Abstract
High-frequency electrical stimulation (HFS) of the human skin induces an increase in both mechanical and heat pain sensitivity in the surrounding unconditioned skin. The aim of this study was to investigate the effect of HFS on the intensity of perception and brain responses elicited by the selective activation of C fibers. HFS was applied to the ventral forearm of 15 healthy volunteers. Temperature-controlled CO2 laser stimulation was used to activate selectively low-threshold C-fiber afferents without concomitantly activating Aδ-fiber afferents. These stimuli were detected with reaction times compatible with the conduction velocity of C fibers. The intensity of perception and event-related brain potentials (ERPs) elicited by thermal stimuli delivered to the surrounding unconditioned skin were recorded before (T0) and after HFS (T1: 20 min after HFS; T2: 45 min after HFS). The contralateral forearm served as a control. Mechanical hyperalgesia following HFS was confirmed by measuring the change in the intensity of perception elicited by mechanical punctate stimuli. HFS resulted in increased intensity of perception to mechanical punctate stimulation and selective C-fiber thermal stimulation at both time points. In contrast, the N2 wave of the ERP elicited by C-fiber stimulation (679 ± 88 ms; means ± SD) was enhanced at T1 but not at T2. The P2 wave (808 ± 105 ms) was unaffected by HFS. Our results suggest that HFS enhances the sensitivity to thermal C-fiber input in the area of secondary hyperalgesia. However, there was no significant enhancement of the magnitude of the C-fiber ERPs at T2, suggesting that quickly adapting C fibers do not contribute to this enhancement.
Keywords: high-frequency stimulation, secondary hyperalgesia, event-related potentials, heat, C fibers
cutaneous tissue injury is often associated with the development of increased pain sensitivity (i.e., hyperalgesia) in the area of actual tissue injury (referred to as primary hyperalgesia) but also in the surrounding uninjured skin (referred to as secondary hyperalgesia). Whereas primary hyperalgesia is thought to predominantly result from a sensitization of peripheral nociceptors, secondary hyperalgesia is thought to mainly result from enhanced responsiveness of the central nervous system (i.e., “central sensitization”; Treede and Magerl 2000; Latremoliere and Woolf 2009).
Secondary hyperalgesia can also be induced experimentally by activating nociceptors in a sustained and intense fashion, for example, using intradermal injection or topical application of capsaicin, a substance that selectively activates primary nociceptive afferents via the transient potential receptor vanilloid 1 (TRPV1) receptor (LaMotte et al. 1991; Magerl et al. 1998; Szolcsányi 1990). Based on the results of some of these studies (Ali et al. 1996), it is believed that secondary hyperalgesia is characterized by enhanced pain to mechanical but not heat stimuli (Ringkamp and Meyer 2009; Ringkamp et al. 2013). However, this textbook notion is challenged by a number of studies demonstrating the presence of both mechanical hyperalgesia and thermal hyperalgesia in the area surrounding the capsaicin-treated (Serra et al. 1998; Yucel et al. 2002) or injured skin (Pedersen and Kehlet 1998).
Recently, we demonstrated that high-frequency electrical stimulation (HFS) of the skin, an experimental pain model that induces a robust secondary mechanical hyperalgesia (Klein et al. 2004, 2008; Vo and Drummond 2013; van den Broeke et al. 2014a; van den Broeke and Mouraux 2014b), also induces a clear secondary heat hyperalgesia (van den Broeke and Mouraux 2014b). Indeed, we showed that, after HFS, brief cutaneous laser heat stimuli applied above the threshold of heat-sensitive Aδ and C-fiber nociceptors are perceived as more intense when they are delivered to the unconditioned surrounding skin. Surprisingly, concomitantly recorded laser-evoked brain potentials (LEPs) were not enhanced after HFS. Although brief and intense laser stimuli coactivate heat-sensitive Aδ and C fibers (resulting in the perception of “first” and “second” pain, respectively), LEPs elicited by such stimuli have been shown to exclusively reflect activity related to the activation of rapidly adapting Aδ-fiber polymodal nociceptors [type II mechanical-heat sensitive A nociceptors (AMHs); Treede et al. 1995]. Indeed, the relatively short latency of the elicited brain responses (200–600 ms when stimulating the hand dorsum; Mouraux et al. 2003) is compatible with the conduction velocity of myelinated Aδ fibers but not with the slower conduction velocity of unmyelinated C fibers (Churyukanov et al. 2012; Jankovski et al. 2013). The aim of the present study was to assess the effect of HFS on the intensity of perception and LEPs elicited by the selective activation of heat-sensitive C-fiber afferents.
Recently, it has been shown that reliable LEPs related to the selective activation of C fibers can be obtained using a temperature-controlled infrared CO2 laser stimulator to selectively activate heat-sensitive C-fiber afferents that have a lower thermal activation threshold than Aδ-fiber afferents (Jankovski et al. 2013). Using such stimuli, we characterized the effect of HFS on the thermal perception and brain responses elicited by the selective activation of heat-sensitive C fibers. The previously described effect of HFS on the perception of nociceptive mechanical stimuli was confirmed by measuring the changes in the intensity of perception elicited by mechanical punctate stimuli.
METHODS
Participants
Fifteen healthy volunteers [8 men and 7 women aged 21–31 yr (mean age: 26 yr)] participated in the experiment. Approval for the experiment was obtained from the local Ethical Committee. All participants signed an informed consent form and received financial compensation for their participation.
Experimental Design
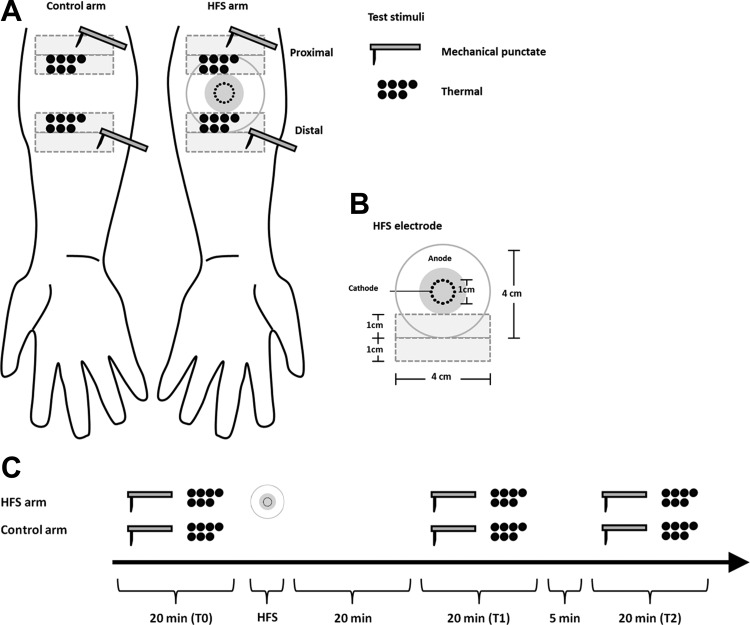
The design of the experiment is summarized in Fig. 1. During the sensory testing and the HFS conditioning procedure, the participants were comfortably seated in a chair with their arm resting as comfortable as possible on a pillow.
Fig. 1.
Experimental setup. A: high-frequency stimulation (HFS) was applied to the volar forearm of 1 arm. Test mechanical punctate stimuli and thermal stimuli were applied to the skin surrounding the area onto which HFS was applied as well as to the same skin area on the contralateral arm that served as a control. B: electrode used to deliver HFS consisted in 16 blunt stainless steel pins placed in a 10-mm diameter circle (cathode), surrounded by a concentrically located stainless steel anode. The heterotopic test area is shown in light grey. C: effect of HFS on the responses elicited by the test stimuli was assessed at 3 different time points: before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2).
Conditioning stimulation: HFS.
HFS was delivered to the volar forearm, 10 cm distal to the cubital fossa. The stimulation consisted of five trains of electrical pulses (pulse width: 2 ms) delivered at a 100-Hz rate during 1 s. The time interval between each train was 10 s. The intensity of stimulation was individually adjusted to 20 times the detection threshold to a single pulse (0.24 ± 0.05 mA; means ± SD). The stimulation trains were generated by a constant current electrical stimulator (Digitimer DS7A; Digitimer) and delivered to the skin using a specifically designed electrode previously demonstrated to activate nociceptive afferents (Klein et al. 2004). The electrode, designed and built at the Centre for Sensory-Motor Interaction (Aalborg University, Aalborg, Denmark), consists of 16 blunt stainless steel pins with a diameter of 0.2 mm protruding 1 mm from the base. The 16 pins are placed in a circle with a diameter of 10 mm and serve as a cathode. A stainless steel reference electrode serving as anode is concentrically located and has an inner diameter of 22 mm and an outer diameter of 40 mm. To avoid any confounding effect of handedness, the arm onto which HFS was applied (dominant vs. non dominant) was counterbalanced across subjects. Handedness was assessed using the Flinders Handedness Survey (Nicholls et al. 2013).
Heterotopic test stimulation.
The heterotopical effect of HFS was characterized using two different types of sensory stimuli: mechanical punctate and thermal stimuli. The test stimuli were applied to the skin surrounding the area onto which HFS was applied as well as to the same skin area on the contralateral arm that served as control to take into account a possible time-dependent habituation. The measurements were performed before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2). The order of presentation of the two types of test stimuli was balanced across measurements and participants. The arm onto which the stimuli were applied first (HFS vs. control arm) was also balanced across measurements and participants.
Mechanical punctate stimuli were delivered by pressing a calibrated sharp-tipped Semmes-Weinstein monofilament (size: 5.18, 15 g, target force: 147 mN) with a 90° angle to the skin surface until it bends. The stimuli were applied twice within an area of 4 cm2 at a distance of 2.0 cm distal and proximal relative to the center of the conditioning stimulation.
Thermal stimuli consisted of pulses of radiant heat generated by a temperature-controlled CO2 laser whose power is pulse-width regulated using a closed-loop control based on an online measurement of skin temperature performed using a radiometer whose field of view is collinear with the laser beam (Laser Stimulation Device; SIFEC). By vibration of the optical fiber at some distance from the source, a quasi-uniform spatial distribution of radiative power within the stimulated area is obtained. At the end of the fiber, optics collimate the beam, resulting in a 6-mm beam diameter at target site. Importantly, the very small lag in the feedback control makes it possible to generate very rapid increases in skin temperature (Churyukanov et al. 2012). The stimulator was used to generate 100-ms radiant heat stimuli consisting of a 10-ms heating ramp during which the skin was brought to the desired target temperature, followed by a plateau during which skin temperature was maintained at target temperature for 90 ms. To avoid skin overheating and minimize stimulus-induced sensitization or habituation of heat-sensitive afferents, the target of the laser beam was displaced manually by the experimenter after each stimulus.
At the beginning of the experiment, the thermal detection thresholds of Aδ and C fibers were determined at both arms, using two interleaved staircase algorithms with reaction time (RT) as criterion to distinguish between detection mediated by Aδ-fiber input (RT <650 ms, compatible with the conduction velocity of myelinated Aδ fibers) and detection mediated by C-fiber input (RT ≥650 ms, compatible with the conduction velocity of unmyelinated C fibers) (Churyukanov et al. 2012). This method is based on a previous study that showed that RTs can be used to effectively discriminate between behavioral responses to C-fiber and Aδ-fiber inputs (Churyukanov et al. 2012). Participants were asked to respond as quickly as possible by pressing a button attached to the arm of the chair as soon as they perceived the thermal stimulus. The staircase used to estimate C-fiber detection threshold started at 41°C. If the stimulus was detected, the temperature of the subsequent stimulus was decreased by 1°C. If the stimulus was undetected, the temperature of the subsequent stimulus was increased by 1°C. Therefore, this staircase converged towards the absolute detection threshold. The staircase used to determine the Aδ-fiber detection threshold started at 46°C. If the stimulus was detected with a RT <650 ms, the temperature of the temperature of the subsequent stimulus was decreased by 1°C. Conversely, if it was detected with a RT ≥650 ms or if it was undetected, the temperature of the subsequent stimulus was increased by 1°C. Therefore, this staircase converged towards the detection threshold of myelinated Aδ fibers.
The stimulus temperature used to assess the effect of HFS on the responses to C-fiber input (Ttest) was then defined individually to a value above the threshold of C fibers but below the threshold of Aδ fibers as follows: Ttest = (TC-threshold + TAδ-threshold)/2, where TC-threshold corresponded to the highest estimate of C-fiber threshold at the two arms and TAδ-threshold corresponded to the lowest estimate of Aδ-fiber threshold at the two arms.
At each time point (T0, T1, and T2) and each arm (HFS and control), the thermal stimuli were then repeated until 20 trials were collected with a RT ≥650 ms, with a maximum of 50 trials. In each condition and subject we were able to collect 20 trials detected with a RT ≥650 ms. The stimuli were delivered using a random interstimulus interval ranging from 3 to 5 s, and delivered to an area of 4 cm2, within a distance of 1–2 cm distal or proximal (balanced across subjects) relative to the center of the conditioning stimulation.
Intensity of Perception
The effect of HFS on the intensity of perception elicited by the two types of test stimuli was assessed by asking participants to rate the intensity of the stimuli on a numerical rating scale (NRS) ranging from 0 (no perception) to 100 (maximal pain), with 50 representing the transition from nonpainful to painful domains of sensation. Inclusion of both the nonpainful and painful domains of sensation allowed us to use the same scale for every type of stimulus. For mechanical punctate stimuli, ratings were obtained following the delivery of each stimulation pair. For thermal stimuli, ratings were obtained after each block.
C-Fiber LEPs
The electroencephalogram (EEG) was recorded using 32 actively shielded Ag-AgCl electrodes mounted in an elastic electrode-cap and arranged according to the international 10–20 system (Waveguard32 cap; Advanced Neuro Technologies). Participants were instructed to keep their gaze fixed on a black cross displayed at a distance of ∼1 m at an angle of 30° below eye level and to sit as still as possible without making any movements. The EEG signals were amplified and digitized using a sampling rate of 1,000 Hz and an average reference (HS64; Advanced Neuro Technologies). Eye movements were recorded using two surface electrodes placed at the upper-left and lower-right sides of the left eye. Impedance was kept under the 10 kΩ for all leads.
The EEG was analyzed offline using Brain Vision Analyzer v. 1.05 (Brain Products). As a first step, the continuous EEG was filtered using a 0.5- to 30-Hz zero-phase Butterworth band-pass filter as well as a 50-Hz notch filter. The EEG was then segmented into epochs extending from −500 to +2,000 ms relative to stimulus onset. Epochs containing ocular artifacts (i.e., eye movements and eye blinks) were corrected using the Gratton-Coles method (Gratton et al. 1983). After baseline correction (reference interval: −500 to 0 ms), segments with amplitude values exceeding ±100 μV were rejected as these were likely to be contaminated by artifacts. Average waveforms were computed including only the trials where the stimulus was detected with a RT ≥650 ms (i.e., trials where the stimulus most probably activated C-fiber afferents selectively). Separate waveforms were computed for each participant, time point (T0, T1, and T2), and stimulation site (HFS and control). Based on the latency and topographic distribution of C-fiber LEPs reported in a previous study (Jankovski et al. 2013), two distinct peaks were identified in the LEP waveforms as follows. The N2 was defined as a negative peak maximal at the scalp vertex and occurring ∼600–800 ms after stimulus onset. The P2 was defined as a positive peak also maximal at the scalp vertex, following the N2 peak and occurring ∼900–1100 ms after stimulus onset. Peak amplitudes (expressed relative to baseline) and latencies (expressed relative to stimulus onset) were measured at electrode Cz.
Skin Temperature
Before each thermal stimulus was delivered to the skin, the baseline skin temperature was measured for a period of 100 ms, using the radiometer collinear with the laser beam.
Statistical Analyses
Statistical analyses were conducted using SPSS 18 (SPSS, Chicago, IL, USA). To check whether the data were normally distributed, we inspected the frequency distribution of the data, skewness, and kurtosis values and applied the Shapiro-Wilk test.
To characterize the effect of HFS on the behavioral (intensity of the percept elicited by mechanical punctate and thermal stimuli, as measured using the NRS) and electrophysiological measures (N2 and P2 waves of LEPs) and baseline skin temperature, a general linear model (GLM) repeated-measures ANOVA analysis was performed using two within-subject factors: time (T0, T1, and T2) and treatment (control vs. HFS arm). In this model, the specific effect of HFS can be isolated from time-dependent effects such as habituation by assessing the interaction between the factors time and treatment. For the statistical evaluation of the intensity of percept obtained during mechanical punctate stimulation, we also included the factor area (distal vs. proximal) in the repeated-measures ANOVA analysis.
The assumption of sphericity was tested using Mauchly's test of sphericity. In those cases where the data violated the assumption of sphericity, F values were corrected using the Greenhouse-Geisser procedure. For post hoc tests, P values were Bonferroni corrected for the number of tests. The level of significance was set at P < 0.05 (two-sided).
RESULTS
Aδ- and C-fiber detection thresholds
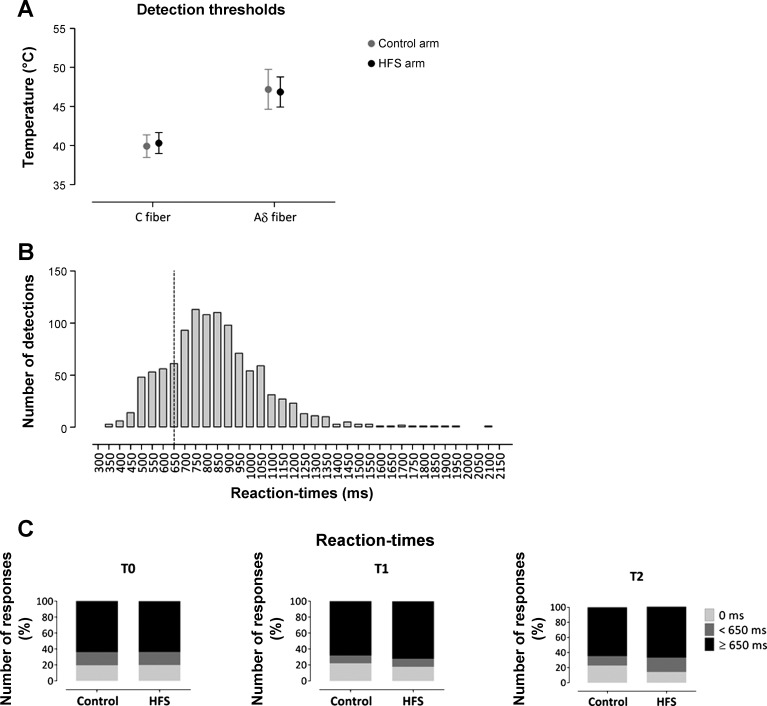
The estimated Aδ- and C-fiber detection thresholds are shown in Fig. 2. The target temperature of the test stimuli used to elicit C-fiber responses before and after HFS was 43 ± 1°C.
Fig. 2.
A: Aδ- and C-fiber detection thresholds estimated at the beginning of the experiment. B: distribution of reaction times (RTs) to the thermal laser stimuli delivered at all time points (T0, T1, and T2) and stimulated arms (control and HFS). Note that the distribution peaks at latencies compatible with the conduction velocity of C fibers and that it is after the criterion of 650 ms used to distinguish between stimuli coactivating Aδ and C fibers from stimuli selectively activating C fibers. C: percentages of trials detected with a RT <650 ms (compatible with the detection of Aδ-fiber input), trials with a RT ≥650 ms (compatible with the detection of C-fiber input), and trials with no detection at each time point (T0, T1, and T2) and stimulated arm (control and HFS).
Perception of Mechanical Punctate Stimuli
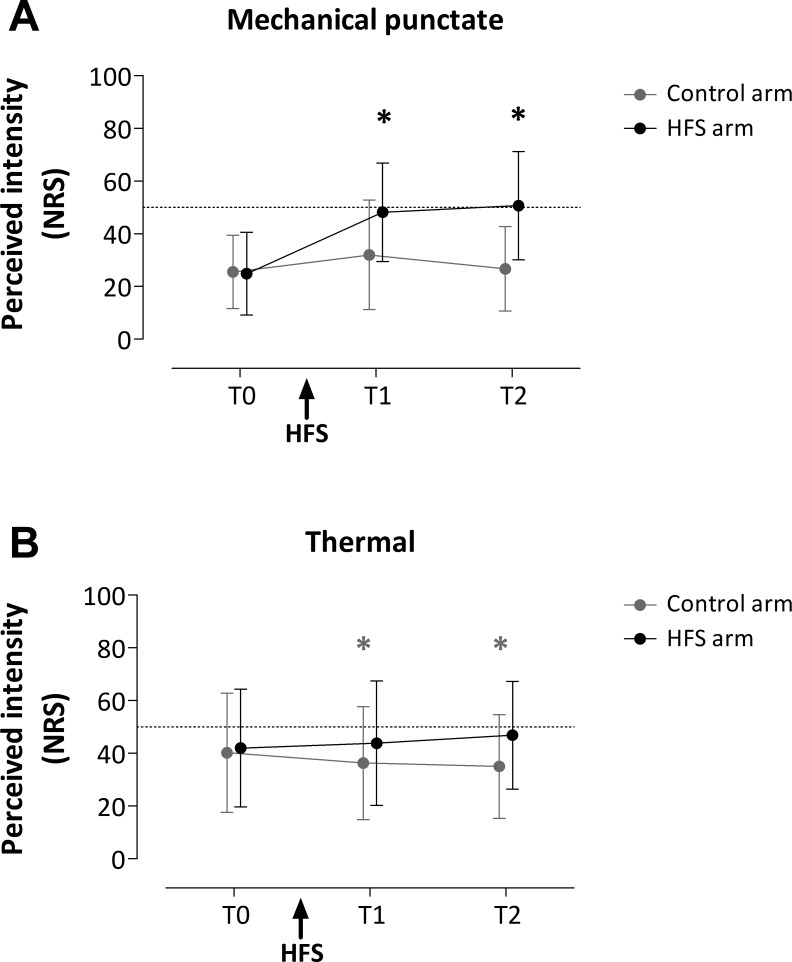
Mechanical hyperalgesia was present in all subjects. The perception elicited by mechanical punctate stimuli delivered to the control and HFS-conditioned arm before (T0) and after (T1, T2) conditioning is shown in Fig. 3.
Fig. 3.
Effect of HFS on the perception of mechanical punctate stimuli (delivered to the distal and proximal area relative to the site of HFS; A) and thermal laser stimuli selectively activating C fibers (B). Group-level means ± SD of the intensity of perception [numerical rating scale (NRS) scores] obtained at the 3 different time points: before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2). Note that HFS significantly enhanced the perception of mechanical punctate and thermal stimuli at both T1 and T2. *P < 0.05, statistical significance of post hoc tests.
The repeated-measures ANOVA revealed a statistically significant time × treatment interaction [F(2,28) = 15.585, P < 0.001, η2 = 0.527]. This interaction shows that the intensity of the percept elicited by mechanical punctate stimuli was significantly different between the two arms at the different measurement times. The univariate within-subject contrasts revealed that the perceived intensity was significantly enhanced at the conditioned arm after HFS, both at T1 [F(1,14) = 18.263, P = 0.001, η2 = 0.566] and at T2 [F(1,14) = 55.626, P < .001, η2 = 0.799]. The area × time × treatment interaction was not significant, indicating that there were no differences in perceived intensity after HFS between the proximal and distal areas. Post hoc tests (on the pooled data) revealed a statistically significant increase of perception at T1 [paired t-test; t(14) = −7.179, P < 0.05] and T2 [paired t-test; t(14) = −8.061, P < 0.05] on the conditioned arm.
Perception of C-Fiber Thermal Stimuli
The perception elicited by thermal stimuli delivered to the control and HFS-conditioned arm before (T0) and after (T1 and T2) conditioning is shown in Fig. 3. The repeated-measures ANOVA revealed a significant time × treatment interaction [F(2,28) = 7.407, P = 0.003, η2 = 0.346]. The univariate within-subject contrasts revealed that the perceived intensity was significantly enhanced at the conditioned arm after HFS, both at T1 [F(1,14) = 9.024, P = 0.009, η2 = 0.392] and at T2 [F(1,14) = 14.103, P = 0.002, η2 = 0.502]. Post hoc tests revealed a statistically significant decrease of perception at T1 [paired t-test; t(14) = 3.490, P < 0.05] and T2 [paired t-test; t(14) = 2.923, P < 0.05] on the control arm.
C-Fiber LEPs
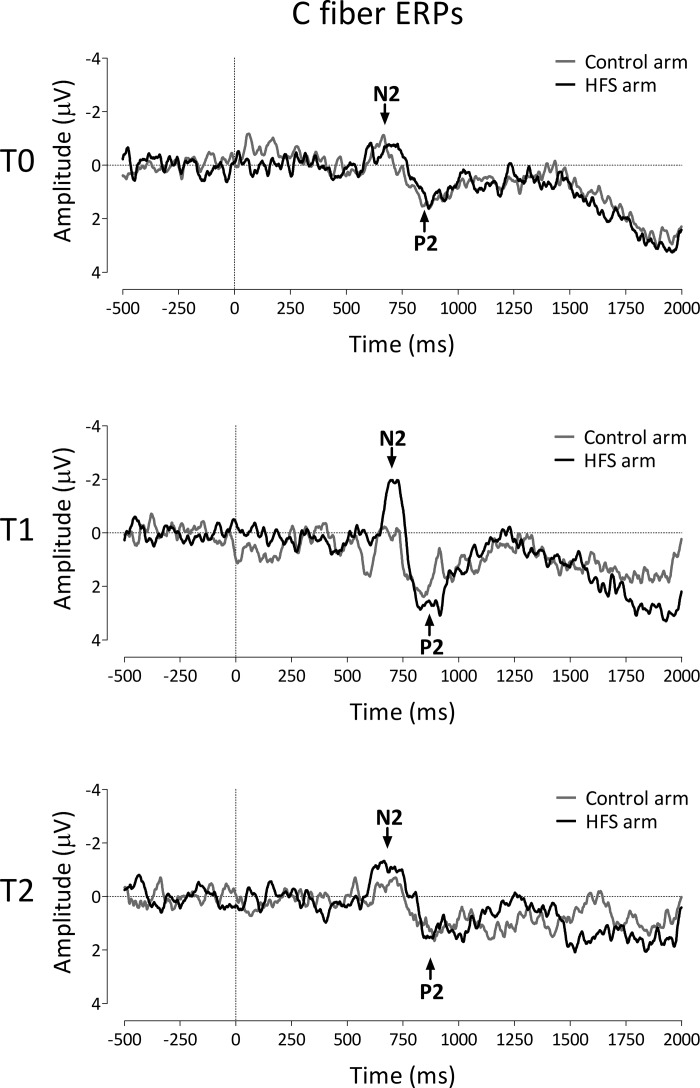
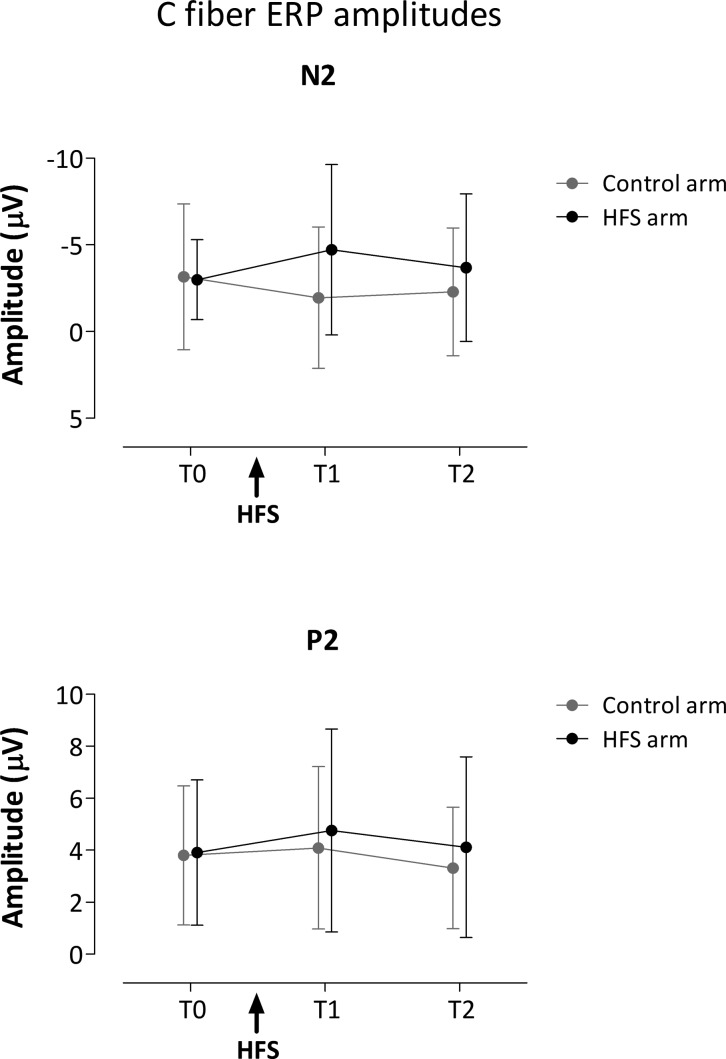
Group-level average waveforms of the LEPs elicited by thermal stimuli detected with RTs compatible with the selective activation of C fibers are shown in Fig. 4. The mean (and SD) amplitudes of the N2 and P2 waves obtained at the different time points (T0, T1, and T2) and stimulation sites (control and HFS arm) are shown in Fig. 5. The N2 and P2 latencies are summarized in Table 1. The repeated-measures ANOVA revealed a significant time × treatment interaction [F(2,28) = 4.274, P = 0.024, η2 = 0.234] for the LEP N2 wave. The univariate within-subject contrasts revealed that the N2 amplitude was significantly enhanced at T1 [F(1,14) = 5.930, P = 0.029, η2 = 0.298] at the conditioned arm after HFS. Post hoc tests were not statistically significant. No statistically significant changes were observed for the N2 latency and P2 amplitude and latency.
Fig. 4.
Effect of HFS on the event-related brain potentials (ERPs) elicited by the thermal laser stimuli selectively activating C fibers. The waveforms show the group-level average ERP waveforms of the signals measured from Cz vs. average reference, before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2), following stimulation of the HFS-treated arm (black) and the control arm (grey). Note the increase of the N2 wave at the treated arm at T1.
Fig. 5.
Mean (and SD) amplitude of the N2 and P2 waves of C-fiber laser-evoked potentials before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2), following stimulation of the HFS-treated arm (black) and the control arm (grey). Note that the amplitude of the N2 wave was significantly enhanced at T1 compared with control and T0.
Table 1.
N2 and P2 latencies
|
T0 |
T1 |
T2 |
||||
|---|---|---|---|---|---|---|
| Latency, ms | Control arm | HFS arm | Control arm | HFS arm | Control arm | HFS arm |
| C-fiber ERP N2 | 658.6 (91.6) | 684.0 (107.2) | 667.3 (83.9) | 682.1 (55.3) | 696.7 (104.0) | 685.7 (88.7) |
| C-fiber ERP P2 | 806.7 (111.6) | 791.2 (110.6) | 800.1 (90.1) | 818.8 (89.1) | 810.2 (124.7) | 818.3 (112.3) |
Values are means (SD).
HFS, high-frequency electrical stimulation; ERP, event-related brain potential.
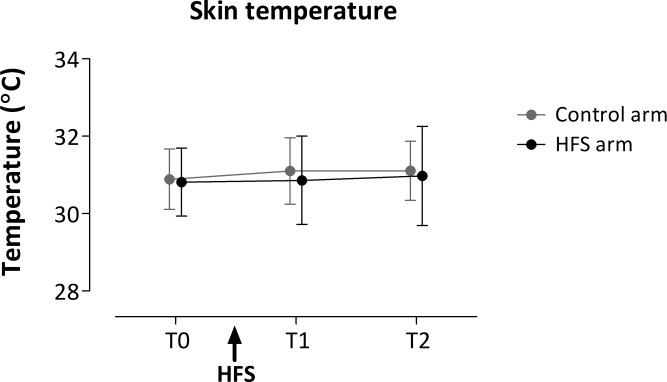
Baseline Skin Temperature
The baseline skin temperature observed at both arms (control and HFS) at the different time points (T0, T1, and T2) is shown in Fig. 6. The GLM repeated-measures ANOVA revealed no statistically significant changes in baseline skin temperature after HFS.
Fig. 6.
Mean (and SD) baseline skin temperature before HFS (T0), 20 min after HFS (T1), and 45 min after HFS (T2) following stimulation of the HFS-treated arm (black) and the control arm (grey).
DISCUSSION
The aim of this study was to examine whether, in addition to enhancing the responses to mechanical punctate stimuli, HFS also enhances the responses to thermal stimuli selectively activating heat-sensitive C-fiber afferents. After HFS, the intensity of perception to both mechanical punctate stimuli and thermal stimuli was significantly enhanced, thus confirming the presence of mechanical hyperalgesia and demonstrating the concomitant presence of enhanced thermal sensitivity to C-fiber input in the surrounding unconditioned skin. The time courses of the enhanced perception to mechanical and thermal stimuli were similar, being present both 20 min (T1) and 45 min (T2) after HFS.
In contrast, the magnitude of the N2 wave of C-fiber LEPs was significantly enhanced following HFS at T1 but not at T2. Furthermore, the magnitude of the P2 wave was unaffected by HFS. This suggests that the effects of HFS on the perception and ERPs elicited by C-fiber stimuli are functionally distinct.
Effect of HFS on the Percept Elicited by Mechanical Punctate Stimuli
In agreement with previous reports (Klein et al. 2004, 2008; Vo and Drummond 2013; van den Broeke et al. 2014a; van den Broeke and Mouraux 2014b), we demonstrate that mechanical punctate stimuli delivered to the skin surrounding the conditioned area are perceived as more intense, both 20 min and 45 min after applying HFS. Previous studies have provided evidence that this secondary mechanical hyperalgesia is primarily mediated by an enhancement of the responses to mechano-sensitive nociceptors (Ziegler et al. 1999).
Effect of HFS on the Perception of Thermal Stimuli Selectively Activating C Fibers
After HFS, brief thermal stimuli selectively activating C fibers delivered to the heterotopic skin area were also perceived as more intense. The time course of the effect of HFS on the percept elicited by the activation of heat-sensitive C fibers was similar to the time course of the effect of HFS on the percept elicited by mechanical punctate stimuli, a significant enhancement being present both 20 and 45 min after HFS (Fig. 3). This demonstrates that, in addition to inducing mechanical hyperalgesia, HFS also induces an enhanced thermal sensitivity to C-fiber input in the surrounding unconditioned skin.
Before HFS, the responses elicited by brief thermal stimuli delivered at an intensity below the detection threshold of Aδ fibers were probably related to the preferential activation of C-warm receptors or to the combined activation of C-warm receptors and mechanical-heat sensitive C nociceptors (CMHs). Indeed, C-warm receptors have a low thermal activation threshold (<1°C above skin temperature; LaMotte and Campbell 1978) and their activity monotonically increases over the temperature ranges 39–43°C (LaMotte and Campbell 1978). Although, it is generally agreed that CMHs have a lower thermal activation threshold than AMHs, there is a large variability in the estimated threshold of nonsensitized CMHs across studies, ranging between ∼40°C (e.g., Weidner et al. 1999) and 45°C (e.g., LaMotte and Campbell 1978; Hallin et al. 1981). This variability could be due to differences in the methods used to deliver the thermal stimuli and/or to the method used to assess fiber activation.
After HFS, the thermal stimuli were perceived as more intense, both 20 min (T1) and 45 min (T2) after HFS. The RTs to these stimuli remained compatible with the conduction velocity of C fibers. This enhanced sensitivity to C-fiber input in the area of secondary hyperalgesia could result from enhanced responsiveness of C-fiber input of the central nervous system, i.e., central sensitization.
However, this enhanced sensitivity to C-fiber input could also result, at least in part, to peripheral sensitization of heat-sensitive C-fiber afferents. Indeed, after HFS (Klein et al. 2004), or after intradermal injection of capsaicin (Magerl et al. 2001), one can observe a reddening of the skin progressively extending several centimeters around the treated area (i.e., extending in the area of secondary hyperalgesia). This flare response results from the release of inflammatory mediators by peptidergic nociceptive afferents activated by the conditioning stimulus. The spreading of the flare response beyond the treated area is of peripheral origin (Groetzner and Weidner 2010) and mediated by mechano-insensitive C fibers (Schmelz et al. 2000a). The flare response may be explained by antidromic activation of neighboring branching fibers through an axon reflex, thereby leading to the remote release of inflammatory mediators and/or by passive diffusion of these mediators (Lewis 1936; Groetzner and Weidner 2010). Therefore, as inflammation is likely to induce a sensitization of peripheral afferents, the spreading of the flare response beyond the treated area raises the possibility that peripheral sensitization contributes to the phenomenon of increased sensitivity in the area of secondary hyperalgesia (Serra et al. 2004).
At the peripheral level, there is substantial evidence that CMHs are not sensitized in the area of secondary hyperalgesia induced by intradermal capsaicin (Schmelz et al. 2000b; Serra et al. 2004). However, using microneurography, Serra et al. (2004) found that mechano-insensitive C fibers became responsive to mechanical stimulation after remote intradermal injection of capsaicin. Moreover, heat stimuli (heated rod at ∼45°C) delivered to the receptive fields of these mechano-insensitive C fibers elicited prolonged discharges. However, this result is in contradiction with that of Schmelz et al. (2000b), who did not observe sensitization of mechano-insensitive C fibers in the area adjacent to the site of intradermal capsaicin injection. Specifically, injection of capsaicin in one part of the receptive field of a mechano-insensitive C fiber did not change the activity of the same C fiber when stimulating another part of the receptive field. However, Schmelz et al. (2000b) tested mechanical sensitivity but not heat sensitivity of mechano-insensitive afferents in the remote area. Furthermore, they used a much smaller dose of capsaicin to induce sensitization (20 μg instead of 100 μg).
Therefore, and not excluding a possible contribution of central sensitization, the enhanced responses to heat-evoked C-fiber input observed in the present study could also have resulted from a peripheral sensitization of mechano-insensitive C fibers.
Effect of HFS on C-Fiber LEPs
In the present study, the laser stimuli generated increases in skin temperature above the thermal detection threshold of C fibers but below the thermal detection threshold of Aδ fibers, both before and after HFS. At all time points and stimulation sites, the recorded LEPs were exclusively related to the activation of C fibers, as demonstrated by the long latencies of the N2 and P2 waves compatible with the slow conduction velocity of unmyelinated C fibers and the absence of any earlier response compatible with the conduction velocity of Aδ fibers (Mouraux et al. 2003).
Following HFS, the magnitude of the N2 wave of C-fiber LEPs was significantly enhanced at T1 (20 min after HFS) but not at T2 (45 min after HFS). This time course contrasts with the time course of the effect of HFS on the intensity of the percept elicited by the same stimuli, which was significantly enhanced at both time points. A previous study has shown that secondary hyperalgesia following HFS is relatively long lasting (half-life between 3–5 h; Pfau et al. 2011). Therefore, the mechanism explaining the transient enhancement of the C-fiber N2 wave is most probably distinct from the mechanism explaining the enhancement of thermal perception. One possible explanation for the transient increase of the C-fiber N2 wave could be an effect of attention. Indeed, it could be expected that, following HFS, participants tend to have their attention focused towards the treated arm. Such an interpretation could also explain our recent finding that HFS induces a transient enhancement of the N1 wave of nonnociceptive vibrotactile ERPs.
More generally, the finding that HFS did not enhance C-fiber LEPs at both time points indicates that the HFS-induced enhanced thermal sensitivity is mediated by afferents that do not significantly contribute to C-fiber LEPs. This could be explained by the fact that the recording of ERPs requires a time-locked and phasic afferent volley and, hence, that C-fiber LEPs most likely reflect cortical activity related only to the activation of rapidly adapting C fibers. In contrast, the percept elicited by the stimuli used to elicit C-fiber LEPs most likely integrates inputs conveyed by both rapidly and slowly adapting C fibers. Indeed, based on their responses to sustained noxious heat (e.g., 53°C for 30 s; Meyer and Campbell 1981), C fibers have been categorized as either slowly adapting or rapidly adapting. Slowly adapting C fibers respond gradually following the onset of the thermal stimulus and exhibit little or no adaptation when the thermal stimulus is maintained over time. In contrast, rapidly adapting C fibers respond immediately after the onset of the thermal stimulus but quickly adapt if the thermal stimulus is maintained over time (Meyer and Campbell 1981).
Conclusion
The present study shows that, in addition to mechanical hyperalgesia, HFS induces a concomitant enhancement of thermal sensitivity to selective C-fiber input in the surrounding unconditioned skin. This enhanced thermal sensitivity could result from peripheral sensitization of mechano-insensitive C fibers and/or from enhanced responsiveness of neurons at the level of the central nervous system relaying that input to the cortex.
GRANTS
E. N. van den Broeke is supported by the Belgian National Foundation for Scientific Research (FNRS). A. Mouraux is supported by an ERC “Starting Grant” (PROBING-PAIN 336130).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.N.V.D.B. and A.M. conception and design of research; E.N.V.D.B. performed experiments; E.N.V.D.B. analyzed data; E.N.V.D.B. and A.M. interpreted results of experiments; E.N.V.D.B. prepared figures; E.N.V.D.B. drafted manuscript; E.N.V.D.B. and A.M. edited and revised manuscript; E.N.V.D.B. and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ole Kæseler Andersen for providing the conditioning electrode.
REFERENCES
- Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following capsaicin injection in hairy skin. Pain 68: 401–411, 1996. [DOI] [PubMed] [Google Scholar]
- Churyukanov M, Plaghki L, Legrain V, Mouraux A. Thermal detection thresholds of Aδ- and C-fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PLoS One 7: e35817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55: 468–484, 1983. [DOI] [PubMed] [Google Scholar]
- Groetzner P, Weidner C. The human vasodilator axon reflex–an exclusively peripheral phenomenon? Pain 149: 71–75, 2010. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE, Wiesenfeld Z. Nociceptors and warm receptors innervated by C fibers in human skin. J Neurol Neurosurg Psychiatry 44: 313–319, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Plaghki L, Mouraux A. Reliable EEG response to the selective activation of C-fiber afferents using a temperature-controlled infrared laser stimulator in conjunction with an adaptive staircase algorithm. Pain 154: 1578–1587, 2013. [DOI] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hopf HC, Sandkühler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci 24: 964–971, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Stahn S, Magerl W, Treede RD. The role of heterosynaptic facilitation in long-term potentiation (LTP) of human pain perception. Pain 139: 507–519, 2008. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol 41: 509–528, 1978. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10: 895–926, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. Experiments relating to cutaneous hyperalgesia and its spread through somatic nerves. Clin Sci 2: 373–421, 1936. [Google Scholar]
- Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain 74: 257–268, 1998. [DOI] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 124: 1754–1764, 2001. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Campbell JN. Evidence for two distinct classes of unmyelinated nociceptive afferents in monkey. Brain Res 224: 149–152, 1981. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guérit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between Aδ- and C-fibre afferent volleys. Clin Neurophysiol 114: 710–722, 2003. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Thomas NA, Loetscher T, Grimshaw GM. The Flinders Handedness survey (FLANDERS): A brief measure of skilled hand preference. Cortex 49: 2914–2926, 2013. [DOI] [PubMed] [Google Scholar]
- Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain 76: 377–384, 1998. [DOI] [PubMed] [Google Scholar]
- Pfau DB, Klein T, Putzer D, Pogatzki-Zahn EM, Treede RD, Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain 152: 1532–1539, 2011. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Meyer RA. Physiology of nociceptors. In: Science of Pain, edited by Basbaum IA, Bushnell MC. Oxford, UK: Elsevier, 2009, p. 97–114. [Google Scholar]
- Ringkamp M, Raja SN, Campbell JN, Meyer RA. Peripheral mechanisms of cutaneous nociception. In: Textbook of Pain (6th ed.), edited by McMahon SB, Koltzenburg M, Tracey I, Turk DC. Philadelphia, PA: Elsevier: 2013, p. 1–30. [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schmid R, Torebjörk HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 11: 645–8, 2000a. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmid R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibers. Brain 123: 560–571, 2000b. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J. Flare and hyperalgesia after intradermal capsaicin injection in human skin. J Neurophysiol 80: 2801–2810, 1998. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Bostock H, Ochoa J. Two Types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol 91: 2770–2781, 2004. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. Capsaicin, irritation, and desensitization: neurophysiological basis and future perspectives. In: Chemical Senses, edited by Green BG, Mason JR, Kare MR. New York: Marcel Dekker, 1990, p. 141–169. [Google Scholar]
- Treede RD, Magerl W. Multiple mechanisms of secondary hyperalgesia. Prog Brain Res 129: 331–341, 2000. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483: 747–758, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo L, Drummond PD. High frequency electrical stimulation concurrently induces central sensitization and ipsilateral inhibitory pain modulation. Eur J Pain 17: 357–368, 2013. [DOI] [PubMed] [Google Scholar]
- van den Broeke EN, Geene N, Van Rijn CM, Wilder-Smith OH, Oosterman J. Negative expectations increases mechanical hyperalgesia after high frequency conditioning stimulation of human skin. Eur J Pain 18: 86–91, 2014. [DOI] [PubMed] [Google Scholar]
- van den Broeke EN, Mouraux A. High frequency electrical stimulation of the human skin induces heterotopical mechanical hyperalgesia, heat hyperalgesia and enhanced responses to non-nociceptive vibrotactile input. J Neurophysiol 111: 1564–1573, 2014b. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjörk HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci 19: 10184–10190, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel A, Andersen OK, Nielsen J, Arendt-Nielsen L. Heat hyperalgesia in humans: assessed by different stimulus temperature profiles. Eur J Pain 6: 357–364, 2002. [DOI] [PubMed] [Google Scholar]
- Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 122: 2245–2257, 1999. [DOI] [PubMed] [Google Scholar]