Abstract
Objectives
This study used a rat contact lens (CL) model to test if high vs. low Dk lens wear caused changes in: 1) conjunctival Langerhans cell (LC) number or location; 2) Bcl-2 expression; and 3) infection risk.
Methods
Female, Lewis rats wore a high or low Dk CL continuously for 2 weeks. Afterward, corneas were harvested and processed for ADPase activity to identify Langerhans cells (LC), for immunostaining and for real time RT-PCR. CL wearing rats also were challenged with Pseudomonas aeruginosa by placing a bacterial-soaked CL on the eye followed by topical delivery of bacteria. After 48 hours, slit lamp examination and real time RT-PCR were used to evaluate the corneal response.
Results
Conjunctival LC were significantly increased after low vs. high Dk CL wear (p<0.0001). In contrast, conjunctival LC in non-lens wearing rats was not significantly different from the high Dk lens wearing group. Bcl-2 mRNA levels were significantly decreased in low vs. high Dk Cl wearing rats, while Bax, FasL, caspase 3 and caspase 9 levels were unchanged. Immunostaining for Bcl-2 showed fewer positively stained epithelial cells in the low vs. high Dk lens wearing group. After bacterial challenge, 30% of low vs. none of the high Dk CL wearing corneas became infected and showed increased mRNA levels for several pro-inflammatory cytokines/chemokines, inducible nitric oxide synthase (iNOS) and matrix metalloproteinase (MMP)-9.
Conclusion
Low vs. high Dk and/or no CL wear led to an increased number of conjunctival LC, decreased Bcl-2 levels, and increased the risk of bacterial infection.
Keywords: rat, contact lens, Langerhans cells, conjunctiva, P. aeruginosa
INTRODUCTION
Contact lenses impede the movement of oxygen to the anterior of the cornea, and create a lens-induced hypoxia1 Chronic corneal hypoxia is a major issue because it causes corneal edema, which may be manifested clinically as central corneal clouding, striae and folds. In the long term, contact lens induced hypoxia may lead to corneal exhaustion syndrome and discontinued contact lens wear.2 It also has been found that contact lenses that do not meet the cornea’s oxygen needs cause impaired corneal metabolism and integrity, decreased epithelial thickness, stromal thinning, increased endothelial polymegathism and limbal redness and vascularization.3,4 Other studies show that hypoxia causes increased bacterial adhesion to epithelial cells5–8 and overnight hypoxia increases the risk for infection.9 In experimental animal models, extended wear of hydrogel lenses has been shown to induce migration of Langerhans cells (LC) into the central cornea.10 Another study showed that LC are critical to the innate immune response to P. aeruginosa and if LC are induced into the cornea before infection, disease outcome is worsened. 11 It was concluded that one of the consequences of LC in the central cornea before infection may have resulted in priming the cornea to respond more rapidly and severely to insults and enhanced immune responsiveness. Low Dk rigid gas permeable lenses may also markedly decrease shedding of the corneal epithelium and appeared to do so in an experimental rabbit model by blocking changes in Bcl-2, an anti-apoptotic factor. In order to examine further the relationship between oxygen transmissibility and selected features described above, this study used a rat model and tested high vs. low Dk lens wear to determine if they disparately affected 1) conjunctival Langerhans cell (LC) number or location; 2) Bcl-2 expression; and 3) risk of infection.
MATERIALS AND METHODS
Contact Lens
A lotrafilcon B silicone hydrogel CL (33% water with a Dk of 110; a high Dk CL) (CIBA Vision, Duluth, GA), lot number P-257T-28905-01; and a nelfilcon A (69% water with a Dk of 26; a low Dk CL) (CIBA), lot number NB# 2956 were used. All lenses in this study were made specifically for the rat cornea with the following dimensions: 2.4 mm base curve, 80 μm center thickness, 40 μm edge thickness, and 5.34 mm diameter.12
Animals
Two-three month old, female Lewis rats, purchased from Harlan, Indianapolis, IN were housed in accordance with the National Institutes of Health guidelines. Rats were lightly anesthetized with ethyl ether (Fisher Scientifics, Fairlawn, NJ) and a new, sterile CL was placed onto the left eye of each rat for 2 weeks of extended wear. Three-month-old rats (weight 175–200g) were used for the low Dk test group, while 2-month-old rats (weight 100–125g) were used for high Dk test group based upon lens fitting and retention. The contralateral eye which was not subjected to CL wear provided an internal control.
LC staining and quantitation
After two weeks of extended CL wear, rats were sacrificed (described below) and eyes from each of the CL wearing groups (n=6/group) and their contralateral non-lens wearing eyes were hemisected and the anterior segment tissue placed in 0.02 M EDTA-PBS buffer, pH 7.2 for 1 hour at 37°C to allow epithelial removal for LC staining. Epithelial sheets were fixed in cacodylate-buffered formaldehyde for 20 min at 4°C, washed four times with cold 0.1 M cacodylate buffer, and then incubated in ADPase substrate solution containing ADPase buffer, 2% lead nitrate, and ADP (5 mg/mL; Sigma) for 15 min at 37° C. Sheets were next washed four times with Tris-maleate buffer (pH 7.2), developed for 5 min in a 1:10 ammonium sulfide solution, washed again three times with buffer, mounted onto slides with glycerol, flattened by 2–4 cruciate cuts, and coverslipped. Epithelial sheets were photographed with a Zeiss Axiophot with Axiocam digital imagery (Carl Zeiss, Morgan Instruments, Cincinnati, OH) at 25X, printed and LC number quantitated per field (n=10 fields/group).10 Data were statistically analyzed using an unpaired Student’s t test.
Bacterial challenge
After 2 weeks extended wear of either high or low Dk CL, the worn lens was removed from the rat eye (n=10/group), and soaked for one hour in a suspension containing 1.0 × 108 CFU/μl P. aeruginosa (cytotoxic strain 19660, American Type Culture Collection, Manassas, VA), replaced onto the respective eye, followed by topical challenge with 10 μl of 1.0 × 108 CFU/μl bacteria four hours later. Rats were observed at 24 and 48 hours after bacterial challenge, and at the later time slit lamp and clinical score grading were performed. Rats were anesthetized with ether and sacrificed by lethal intracardiac injection of 4M KCl (Fisher Scientific, Fairlawn, NJ). Corneal tissue was collected and stored in RNA later RNA Stabilization Reagent (Qiagen, Valencia, CA) for real-time RT-PCR analysis.
Ocular response to bacterial challenge
After bacterial challenge, corneal disease was graded using an established scale13: 0, clear or slight opacity partially covering the pupil; +1, slight opacity fully covering the entire anterior segment; +2, dense opacity partially or fully covering the pupil; +3, dense opacity covering the entire anterior segment; and +4, corneal perforation. Slit lamp was used to document the corneal response.
Bacterial Plate Count
Infected corneas (n = 3) were excised and the number of viable bacteria quantitated. Individual corneas were homogenized in sterile 0.9% saline containing 0.25% BSA. Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar (Difco, Detroit, MI) in triplicate and plates were incubated overnight at 37°C. Results are reported as log10 number of CFU/sample ± SEM.
Real time RT-PCR
Total RNA was extracted (n=6/group) using RNA Stat-60® reagent (Tel-Test, Friendsville, TX) following the manufacturer’s protocol. The cDNA was synthesized from 1 μg of total RNA using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA). Primers (Table 1) were designed using the Primer3 program, and spanned 1 kb or more introns to prevent genomic DNA from being amplified. Specificity of the primers was confirmed by BLAST analysis. cDNA was diluted 25-fold with diethylpyrocarbonate (DEPC)-treated H2O and 2 μl of cDNA was added to a 18 μl iQ™ SYBR® Green Supermix (Bio-Rad, Hercules, CA) with a corresponding primer pair. The quantitative PCR reactions were performed in duplicate on a MyiQ™ Single-Color Real-Time PCR Detection System (Bio-Rad), and expression of RPL19 was used for normalization. Thermal cycling parameters were as follows: 95°C for 3 min, 40 cycles of denaturing at 95°C for 10 sec, and annealing/extension for 30 sec at 60°C.
Table 1.
| Primer | GenBank No. | Sequence (5′ – 3′) | Size (bp) |
|---|---|---|---|
| RPL19 | NM_031103 | F – CCC CAA TGA AAC CAA CGA AA R – ATG GAC AGT CAC AGG CTT C |
100 |
| IL-1α | NM_017019 | F – CAG CCC TTT ACT GAA GAT GAC C R – TGA TGA ACT CCT GCT TGA CG |
136 |
| IL-1β | NM_031512 | F – ACC CAA GCA CCT TCT TTT CC R – AGA CAG CAC GAG GCA TTT TT |
149 |
| IL-1RA | NM_022194 | F – CTC TCT CCT TCT CAT CCT TCT GTT R – CAC ATT CCG AAA GTC AAT AGG C |
211 |
| IL-6 | NM_012589 | F – ACC ACC CAC AAC AGA CCA GT R – CTC CAG AAG ACC AGA GCA GAT T |
243 |
| IL-10 | NM_012854 | F – GAA TAA AAG CAA GGC AGT GGA G R – TTT GAG TGT CAC GTA GGC TTC T |
127 |
| MMP-9 | NM_031055 | F – GCT TGG ATA ACG AGT TCT CTG G R – GCA GGA GGT CAT AGG TCA CG |
162 |
| CINC-1 | NM_030845 | F – AGT GGC AGG GAT TCA CTT CAA R – GCC ATC GGT GCA ATC TAT CT |
208 |
| TNF-α | NM_012675 | F – CAT CTT CTC AAA ACT CGA GTG ACA A R – TGG GAG TAG ATA AGG TAC AGC CC |
175 |
| iNOS | NM_012611 | F – AGG GAG TGT TGT TCC AGG TG R – TCT GCA GGA TGT CTT GAA CG |
81 |
| Bcl-2 | NM_016993 | F – AGT ACC TGA ACC GGC ATC TG R – CAT GCT GGG GCC ATA TAG TT |
83 |
| Bax | NM_017059 | F – CTA GCA AAC TGG TGC TCA AGG R – CAC AAA GAT GGT CAC TGT CTG C |
182 |
| FasL | NM_012908 | F – CAC ACC CTC TGA AAC CAA AAA R – CCA GAG ATC AAA GCA GTT CCA |
114 |
| Casp3 | NM_012922 | F – TTA CCC TGA AAT GGG CTT GT R – TAG CTG CAT CGA CAT CGG TA |
98 |
| Casp9 | NM_031632 | F – CTG TGT CCA TCG AGA GAA TTG R – AGA GGA AGT GAA GGC CAC CT |
140 |
Immunostaining
Eyes were enucleated from both groups (n=3/group) of CL wearing rats, hemisected and embedded in O.C.T., frozen in liquid nitrogen, stored at −20°C and sectioned (10 μm). Sections were dried overnight, fixed in acetone (2 min), washed in 0.01M phosphate buffered saline (PBS) and nonspecific staining blocked in PBS containing 2.5% bovine serum albumin, and 1:100 donkey IgG (30 min). Sections were rinsed and incubated with rabbit anti-rat/mouse Bcl-2 polyclonal antibody (BD PharMingen) (1:800) in blocking agent, rinsed with PBS and then incubated with 0.05 M Tris-HCl buffer and the secondary antibody (Alexa Fluor 594 conjugated donkey anti-rabbit Ab, Invitrogen) diluted 1:1500 in .05 M Tris-HCl buffer. Slides were rinsed, counterstained with SYTOX GREEN nuclear label (1:200,000) for 2 min and coverslipped using VECTASHIELD mountant (Vector Laboratories). Control sections were similarly treated but without primary antibody. Sections were visualized and digital images were captured with a Leica TSC SP2 confocal laser scanning microscope (Leica Microsystems, Mannheim, Germany).
Statistical analysis
The difference in clinical score between the two-groups was tested by the Mann-Whitney U test. An unpaired, two-tailed Student’s t-test was used to determine the significance for LC number and mRNA data. Differences were considered significant at p ≤ 0.05. The data are expressed as the mean ± the standard error of the mean (SEM). Each experiment was repeated at least once, and data from a typical experiment are shown.
RESULTS
Langerhans cell detection and quantitation
After 2 weeks extended CL wear, centripetal migration of LC into the cornea was rarely seen (few round, less dendritic shaped cells) in either high or low Dk lens wearing rats and was never seen in the non-lens wearing animals. However, when examining LC in the conjunctiva (limbal area) and quantitating the number of cells (Figure 1A–D) following CL usage, differences were detected. LC number in the low Dk CL wearing rats (Figure 1C, D) was significantly higher (47 vs. 29/field; p<0.0001) than in the high Dk wearing (Figure 1B, D) or non-lens wearing (Figure 1A, D; p<0.0001) rats. In addition, the number of LC in the conjunctiva of non-lens wearing rats (27 vs. 29/field) did not significantly differ from the high Dk lens wearing animals (p=0.16) (Figure 1D).
Figure 1.
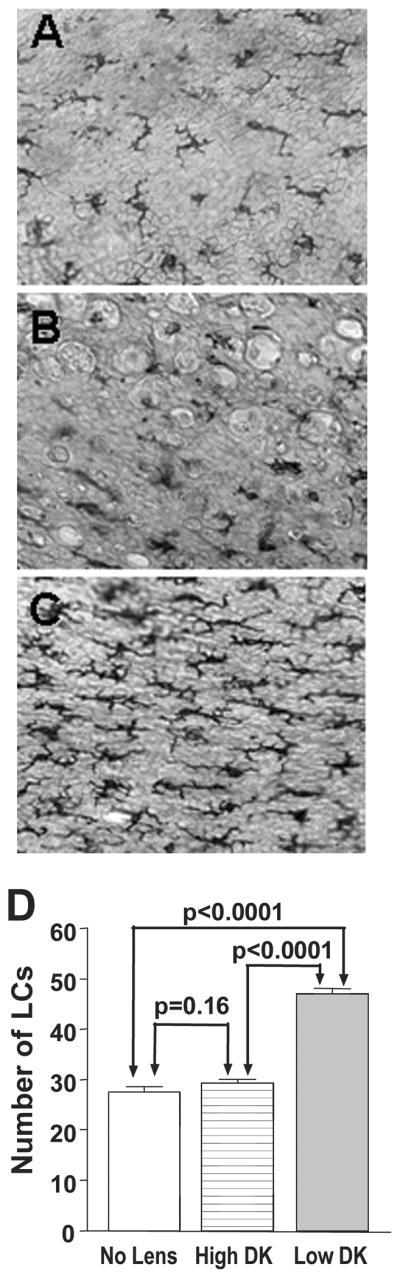
A–D. After 2 weeks extended CL wear, similar ADPase positive dendritic-shaped LC were seen in the limbal conjunctiva of non-lens wearing (A) and high Dk (B) wearing rats, but appeared to be more numerous in low Dk (C) lens wearing animals. Few (less dendritic, more rounded) cells were observed. Magnification=160X (E) When quantitating conjunctival LC number, LC were significantly increased in the conjunctiva (limbal) of low Dk when compared with high Dk (p<0.0001) or non-lens wearing rats (p<0.0001). The number of LC in the conjunctiva of non-lens wearing rats was not significantly different from the high Dk wearing animals (p=0.16).
Bacterial challenge and plate counts
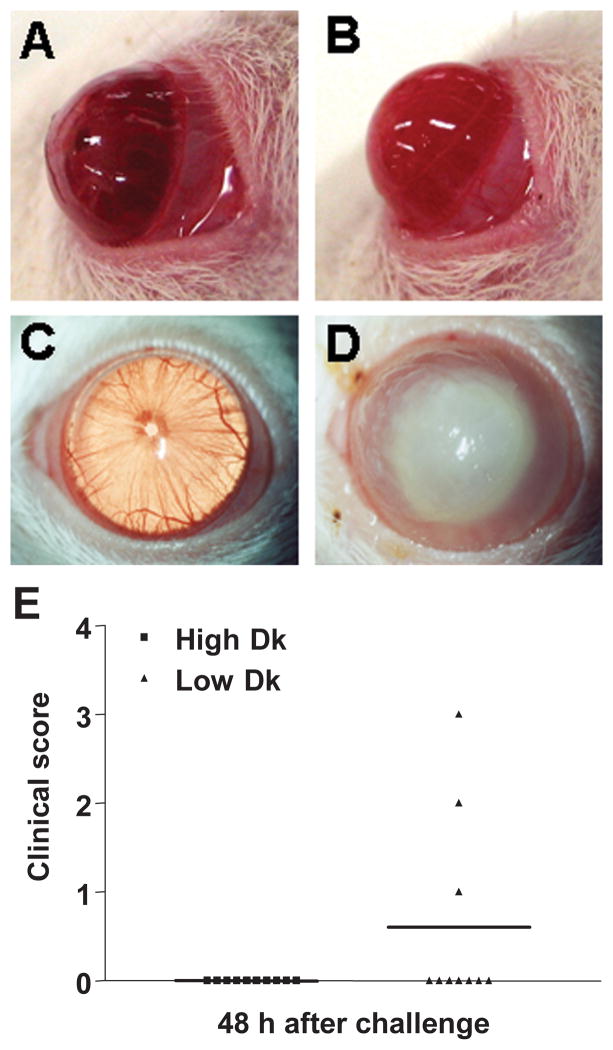
To test whether the increased number of LC in the conjunctiva of low Dk CL wearing rats enhanced corneal susceptibility to bacterial infection when compared with high Dk CL wearing animals, rats were challenged with P. aeruginosa after 2 weeks extended wear. Rats wearing the high (Figure 2A) or low (Figure 2B) Dk CL were photographed using slit lamp before (Figure 2A and B) and at 48 hours after bacterial challenge (Figure 2C and D). Although the data were not significant (p=0.09), three of the ten rats wearing a low Dk CL (Figure 2D) vs. none of the ten high Dk CL wearing rat corneas (Figure 2C) became infected. Disease was graded for severity (Figure 2E) and the clinical scores showed that in the infected corneas, opacity ranged from a mildly opaque (clinical score of +1) to a fully opaque cornea (clinical score of +3). None of the infected corneas perforated. Bacterial plate counts from infected corneas contained an average of 930,000 CFU/ml from the infected corneas; no bacteria were detected in challenged, uninfected corneas.
Figure 2.
(A–E). Slit lamp photomicrographs (A–D). Lewis rat wearing high Dk lotrafilcon B lens before (A) and after (C) bacterial challenge or low Dk nelfilcon A lens before (B) and after (D) bacterial challenge. Magnification = A,B= 3X; C,D=4X. (E) Clinical scoring of disease at 48 hours after bacterial challenge showed that 3 of 10 low Dk wearing rat corneas vs. none of 10 of the high Dk wearing rat corneas infected. These data, showing a trend toward infection with low DK lens wear, were not significant (p=0.09).
Real time RT-PCR
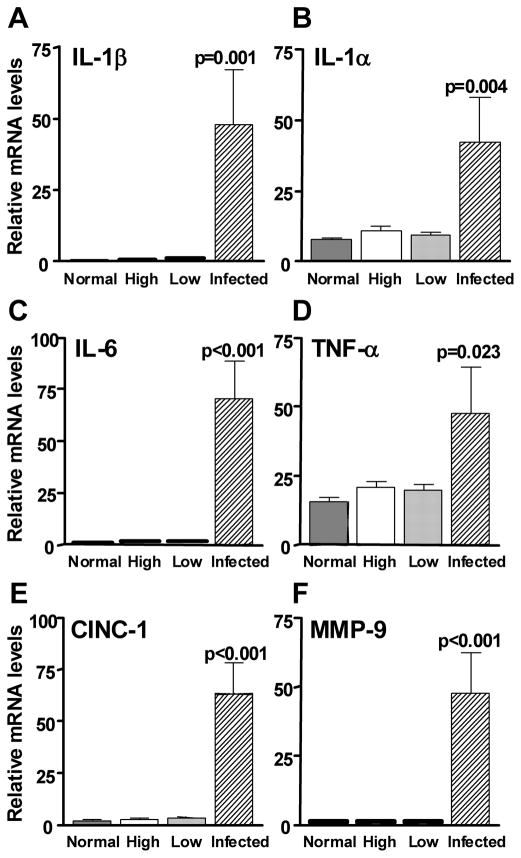
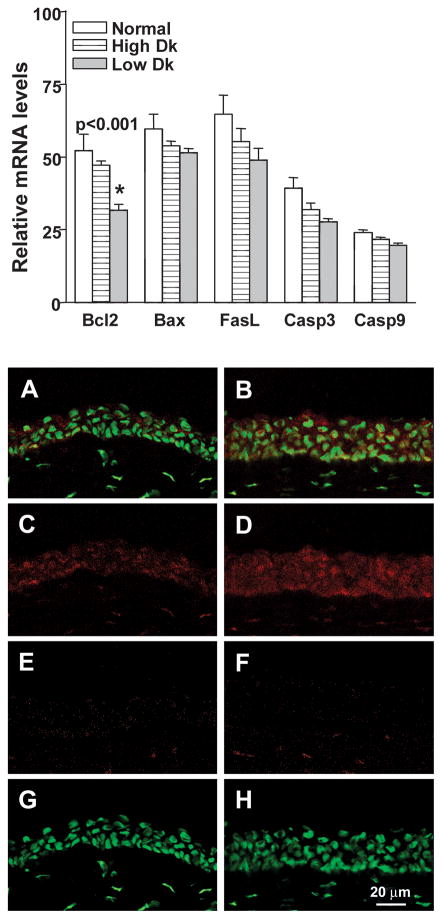
Figure 3(A–F) shows that mRNA levels of proinflammatory cytokines/chemokines IL-1β (A, p=0.001), IL-1α (B, p=0.004), IL-6 (C, p<0.001), TNF-α (D, p=0.023), and CINC-1 (E, p<0.001) (rat IL-8), as well as MMP-9 (F, p<0.001) which can amplify the effects of IL-1, were significantly elevated in the infected cornea of low Dk CL wearing rats when compared with uninfected low or high Dk wearing animals. Figure 4(A–C) shows that mRNA levels of the physiological antagonist of IL-1 receptor, IL-1RA (A, p=0.003), the anti-inflammatory cytokine IL-10 (B, p<0.001), and the bactericidal compound, iNOS (C, p=0.004) also were significantly higher in the infected low Dk CL wearing rat corneas when compared with uninfected low or high Dk wearing groups. No differences were detected between high or low DK wearing groups for these cytokines. Apoptosis related genes also were analyzed in the challenged but uninfected corneas to determine whether or not there were differences in the two CL wearing groups compared with the non-lens wearing group (Figure 5, top illustration). No differences in the two CL wearing groups vs. the normal, non-lens wearing cornea were seen for mRNA levels of Bax, Fas ligand, caspase 3 or caspase 9. mRNA levels of the anti-apoptotic factor Bcl-2 did differ, however, with a significant decrease seen in the low Dk vs. both the high Dk and non lens wearing groups (p<0.001 for each). There was no difference in Bcl-2 mRNA levels in high Dk vs. the non-lens wearing group (p=0.28). Immunostaining (Figure 5, bottom illustration A–H) for Bcl-2 was detected in the corneal epithelium of both low (A,C) and high Dk (B,D) CL wearing groups of rats, but appeared more intense in the high Dk CL wearing rats. Normal corneas were not tested. Slight staining was visible in the control sections of both groups (E, F) in the stroma (keratocytes), but not the epithelium, suggesting that the stromal staining seen in both Figures C and D, was likely nonspecific. SYTOX GREEN nuclear stain (G, H) appeared similar in both groups.
Figure 3.
(A–F). Real time RT-PCR at 48 hours after bacterial challenge. mRNA levels of IL-1β, IL-α, IL-6, TNF- α, CINC-1 (rat IL-8) and MMP-9 were significantly elevated in the infected low Dk wearing corneas when compared with levels in the uninfected low (or high) Dk wearing corneas. mRNA levels in the latter two appeared similar to the non-lens wearing, normal cornea.
Figure 4.
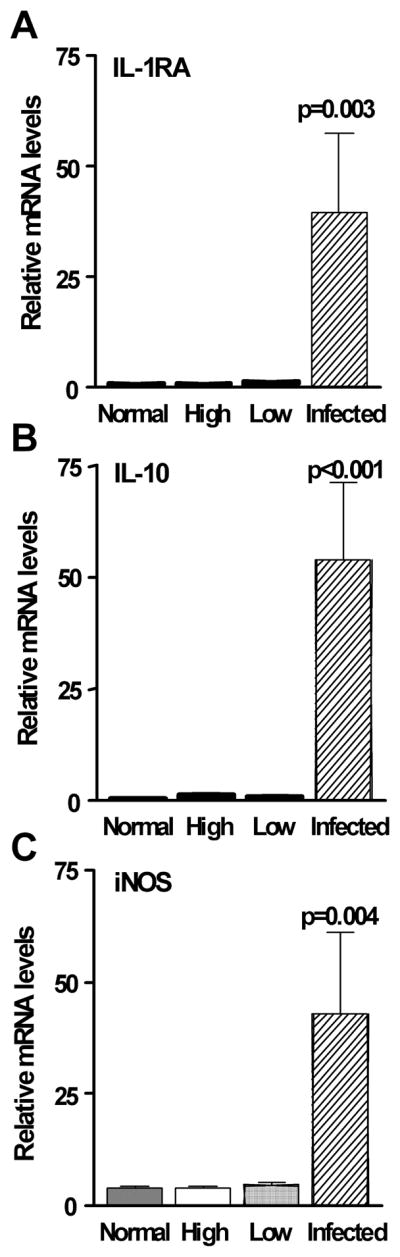
(A–C). Real time RT-PCR at 48 hours after bacterial challenge. mRNA levels of IL-1RA, IL-10 and iNOS were significantly elevated in the infected low Dk wearing corneas when compared with levels in the uninfected low (or high) Dk wearing corneas. mRNA levels in the latter two appeared similar to the non-lens wearing, normal cornea.
Figure 5.
(Top) Real time RT-PCR at 48 hours after bacterial challenge in normal, and uninfected high and low Dk wearing eyes. mRNA levels of Bcl-2 were significantly decreased in the infected low Dk wearing corneas when compared with levels in the normal or challenged but uninfected high Dk CL wearing corneas. No differences among the three groups were seen for Bax, FasL, caspase 3 or caspase 9. (Bottom A–H). More cells in the epithelium appeared to be stained positively for Bcl-2 in the high (B,D) vs low Dk (A,C) CL wearing cornea. (E, F) Control eyes incubated in the absence of the primary antibody showed no staining in the epithelium, but some non-specific keratocyte staining was observed. (G, H) SYTOX GREEN nuclear labeling alone is shown. Magnification= 220X; bar= 20μm.
DISCUSSION
The contact lens wearing rat model allows use of an inbred animal model to study the effects of CL wear in the absence of corneal scarring, used in most rodent models.12 This model was used to assess the impact on the cornea of low vs. high Dk lens wear of 2 weeks regarding several parameters. CL wear increased the number of conjunctival LC, powerful antigen presenting cells in the conjunctiva, of low Dk wearing, but not high Dk lens wearing rats, but did not result in centripetal migration of the cells into the cornea. Hypothetically, this peripheral increase in LC might prime the eye to respond more quickly to antigen challenge. This could result in subsequent migration of LC carrying antigen to regional lymph nodes, presenting antigen to cells in the node, and subsequent migration of effector cells back to the eye, enhancing the ocular innate immune response and leading to an overproduction of inflammatory mediators and increased disease. Comparably, in other species such as the rabbit, two week extended wear of a low Dk CL (61.4% polymacon and 38.6% water) did induce migration of LC into the central cornea and upon removal of the CL, the number decreased after one week, but there were still detectable cells in the central cornea after 2 weeks.10 In the guinea pig, extended wear of hydrogel lenses also was shown to cause increased LC in the peripheral cornea after 2 nights of wear onward and cells were seen in the central cornea from 4 nights onward, suggesting that their presence in the central cornea may also be related to some of the adverse events seen with CL wear.14 Corneal dendritiform cells, possibly dendritic or LC also have been identified in severe corneal immunologic conditions (case of mixed bacterial keratitis with severe immunologic reaction) using in-vivo confocal microscopy.15 In addition, in a large patient study (225 eyes of 130 healthy volunteers vs. 98 eyes of 55 CL wearers) were examined using a Heidelberg Retina Tomograph II and a Rostock Cornea Module.16 LC were found in both the center and periphery of the cornea without differences in distribution between the two groups. However, the CL wearers had an almost twofold higher LC density in both locations implying chronic mechanical irritation of the cornea in response to the CL as a foreign body. In this regard, in an experimental model, LC were induced into the cornea before P. aeruginosa infection in mice that are normally resistant to the disease (cornea heals). The consequences of this were corneal perforation, increased mRNA levels of IFN-γ and an enhanced delayed type hypersensitivity response.11
Development of the rat contact lens wearing model also has been used to assess the effects of challenge with P. aeruginosa17 and in the current study the effect of Dk was assessed. Low vs. high Dk lens wear increased the risk for bacterial infection following challenge with P. aeruginosa. Although not significant (p=0.09), these data suggest there is a higher risk of infection associated with low Dk lens wear, and perhaps this higher risk was associated with the increased number of conjunctival LC seen in the low Dk wearing group. As expected, mRNA levels of proinflammatory cytokines/chemokines, as well as MMP-9, also were significantly elevated in the infected cornea of low Dk CL wearing rats when compared with uninfected low or high DK wearing animals. mRNA levels of anti-inflammatory cytokines and the bactericidal compound, iNOS also were significantly higher in the infected low Dk CL wearing rat corneas when compared with uninfected low or high Dk wearing groups, indicating that the cornea is attempting to restore homeostasis even in the presence of the low Dk lens.
In support of these data in a 3 year prospective, randomized masked clinical trial to evaluate the relationship of CL wear to oxygen transmissibility and bacterial adherence, seven soft and three rigid gas permeable (RGP) lenses with stratified oxygen permeability were evaluated. Evidence was provided that showed that lens oxygen transmissibility properties and not lens type significantly correlated inversely with the binding of P. aeruginosa to human exfoliated corneal epithelial cells after overnight and extended wear. The results establish a significant correlation between CL induced increases in epithelial P. aeruginosa binding and lens oxygen transmissibility in humans. The data suggested that CL constructed from ultra-transmissible oxygen material might offer a significant potential in safety for extended wear.18 Klotz et al.19 also showed that extended wear soft CL are associated with enhanced adherence of P. aeruginosa to the rabbit cornea. Three to eight times as many bacteria adhered to the lens-wearing eye as compared with control eye. Several lectins, also increased in binding, possibly directly contributing to the enhanced bacterial adherence. Our study did not address the factor of adherence of bacteria as a possible contributor to infection.
Others20 have shown in the rabbit that Bcl-2 nuclear staining was altered after either high or low DK lens wear. Low DK lens wear led to decreased basal staining for Bcl-2, while high DK lens wear increased staining basally. In our study, decreased intensity of nuclear staining was observed after low DK, but not high DK lens wear. Some epithelial staining also appeared not associated with nuclei, suggesting that it might reflect non-specific staining. However, it also is possible that this staining may be associated with nuclei out of the plane of focus. This possibility is supported because the control for non-specific staining did not show epithelial staining. On the other hand, changes in Bcl-2 at the mRNA level, might indicate a role for the molecule other than apoptosis. Nonetheless, it appears that Bcl-2 protein plays a role in the regulation of apoptotic cell shedding in the normal rabbit corneal epithelium. Although this may be the case for the rat cornea, we would need to substantiate Bcl-2 staining in the normal cornea which was not done in this study which only compared high with low Dk lens wearing eyes. In another study,21 the effects of open or closed eye and overnight CL wear on rabbit corneal epithelial surface cell death, detected by annexin V binding and propidium iodide labeling were tested. The study reported that low oxygen transmissible RGP test lenses produced a significant increase in nonviable epithelial cells in the central cornea. Furthermore, under very low oxygen tension, combined with lens effect, such as low Dk/t RGP lens wear, surface cell death may be accelerated.
Collectively, the studies suggest that there are major differences induced in the CL wearing cornea during low vs high Dk lens wear and that high oxygen transmissibility is an important contributor to ensure corneal health and homeostasis.
Acknowledgments
Grant support: CIBA Vision Novartis and in part by NIH grant P30EY04068
References
- 1.Holden B, Stretton S, Lazon P, et al. The future of contact lenses: Dk really matters. Contact Lens Spectrum. 2006 clspectrum.com/article.aspx?article=12953.
- 2.Sweeney DF. Corneal exhaustion syndrome with long-term wear of contact lenses. Optom Vis Sci. 1992;69:601–608. doi: 10.1097/00006324-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Sweeney DF, Vannas A, et al. Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci. 1985;26:1489–1501. [PubMed] [Google Scholar]
- 4.Holden BA, Sweeney DF, Swarbick HA, et al. The vascular response to long-term extended contact lens wear. Clin Exp Optom. 1989;69:112–119. doi: 10.1111/j.1444-0938.1986.tb06800.x. [DOI] [PubMed] [Google Scholar]
- 5.Ren DH, Yamamoto K, Ladage PM, et al. Adaptive effects of 30-night wear of hyper-O2 transmissible contact lenses on bacterial binding and corneal epithelium: a 1-year clinical trial. Ophthalmol. 2002;109:27–40. doi: 10.1016/s0161-6420(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding to exfoliated epithelial cells. Ophthalmol. 2001;109:1957–1969. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- 7.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmol. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig SMJ, Efron N, Pier G. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci. 1992;33:2908–2916. [PubMed] [Google Scholar]
- 9.Solomon OD, Loff H, Perla B, et al. Testing hypotheses for risk factors for contact lens-associated infectious keratitis in an animal model. CLAO J. 1994;20:109–113. [PubMed] [Google Scholar]
- 10.Hazlett LD, McClellan SA, Hume EB, et al. Extended wear contact lens usage induces Langerhans cell migration into cornea. Exp Eye Res. 1999;69(5):575–577. doi: 10.1006/exer.1999.0728. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett LD, McClellan SA, Rudner XL, et al. The role of Langerhans cells in Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2002;43:189–197. [PubMed] [Google Scholar]
- 12.Szliter EA, Morris CA, Carney F, et al. Development of a new extended-wear contact lens model in the rat. CLAO. 2002;28:119–123. [PubMed] [Google Scholar]
- 13.Hazlett LD, Moon MM, Strejc M, et al. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987;28:1978–1985. [PubMed] [Google Scholar]
- 14.Sankaridurg PR, Rao GN, Rao HN, et al. ATPase-positive dendritic cells in the limbal and corneal epithelium of guinea pigs after extended wear of hydrogel lenses. Cornea. 2000;19:374–377. doi: 10.1097/00003226-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Su PY, Hu FR, Chen YM, et al. Dendritiform cells found in central cornea by in-vivo confocal microscopy in a patient with mixed bacterial keratitis. Ocul Immunol Inflamm. 2006;14:241–244. doi: 10.1080/09273940600732398. [DOI] [PubMed] [Google Scholar]
- 16.Zhivov A, Stave J, Vollmar B, et al. In vivo confocal microscopic evaluation of langerhans cell density and distribution in the corneal epithelium of healthy volunteers and contact lens wearers. Cornea. 2007;26:47–54. doi: 10.1097/ICO.0b013e31802e3b55. [DOI] [PubMed] [Google Scholar]
- 17.Szliter EA, Barrett RP, Gabriel MM, et al. Pseudomonas aeruginosa-induced inflammation in the rat extended-wear contact lens model. Eye Contact Lens. 2006;32(1):12–18. doi: 10.1097/01.icl.0000167611.03883.58. [DOI] [PubMed] [Google Scholar]
- 18.Ren DH, Petroll WM, Jester JV, et al. The relationship between contact lens permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear. CLAO J. 1999;25:80–100. [PubMed] [Google Scholar]
- 19.Klotz SA, Misra RP, Butrus SI. Contact lens wear enhances adherence of Pseudomonas aeruginosa and binding of lectins to the cornea. Cornea. 1990;3:266–270. [PubMed] [Google Scholar]
- 20.Yamamoto K, Ladage PM, Ren DH, et al. Effects of low and hyper Dk rigid gas permeable contact lenses on Bcl-2 expression and apoptosis in the rabbit corneal epithelium. CLAO J. 2001;3:137–143. [PubMed] [Google Scholar]
- 21.Li L, Ren DH, Ladage PM, et al. Annexin V binding to rabbit corneal epithelial cells following overnight contact lens wear or eyelid closure. CLAO J. 2002;1:48–54. [PubMed] [Google Scholar]