria included maintaining fixation on the LED straight ahead until turned off and executing an eye movement within 700 ms. Spatial criteria involved maintaining eye position with a (4°, 4°) acceptance window set around the initial fixation point for the duration of the delay period and making a saccade with end position falling within a (4°, 4°) acceptance window set around each target. Failure to meet these criteria resulted in termination of the trial at the time the error was made. Thus trials in which a premature response was detected, in which the subject's eye position left the acceptance window around the fixation during the delay period for >75 ms, were immediately terminated.
Fig. 1.
Experimental task. The subject was first required to fixate on a light emitting diode (LED) straight ahead (1). During fixation, 1 of 8 targets was presented for 100 ms elsewhere within the oculomotor range (2). Subsequently, a delay period of randomly determined duration (1–6 s) was imposed (3). The subject had to maintain fixation for the entire duration of this period. Lastly, upon offset of the fixation LED, which constituted the signal to respond, the subject was expected to make a saccadic eye movement to the remembered location of the target (4).
They were allowed to perform as many trials as they wanted in each session so they could drink enough water to achieve or exceed the minimum daily intake established by the IACUC. Experimental sessions were terminated after subjects closed their eyes for 10 consecutive trials. Supplemental water was provided after sessions in which they did not drink the established minimum. Although this approach resulted in experimental sessions with different numbers of trials (see Table 1), it promoted steady day-to-day performance.
Table 1.
Behavioral classification and number of practice and testing trials performed by each subject
| Group/Subject | Number of Practice Trials | Number Testing Trials | Number of Testing Sessions |
|---|---|---|---|
| Calm | |||
| SW | 38,980 | 4,094 | 6 |
| GO | 12,639 | 2,051 | 4 |
| Impulsive | |||
| SH | 38,734 | 1,102 | 4 |
| MI | 67,855 | 2,726 | 5 |
Sample collection, DNA extraction, and DAT genotyping.
Blood samples were collected from the four subjects in Monoject vacutainer tubes (Tyco Health Care Group, Mansfield, MA) with 7.5% EDTA (K3), kept on ice until mixed with RNAlater (Sigma-Aldrich, St. Louis, MO), and then stored at −80°C. The identity of the subjects was unknown to I. Zaitoun and M. L. Epstein, who carried out this part of the project. Genomic DNA was isolated using GenElute Blood Genomic DNA Kit (Sigma-Aldrich). The quality and quantity of DNA were measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). PCR amplification was conducted in a 25-μl reaction volume consisting of 50 ng of genomic DNA, 100 ng of each primer, 200 μM of each deoxynucleoside triphosphate (1 μl; Promega, Madison, WI), 2.5 μl of 10× PCR buffer, 1.5 U of Taq DNA polymerase (New England Biolabs, Ipswich, MA), and molecular biology grade water (Sigma-Aldrich). Primers used for genotyping were reported previously (Miller et al. 2001): U13: 5′ TGTTCAGAGGCATTGGAG 3′; L12: 5′ AAAAAGCCATTCGCAAACAT 3′. The purified bands were used for Sanger sequencing according to manufacturer's protocols. Each sample was sequenced twice, first using the forward primer (U13) and the second using the reverse primer (L12). Sequenced samples were run on an ABI 3730xl DNA Analyzer. Sequence data were displayed using FinchTV Version 1.4.0 (Geospiza, Seattle, WA).
Bisulfite genomic modification and sequencing.
Methylation was measured using DNA extracted from cells in the blood to preserve our subjects for additional studies. Similar methods are used in human studies, which have shown that peripheral DNA methylation correlates with methylation patterns in the brain (Horvath et al. 2012), and with measures of brain protein synthesis (Wang et al. 2012).
To quantify DNA methylation, 2 µg of genomic DNA per subject were bisulfite converted, which changed unmethylated cytosine into uracil, using the Imprint DNA Modification kit (Sigma-Aldrich). Because DNA methylation can occur on either one or both alleles of the DAT gene, the different alleles were expressed separately in individual bacterial colonies. Modified DNA was purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and eluted in 20 μl H2O. Primers for modified DNA amplification were designed manually and had the following sequences: DAT1_BS_F222: GAGGTATTGGAGGATAGGGGTTT, DAT1_BS_R222: AAAAATAAAATTCCAATAAAATCTCTTCCTAAAAA. The target region was PCR amplified using 2 μl of bisulfite-modified DNA. Nested PCR was conducted using 2 μl of the first-round-PCR products (DAT1_BS_F223: GTTTAGGTTATTGTTATTCGGGTAGTT, DAT1_BS_R223: CCAAACAAAATATAATCTACAAACTACCTACATAAA). Nested PCR products were purified from 1.5% agarose gel using a QIAquick Gel extraction kit (Qiagen), and cloned into pGEM-T Easy Vector Systems (Promega, Madison, WI). Cloning vectors were transformed into JM109 competent cells (Promega), which were plated onto LB/ampicillin/IPTG/X-Gal plates and incubated overnight at 37°C. White colonies were selected and amplified in broth medium. Plasmids, including the pGEM-T Easy Vector, were isolated using PerfectPrep Spin Mini Kit (5 PRIME, Gaithersburg, MD). Plasmids were amplified using the following primers in a 25-μl reaction: pUC/M13-For: AACGCCAGGGTTTTCCCAGTCAC; pUC/M13-Rev: TCACACAGGAAACAGCTATGAC. Eighty nanograms of PCR product were used for Sanger sequencing as described earlier, using the T-7 primer (TGTAATACGACTCACTATAGGG). The methylation status was determined from the DNA sequence of several individual colonies for each subject and the percentage of methylated colonies was calculated relative to the total sequenced colonies for each subject.
Positron emission tomography scans.
After the behavioral and genetic parts of the study were complete, DAT availability was measured in all four subjects using the cocaine analog [18F]2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)-nortropane ([18F]FECNT; Murali et al. 2013), a radioligand selective for DAT (Goodman et al. 2000). The scanning protocol has been published (Converse et al. 2013). Briefly, subjects were sedated with a light dose of ketamine (5 mg/kg im) ∼25 min before injection of the radiotracer and transported from the vivarium to the imaging facility in a climate-controlled vehicle. The trip took ∼5 min. Upon arrival the animals were intubated and gas anesthesia was started (1–2% Isoflurane). A catheter was inserted into the cephalic vein for administration of the [18F]FECNT and fluids during recovery from anesthesia. Subsequently, the monkeys were positioned in a micro-PET P4 scanner (Siemens, Knoxville, TN), and a 57Co transmission scan was acquired, after which a 150-min emission scan began. [18F]FECNT was injected intravenously 60 s after the start of the scan. The injected radioactivity was 191 ± 13 MBq (means ± SD, n = 4). Upon recovery from anesthesia the monkeys were returned to their quarters using the same transport method. Scans were completed over 2 days with scanning order counterbalanced across the two groups (2 scans per day; 1 impulsive and 1 calm subject per day in opposite order). The behavioral and genetic classification of the subjects was unknown to A. K. Converse who performed the PET scans and the image analysis.
Image data processing, pharmacokinetic modeling, and statistical analysis.
Images were aligned to an [18F]FECNT template (Converse et al. 2013), and time-activity curves were determined for anatomically defined regions of interest: internal division of the globus pallidus (GPi), external division of the globus pallidus (GPe), and areas of the frontal cortex; and regions defined by radioactivity in the template image: the substantia nigra (SN), ventral tegmental area (VTA), and the striatum (Str; Converse et al. 2013). To better locate the SN and VTA, the anatomically defined regions were warped to fit the region in the [18F]FECNT template image defined by the contour at half of the peak radioactivity concentration found in the midbrain. The [18F]FECNT template was the average FECNT radioactivity image of the control subjects from Converse et al. 2013. [18F]FECNT binding was determined using the Logan graphical reference tissue method with a cerebellar reference region.
Differences in regional DAT availability between the calm and impulsive groups were examined with analysis of covariance (ANCOVA) where the between subjects variable was group (calm/impulsive) and age was the covariate; the subject phenotype was known to J. B. Henriques, who performed the statistical analysis.
RESULTS
Behavioral phenotype.
While training monkeys to perform a memory-guided saccade task configured to create a high level of uncertainty, large differences in performance among the subjects were observed (Fig. 1). Specifically, two subjects frequently responded prematurely before the offset of the fixation LED in many trials. The other two subjects were better able to wait for the signal to respond. The inability of the former subjects to wait for the appropriate time to respond, which led to immediate termination of the trials and loss of reward, resembled the type of responses that characterize individuals clinically labeled as impulsive, i.e., the tendency to act without regard for negative consequences (Moeller et al. 2001). Therefore, we operationally refer to these subjects as “impulsive,” and we refer to the others, who were better able to follow the rules of the task, as “calm.”
The responses of one of the impulsive subjects, MI, are illustrated in Fig. 2A, correct (blue) and incorrect (red). They were characterized by reflexive reactions shortly after the presentation of the target (arrow), followed by numerous small eye movements during the delay period, during which steady fixation was required. The responses of one of the calm subjects, SW, in contrast, illustrate that he was able to wait for the offset of the fixation LED in most trials, and made fewer premature responses (Fig. 2B).
Fig. 2.
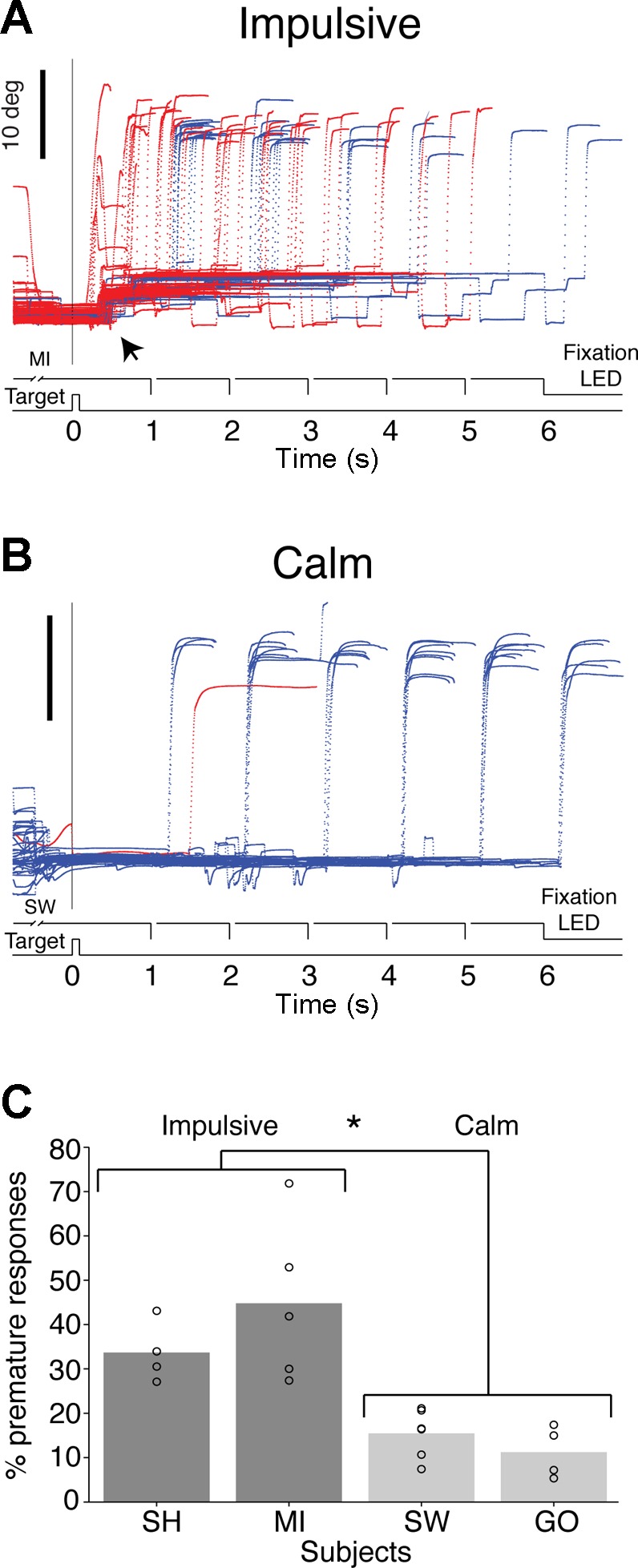
A: representative examples of saccadic eye movements from impulsive subject MI. The vertical or horizontal component of eye movements representing the main direction of movement is plotted as a function of time and synchronized to target presentation (0 ms). Blue traces represent trials in which the subject responded at the appropriate time and red traces represent trials in which the subject responded prematurely. The signal to respond for all 6 delays is illustrated above the abscissa. In this example the target was located at (0, 20°) and premature responses comprised ∼50% of all trials. Note the small eye movements recorded shortly after the presentation of the target (black arrow). This reflexive type of movement, which was present in most trials involving targets in all directions and amplitudes, best illustrates the impulsive nature of this subject's responses. B: representative example of performance by calm subject SW. Note that most eye movements were performed in a timely manner, presenting a stereotyped pattern defined by the six different delays. C: percentage of premature responses measured across 4–6 experimental sessions for each subject. The impulsive group made a significantly larger percentage of premature responses (*P < 0.001), but the subjects within each group were not significantly different (calm: P = 0.275; impulsive: P = 0.289).
The percentage of premature responses made by each subject during testing sessions confirmed that they could be classified into two groups, calm and impulsive (Fig. 2C). The impulsive group made a significantly greater percentage of premature responses compared with the calm group (F1,11.16 = 33.428, P < 0.001, partial η2 = 0.71, linear mixed model; Fig. 2C), while the behavior of the subjects within each group was indistinguishable (calm: F1,8 = 1.370, P = 0.275; impulsive: F1,7 = 1.315, P = 0.289, linear mixed model).
Both groups made the majority of their premature responses shortly after the presentation of the target (time = 0 ms), but the distribution of the timing of these responses was different between the two groups (Fig. 3, A and B). Figure 3B, inset, compares Gaussian fits to the normalized data using the following model: f(x) = a1 × exp{−[(x − b1)/c1]2} + a2 × exp{−[(x − b2)/c2]2}; the data were normalized to compare the shape of the distributions. The c2 coefficient was significantly larger in the fit of the impulsive 1918 [CI: 1610-2225] compared with the calm 732.4 [CI: 621.5-841.2], indicating that this group made more premature responses during the delay period. Interestingly, there were no significant differences in the latency of correct responses between the two groups (P > 0.05).
Fig. 3.
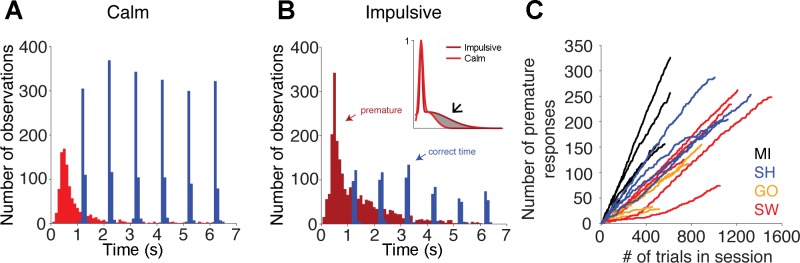
A and B: distribution of response time for both correct and premature responses for the calm and impulsive groups, respectively. B, inset: comparison of the Gaussian fits to the normalized data. The resulting coefficients show that the bell of the Gaussian distribution fit to the data of the impulsive group was significantly wider than that of the calm group (arrow), indicating that the impulsive group made more premature responses during the delay period. Both groups made most premature responses shortly after the presentation of the target, but the proportion of premature responses to correctly timed trials was greater in the impulsive group. C: cumulative functions of the number of premature responses made during the course of testing sessions. Linearity was assessed by computing the average R2 ± 95% confidence interval.
In addition, premature responses were evenly distributed throughout the length of the experimental sessions, as shown by their cumulative functions (Fig. 3C). The R2 coefficients (subject MI: 0.9966 ± 0.0030; SH: 0.9918 ± 0.0079; GO: 0.9753 ± 0.0258; SW: 0.9777 ± 0.0274) indicate that the functions are well-described by straight lines, demonstrating that the subjects did not make more premature responses early in the experimental sessions because they were more thirsty, and thus less able to withhold their responses, or later in the sessions because they were more tired, and thus more prone to making errors.
It could be argued that these behavioral differences resulted from variations in the length of training provided to these subjects, but this was not the case (Table 1). Subjects SH and SW, who were classified as impulsive and calm, respectively, required nearly the same number of practice trials to reach a preset level of performance, defined as the point at which they earned their daily allowance of water, whereas subject MI, who was classified as impulsive, required nearly twice as many practice trials. Subject GO, who was classified as calm, on the other hand, required the fewest number of practice trials.
Genotyping and epigenetics.
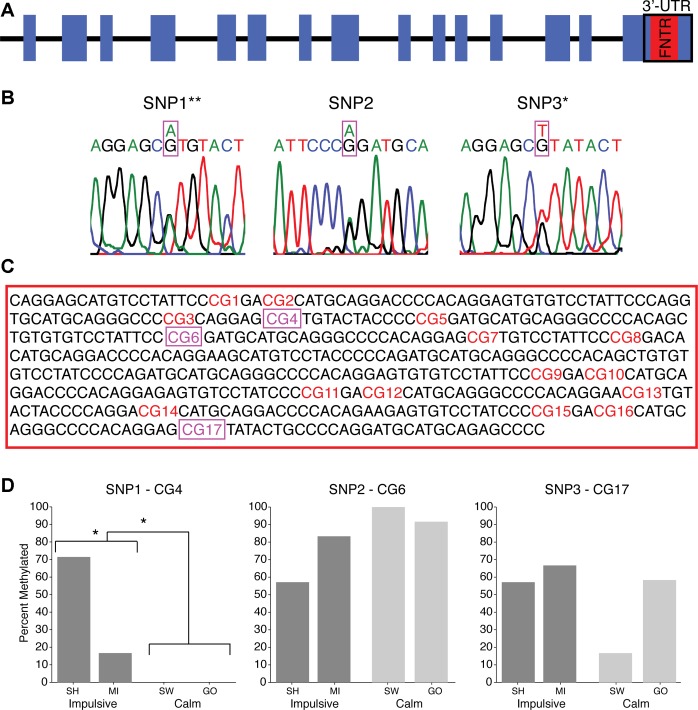
It was hypothesized that the difference between the phenotypes might be explained instead by variation in the DAT gene. Blood samples were obtained from all subjects, and then the FNTR of the 3′-UTR region of the DAT gene was amplified and sequenced under double-blind conditions (Fig. 4A). Three SNPs were found: the first and the second were either adenine (A) or guanine (G), while the third was either thymidine (T) or guanine (G) and was identical to the SNP previously reported in monkeys (Miller et al. 2001; Fig. 4B). Each SNP was examined in each subject for differences in the proportion of PCR products with each version of the two possible alleles, the distribution of which, at both the first and third SNP sites, was significantly different between the calm and impulsive groups [P < 0.0001 at SNP1 and P < 0.010 at SNP3, Fisher's two-tailed exact test; odds ratio (OR) = 0.05, 95% confidence interval (CI) 0.00–0.35 and OR = 0.24, 95% CI 0.00–0.89, respectively; Table 2]. Cramer's Φ was 0.69 and 0.50, respectively, indicating a large effect of both SNP sites, despite the small sample size. Specifically, none of the samples from the calm subjects had a G at SNP1 and none of the samples from the impulsive subjects had a T at SNP3 (Table 2). There was no significant difference between the groups at SNP2 (P = 0.497, Fisher's exact test, two-tailed, OR = 0.24, 95% CI 0.01–5.54; Φ = 0.22).
Fig. 4.
A: schematic representation of the dopamine transporter (DAT) gene of the Rhesus monkey. B: 3 single nucleotide polymorphisms (SNPs) found in the 12-repeat fixed number tandem repeats (FNTR) sequence, revealed by the double peaks at SNP1-G/A, SNP2-G/A, and SNP3-G/T (*P < 0.01, **P < 0.0001). C: nucleotide sequence of the FNTR region. Seventeen CpG dinucleotides were found with the 3 SNPs occurring at CpG numbers 4, 6, and 17 (magenta). D: methylation status of SNP-containing CpGs expressed as a percentage of all methylated clones for each subject in each of the 2 groups. The SNP1-CpG4 site was significantly more methylated in the impulsive group compared with the calm (*P < 0.05). UTR, untranslated region.
Table 2.
Genotypes of the four subjects at the three SNPs
| Phenotype/Subject | SNP1† | SNP2 | SNP3* |
|---|---|---|---|
| Calm | |||
| SW | A/A | A/G | T/G |
| GO | A/A | G/G | T/G |
| Impulsive | |||
| SH | G/G | G/G | G/G |
| MI | A/G | G/G | G/G |
The haplotypes are listed for each animal at each single nucleotide polymorphism (SNP) in the dopamine transporter (DAT) fixed number of tandem repeat region (FNTR). The calm and impulsive groups differed significantly in genotype at SNP1 and SNP3 (
P < 0.01, †P < 0.001, Fisher's exact test). The subjects within each group were not significantly different from each other.
Interestingly, all three SNPs were immediately downstream of a cytosine (C), resulting in either a CpA, CpT, or CpG dinucleotide, the last of which is a substrate for DNA methylation, an epigenetic modification that can alter gene expression (Moore et al. 2013; Robertson 2005). In addition, 14 other CpG dinucleotides were found in the FNTR region (Fig. 4C). The methylation status of the identified CpG sites was examined in the DNA extracted from blood cells. Each SNP was examined in each subject for the proportion of alleles that were methylated. The methylation of the CpG at SNP1 (site 4) was significantly different between the two behavioral phenotypes. While the methylation at SNP1 was different between the two subjects in the impulsive group (P < 0.05, Fisher's exact test, two-tailed), it was completely absent in both subjects of the calm group as a result of the lack of a CpG (P = 0.022, Fisher's exact test, two-tailed; OR = 32.07, 95% CI 1.60–643.44; Φ = 0.57; Fig. 4D). There were no significant differences in methylation at the 14 non-SNP CpG sites between the groups.
PET imaging of DAT.
The methylation status in the regulatory region of a gene may affect expression by altering the binding of transcription factors (Moore et al. 2013; Robertson 2005; Shumay et al. 2010). The differences in methylation between the impulsive and calm groups could result in differences in the expression and thus the availability of DAT, resulting in specific behavioral phenotypes. Accordingly, DAT availability was measured using PET after injecting [18F]FECNT, a selective radioligand for DAT (Goodman et al. 2000).
The mean radioactivity from all four subjects at two different coronal slices illustrates the qualitative distribution of DAT binding and the defined regions of interest (Fig. 5). Because DAT availability decreases with age in humans (Bannon et al. 1992; Costa et al. 2013; Dougherty et al. 1999; Volkow et al. 1994, 1996), an ANCOVA was performed with age as a covariate. The impulsive group had a significantly higher level of DAT available in the GPi (F1,1 = 5791.60, P < 0.01, partial η2 = 1.00), the output nucleus from the basal ganglia to the thalamus (Table 3). A similar trend across groups was found for the GPe (F1,1 = 87.25, P = 0.068, partial η2 = 0.99). As in humans (Ciliax et al. 1999), the highest DAT availability was recorded in the striatum (Fig. 5). However, unlike in humans (Winsberg and Comings 1999; Dougherty et al. 1999; Dresel et al. 2000; Krause et al. 2000), there was no significant difference between the groups in this region (Table 3).
Fig. 5.
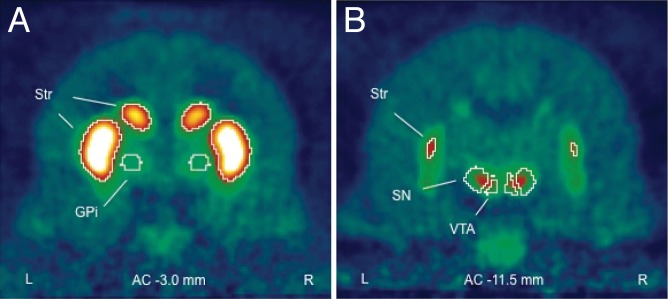
[18F]2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)-nortropane ([18F]FECNT) positron emission tomography (PET) images. Coronal slices of mean radioactivity (n = 4) computed across the entire 150-min scan. Contours delineate regions of interest defined by radioactivity for striatum (Str), by anatomy for the globus pallidus internal (GPi), and by a combination for the substantia nigra (SN) and ventral tegmental area (VTA). Sections taken at 3.0 (A) and 11.5 (B) mm posterior to the anterior commisure of the corpus collosum (AC).
Table 3.
Binding potential of FECNT to DAT measured from PET and results of statistical analysis
| Impulsive (n = 2) |
Calm (n = 2) |
|||||
|---|---|---|---|---|---|---|
| ROI | BP (mean) | SE | BP (mean) | SE | F Value | P Value |
| GPi | 1.659 | 0.001 | 1.584 | 0.001 | 5791.598 | 0.008 |
| GPe | 6.174 | 0.222 | 3.061 | 0.222 | 87.249 | 0.068 |
| Striatum | 8.254 | 0.169 | 7.212 | 0.169 | 17.011 | 0.151 |
| SN | 2.060 | 0.142 | 2.374 | 0.142 | 2.171 | 0.38 |
| VTA | 2.283 | 0.012 | 2.384 | 0.012 | 29.176 | 0.117 |
FECNT, [18F]2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)-nortropane; PET, positron emission tomography; ROI, region of interest; BP (mean), adjusted binding potential mean; GPi, internal globus pallidus; GPe, external globus pallidus; SN, substantia nigra; VTA, ventral tegmental area. The adjusted means represent bilateral binding potentials in each region controlling for age.
DISCUSSION
The data show in the same subjects a linkage between the occurrence of an impulsive phenotype, increased methylation of SNP1 (site 4), and increased DAT availability in the GPi. These findings indicate that mutations in the regulatory region of the DAT gene are substrates for potential epigenetic modification, as suggested by modeling work in the human SLC6A3 (Shumay et al. 2010). The result of these alterations could increase DAT protein levels, which in turn would alter the normal function of basal ganglia/prefrontal cortex circuits leading to impulsive behavior, a hallmark of ADHD and other psychiatric disorders.
The modified memory guided-saccade paradigm used to characterize the subjects' behavioral phenotypes is a very sensitive assay for the characterization of impulsive behavior. The characteristics of eye movements (e.g., latency and kinematics) have been shown to correlate with impulsive behavior in humans (Cirili et al. 2011). Other measures, such as direct observations of the subjects in their home cages (e.g., Miller et al. 2001), are not likely to detect subtle but consistent actions indicative of impulsivity, such as the small eye movements documented in this study (Fig. 2A), and may introduce potential confounding effects. The intruder paradigm, for instance, reveals that the presence of a human in the same room with a nonhuman primate evokes distinct emotional and behavioral responses (Kalin et al. 1991).
It is important to emphasize that the phenotypic classification obtained with the memory-guided saccade task was not dependent on the extent of the behavioral training. The tendency of the impulsive subjects to respond prematurely even after additional training was consistently observed throughout the course of all the experimental sessions, ruling out, therefore, concerns about motivation and fatigue as drivers of premature responses (Fig. 3).
This type of paradigm allows for not only the detection of very subtle behavioral manifestations of impulsive behavior (Fig. 2) but also the collection of large datasets comprising literally tens of thousands of trials (Table 1). However, it requires long periods of training, which preclude testing large numbers of subjects. The sample size of this study is not an exception but the norm in studies of awake behaving monkeys involving combinations of cognitive and molecular measures (e.g., Liu et al. 2004). Nevertheless, to allay any concerns, measures of effect size appropriate for each statistical test, which summarize the results with respect to the population (Cohen 1992), were computed and revealed large effects for all statistically significant results. Therefore, the large effect sizes reported here render moot any concerns about underpowered statistical tests and warrant drawing conclusions from the present data.
Chronic treatment with methylphenidate is known to upregulate the expression of DAT (Shumay et al. 2010). Although the monkeys in this study had participated in previous studies of the effects of methylphenidate on cognitive function (Rajala et al. 2012 and unpublished observations), they were not treated chronically and were only exposed to the drug in single weekly treatments that ended more than 18 mo before this study. Such exposure, therefore, is unlikely to have had a long-lasting effect that could have affected the present results.
The higher availability of DAT documented in the GPi of the impulsive monkeys is consistent with the higher levels of DAT found in the striatum of humans diagnosed with ADHD (Dougherty et al. 1999; Dresel et al. 2000; Krause et al. 2000; Larish et al. 2006) and impulsivity (Costa et al. 2013). It will be important to determine in future studies if differences in DAT availability between phenotypes are limited to nuclei that comprise the basal ganglia (e.g., Dougherty et al. 1999 and the present study), or if they extend to other brain structures where DAT is expressed, such as the prefrontal cortex.
Polymorphisms within the 3′-UTR of both the human and monkey DAT genes have been shown to alter DAT expression, but no specific mechanism has been identified (Miller and Madras 2002). Because DNA methylation is thought to silence gene expression by preventing transcription factors from binding to DNA (Robertson 2005), it is hypothesized that the greater availability of DAT in the impulsive group results from methylation preventing the binding of a transcription factor produced by a suppressor gene, which reduces DAT expression. An example of this is Hesr1/Hey1, which binds to the human DAT gene (SLC6A3) and downregulates its expression in human neural cell lines (Fuke et al. 2005). Variability in the encoding of such transcription factors could also contribute to an impulsive phenotype (Fuke et al. 2005).
The occurrence of ADHD and increased DAT availability have been associated with the 10R allele of the VNTR polymorphism in the 3′-UTR of the human DAT gene (SLC6A3; Cook et al. 1995; Daly et al. 1999; Gill et al. 1997; Heinz et al. 2000; Waldman et al. 1999; Winsberg and Comings 1999). However, this allele is the most prevalent in the general population, in which the occurrence of ADHD is much lower (Mitchell et al. 2000). The present results from the monkey suggest that a polymorphism may provide a substrate for epigenetic changes, which could contribute and/or potentially account for this discrepancy.
The environment may influence genetics via epigenetic mechanisms such as DNA methylation, both during development (van Heesbeen et al. 2013) and dynamically throughout life (Moore et al. 2013). It is unlikely, however, that environmental conditions throughout the duration of the study confounded the results because all the monkeys were housed under virtually identical conditions.
While differences in DAT encoding and availability are not solely responsible for impulsive behavior, the question remains as to how might higher levels of DAT in the GPi alter neural processing and thereby contribute to an impulsive phenotype. Dopamine signals, which encode reward prediction error (Schultz et al. 1997) and are modulated by uncertainty (de Lafuente and Romo 2011), can facilitate behavioral responses to relevant external and internal stimuli. Increased levels of extracellular DA in the striatum increase evoked responses relative to spontaneous activity and, therefore, increase signal-to-noise ratio (Kiyatkin and Rebec 1996; Nicola et al. 2000; Rolls et al. 1984). Accordingly, higher availability of DAT should result in lower levels of extracellular DA (Heinz et al. 1999) and decreased signal-to-noise ratio, which, in turn, should lead the circuit to reach the threshold for responding more easily. The increased levels of DAT in the GPi documented here could facilitate the initiation of actions in response to external (or internal) signals via the direct (GO) pathway, and faster termination of ongoing actions, facilitating the initiation of subsequent actions, via the indirect (NO-GO) pathway. Essentially, we hypothesized that lower levels of DA brought about by higher DAT availability should result in reaching the threshold for responding more easily and contributing to impulsive behavior. The fact that the experimental task used to characterize the impulsive and calm phenotypes in this study was purely oculomotor (Fig. 1) seems to contradict the accepted view of the motor systems' specific output pathways of the basal ganglia. Specifically, that the initiation of saccadic eye movements relies on output from the substantia nigra pars reticulata (SNr) not the GPi (Hikosaka and Wurtz 1983a,b, 1985; Hikosaka et al. 2000). However, single unit recordings from neurons in the GPi and GPe of monkeys reveal activity related to different aspects of oculomotor behavior (Hong and Hikosaka 2008; Kato and Hikosaka 1995; Shin and Sommer 2010; Yoshida and Tanaka 2009), supporting the hypothesis that this output of the basal ganglia is also involved in the generation and control of eye movements (Kato and Hikosaka 1995; Shin and Sommer 2010; Yoshida and Tanaka 2009).
In conclusion, our data suggest that epigenetic regulation of DAT gene expression may provide a major unexplored component to the manifestation of specific behavioral phenotypes known to be associated with psychopathologies (Moeller et al. 2001) and offer a new perspective for investigating the basic mechanisms underlying dopaminergic neurotransmission.
GRANTS
This work was supported by National Science Foundation Grant IOB-0517458 and National Institutes of Health Grant DC-003693. It was also supported by National Institutes of Health Grants T32-GM-007507 (to A. Z. Rajala), R01-DK-081634 (to I. Zaitoun and M. L. Epstein), and R01-AA-12277, R01-AA-10079, and S10-RR-015801 (to A. K. Converse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.Z.R., I.Z., M.L.E., and L.C.P. conception and design of research; A.Z.R., I.Z., A.K.C., D.M., and L.C.P. performed experiments; A.Z.R., I.Z., J.B.H., A.K.C., and L.C.P. analyzed data; A.Z.R., J.B.H., A.K.C., M.L.E., and L.C.P. interpreted results of experiments; A.Z.R., I.Z., A.K.C., and L.C.P. prepared figures; A.Z.R., M.L.E., and L.C.P. drafted manuscript; A.Z.R., M.L.E., and L.C.P. edited and revised manuscript; A.Z.R., I.Z., J.B.H., A.K.C., D.M., M.L.E., and L.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John K. Harting for unwavering support. We also thank John Fallon, John Harting, Amanda Barlow, Brad Postle, and June Dahl for comments on an earlier version of this manuscript, Elizabeth Ahlers for micropet scanning, the University of Wisconsin-Madison Medical Physics cyclotron staff for radioisotope production, Yonghe Yan and Janet Sekulski for computer programming, and C Daly, P Esser, M Gallardo, K Sayles, and C Tegt for outstanding animal care.
REFERENCES
- Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci USA 89: 7095–7099, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 14: 21–27, 1991. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev 29: 399–419, 2005. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 409: 38–56, 1999. [DOI] [PubMed] [Google Scholar]
- Cirilli L, de Timary P, Lefèvre P, Missal M. Individual differences in impulsivity predict anticipatory eye movements. PLoS One 6: e26699, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull 112: 155–159, 1992. [DOI] [PubMed] [Google Scholar]
- Converse AK, Moore CF, Moirano JM, Ahlers EO, Larson JA, Engle JW, Barnhart TE, Murali D, Christian BT, DeJesus OT, Holden JE, Nickles RJ, Schneider ML. Prenatal stress induces increased striatal dopamine transporter binding in adult nonhuman primates. Biol Psychiatry 74: 502–510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet 56: 993–998, 1995. [PMC free article] [PubMed] [Google Scholar]
- Costa A, la Fougère C, Pogarell O, Möller HJ, Riedel M, Ettinger U. Impulsivity is related to striatal dopamine transporter availability in healthy males. Psychiatry Res 211: 251–256, 2013. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry 4: 192–196, 1999. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Dopamine neurons code subjective sensory experience and uncertainty of perceptual decisions. Proc Natl Acad Sci USA 108: 19767–19771, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 354: 2132–2133, 1999. [DOI] [PubMed] [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbäumer K, Kung HF, Hahn K, Tatsch K. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med 27: 1518–1524, 2000. [DOI] [PubMed] [Google Scholar]
- Fuke S, Sasagawa N, Ishiura S. Identification and characterization of the Hesr1/Hey1 as a candidate trans-acting factor on gene expression through the 3' non-coding polymorphic region of the human dopamine transporter (DAT1) gene. J Biochem 137: 205–216, 2005. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry 2: 311–313, 1997. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol 27: 1–12, 2000. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Clinical effects of stimulant medication in ADHD. In: Stimulant Drugs and ADHD: Basic and Clinical Neuroscience, edited bySolanto MV, Arnsten AF, Castellanos FX. Oxford, UK: Oxford Univ Press, 2001, p. 31–71. [Google Scholar]
- Heinz A, Saunders RC, Kolachana BS, Jones DW, Gorey JG, Bachevalier J, Weinberger DR. Striatal dopamine receptors and transporters in monkeys with neonatal temporal limbic damage. Synapse 32: 71–79, 1999. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 22: 133–139, 2000. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301, 1983a. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res 272: 368–372, 1983b. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABArelated substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol 53: 292–308, 1985. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron 60: 720–729, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, van den Berg LH, Ophoff RA. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol 13: R97, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry 157: 1700–1703, 2000. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev 62: 1175–1183, 1991. [PubMed] [Google Scholar]
- Kato M, Hikosaka O. Function of the indirect pathway in the basal ganglia oculomotor system: visuo-oculomotor activities of external pallidum neurons. In: Age-Related Dopamine-Dependent Disorders (Monographs in Neural Sciences), edited by Segawa M, Nomura Y. Basel: Karger, 1995, p. 178–187. [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol 75: 142–153, 1996. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett 285: 107–110, 2000. [DOI] [PubMed] [Google Scholar]
- Larisch R, Sitte W, Antke C, Nikolaus S, Franz M, Tress W, Müller HW. Striatal dopamine transporter density in drug naive patients with attention-deficit/hyperactivity disorder. Nucl Med Commun 27: 267–270, 2006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Richmond BJ, Murray EA, Saunders RC, Steenrod S, Stubblefield BK, Montague DM, Ginns EI. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proc Natl Acad Sci USA 101: 12336–12341, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Madras BK. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry 7: 44–55, 2002. [DOI] [PubMed] [Google Scholar]
- Miller GM, La Garza de R, Novak MA, Madras BK. Single nucleotide polymorphisms distinguish multiple dopamine transporter alleles in primates: implications for association with attention deficit hyperactivity disorder and other neuropsychiatric disorders. Mol Psychiatry 6: 50–58, 2001. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, Briceno I, Papiha SS, Osipova L, Livshits G, Leonard WR, Crawford MH. Distribution of the 3′ VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol 72: 295–304, 2000. [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry 158: 1783–1793, 2001. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology 38: 23–38, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali DD, Barnhart TE, Vandehey NT, Christian BT, Nickles RJ, Converse AK, Larson JA, Holden JE, Schneider ML, Dejesus OT. An efficient synthesis of dopamine transporter tracer [(18)F]FECNT. Appl Radiat Isot 72: 128–132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215, 2000. [DOI] [PubMed] [Google Scholar]
- Populin LC. Monkey sound localization: head-restrained versus head-unrestrained orienting. J Neurosci 26: 9820–9832, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala AZ, Henriques JB, Populin LC. Dissociative effects of methylphenidate in nonhuman primates: trade-offs between cognitive and behavioral performance. J Cogn Neurosci 24: 1371–1381, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD. DNA methylation and human disease. Nat Rev Genet 6: 597–610, 2005. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Thorpe SJ, Boytim M, Szabo I, Perrett DI. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neuroscience 12: 1201–1212, 1984. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. [DOI] [PubMed] [Google Scholar]
- Shin S, Sommer MA. Activity of neurons in monkey globus pallidus during oculomotor behavior compared with that in substantia nigra pars reticulata. J Neurophysiol 103: 1874–1887, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Fowler JS, Volkow ND. Genomic features of the human dopamine transporter gene and its potential epigenetic States: implications for phenotypic diversity. PLoS One 5: e11067, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94: 127–152, 1998. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res 130: 65–71, 2002. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, Batchelder H, Clarke A, Fischman AJ. Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biol Psychiatry 74: 84–89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics 14: 1104–1106, 1992. [DOI] [PubMed] [Google Scholar]
- van Heesbeen HJ, Mesman S, Veenvliet JV, Smidt MP. Epigenetic mechanisms in the development and maintenance of dopaminergic neurons. Development 140: 1159–1169, 2013. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Logan J, Schlyer D, MacGregor R, Hitzemann R, Wolf AP. Decreased dopamine transporters with age in healthy human subjects. Ann Neurol 36: 237–239, 1994. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields SD, Gur R. Dopamine transporters decrease with age. J Nucl Med 37: 554–559, 1996. [PubMed] [Google Scholar]
- Waldman ID, Rowe D, Abramowitz A, Kozel S, Mohr J, Sherman S, Cleveland H, Sanders M, Gard J, Stever C. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet 63: 1767–1776, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Szyf M, Benkelfat C, Provençal N, Turecki G, Caramaschi D, Côté SM, Vitaro F, Tremblay RE, Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS One 7: e39501, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsberg BG, Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry 38: 1474–1477, 1999. [DOI] [PubMed] [Google Scholar]
- Wise S. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31: 599–608, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Tanaka M. Enhanced modulation of neuronal activity during antisaccades in the primate globus pallidus. Cereb Cortex 19: 206–217, 2009. [DOI] [PubMed] [Google Scholar]