Abstract
Liver Tolerance is manifest as a bias towards immune unresponsiveness, both in the context of a Major Histocompatibility Complex-mismatched liver transplant and in the context of liver infection. Two broad classes of mechanisms account for liver tolerance. The presentation of antigens by different liver cell types results in incomplete activation of CD8+ T cells, usually leading to initial proliferation followed by either clonal exhaustion or premature death of the T cell. Many liver infections result in relatively poor CD4+ T cell activation, which may be because liver antigen-presenting cells express a variety of inhibitory cytokines and co-inhibitors ligands. Poor CD4+ T cell activation by liver antigens likely contributes to the abortive activation, exhaustion and early death of the CD8+ T cells. In addition, a network of active immunosuppressive pathways in the liver is mediated mostly by myeloid cells. Kupffer cells, myeloid-derived suppressor cells and liver dendritic cells both promote the activation of regulatory T cells, and suppress CD8+ and CD4+ effector T cells. This suppressive network responds to diverse inputs, including signals from hepatocytes, sinusoidal endothelial cells, and hepatic stellate cells. While liver tolerance may be exploited by pathogens, it serves a valuable purpose. Hepatitis A and B infections occasionally elicit a powerful immune response sufficient to cause fatal massive liver necrosis. More commonly, the mechanisms of liver tolerance limit the magnitude of intra-hepatic immune responses, allowing the liver to recover. The cost of this adaptive mechanism may be incomplete pathogen eradication, leading to chronic infection.
Introduction
The concept that immune responses in the liver are biased towards tolerance comes from early experiment in orthotopic liver transplantation. Thus while other organs transplanted between unrelated pigs were promptly rejected unless under the influence of powerful immunosuppressive drugs, allogeneic liver transplants were generally tolerated (1). The tolerance induced by the transplanted liver was not simply due to a lack of relevant antigens, since the liver transplant conferred on the recipient tolerance to other transplanted organs from the same donor (2, 3). Thus, the transplanted liver was imposing systemic immune tolerance. From these early experiments derive two central questions. First, what is the mechanism of this liver transplantation tolerance? Second, is this kind of tolerance relevant to liver diseases?
Local T cell activation promotes tolerance
The liver acts as a secondary lymphoid organ, priming CD8+ T cells locally rather than in draining lymph nodes. Thus, in mouse models CD8+ T cells specific for liver antigens are rapidly activated locally in the liver (4), while the transplanted liver supports CD8+ T cell activation in a recipient that cannot activate the T cells (5). However, the consequences of such activation may be transient activation followed by T cell apoptosis (6). In tissue culture, hepatocytes activate CD8+ T cells in a similar, abortive way. Such CD8+ T cells undergo premature inactivation and death, from which they may be rescued by the addition of Interleukin-2 (IL-2) (7), a product of CD4+ T-helper cells and important in the delivery of CD4+ T cell help to CD8+ T cells during priming in vivo (8).
The contribution of CD4+ T cells to CD8+ T cell immunity is complex, and context-dependent. Thus, when the antigen creates little tissue damage or inflammation, CD4+ T cell help may be essential for a primary immune response (9). Conversely, with viral and bacterial pathogens that engage innate pathogen receptors and cause tissue injury, CD4+ T cell help may be needed either for CD8+ T cells to develop full effector function, or for them survive after the primary infection, and function as memory cells (10, 11).
In liver infections, CD8+ T cells may show features of cells that did not receive sufficient help. Thus in chronic LCMV in mice, failure to eliminate the virus is associated with “exhausted” T cells that persist, but do not function (12). These cells express a characteristic surface phenotype, including the markers PD-1, Tim3 and Lag3 (13, 14), which are also expressed on human exhausted T cells (15). In chronic HCV infection, the lack of a detectable CD4+ T cell response is one of the clearest correlates of failure to eliminate the virus (16, 17). HCV-infected individuals also harbor “exhausted” or “stunned” CD8+ T cells, defined both functionally as cells that cannot make effector cytokines (18, 19), and phenotypically as cells that express PD-1 and Tim-3 (20).
Based on these data, one plausible model for liver tolerance is that, when CD8+ T cells are primed in the liver, appropriate CD4+ T cell help may not always be available. The consequence is dysfunctional, “exhausted” CD8+ T cells, and thus failure to eliminate the pathogen. However, many other factors complicate this satisfyingly simple model; in particular the prevalence of liver APCs that express co-inhibitory ligands such as PD-L1, and which stimulate T-reg cells. All of these factors may contribute to immune failure through parallel mechanisms.
Liver dendritic cells
Dendritic cells (DCs) are a diverse collection of cells specialized for antigen presentation. The liver contains multiple subsets of these cells, including the two major subsets found in the blood and in many tissues: myeloid DCs and plasmacytoid DCs (mDCs and pDCs). Myeloid DCs may arise from blood monocytes, or from bone marrow progenitor cells. These cells pass through an immature phase during which they avidly take up antigen (21), and then differentiate either to a state that strongly promotes T cell tolerance, or a state that strongly induces effective immunity (22). Immunogenic mDCs also secrete cytokines that influence T cell fate, such as IL-12 and IL-23. In making this cell fate decision, the mDCs integrate diverse environmental signals, including the presence of pro- and anti-inflammatory cytokines, and signals from cell injury sensors and innate immune sensors, including Toll-like receptors (TLRs) and RIG-like receptors (RLRs) (23). In contrast, pDCs emphasize the sensing of viral motifs and the secretion of pro-inflammatory cytokines, particularly IFN-α and IFN-β. They have the capacity of present antigen to T cells, but require special conditions to be strongly immunostimulatory (24).
Murine and human DCs subsets in the liver do not precisely correspond. In humans, classic mDCs (BDCA1+) are present in the liver, and depleted from the blood but enriched in the liver in the context of HCV infection (25), where they undergo classic activation with the increased expression of multiple co-stimulatory molecules (26). Human liver pDCs express BCDA2 and CD123, and respond to HCV be secreting IFN-α/β (27). A distinctive subset of human CD11c+ DCs, which express BDCA3+ (CD141+), and are rare in blood but more abundant in liver (28). These cells express both mDC and pDC properties, but with a twist: they both present soluble antigens (termed cross-presentation) (29), and make an antiviral cytokine, in this case IL-28B (IFN-λ) in response to HCV (30).
In the liver, there is evidence that DCs are biased towards the induction of tolerance, rather than immunity (31–33). In addition to their well-understood anti-viral role through secretion of IFN-α/β, liver pDCs induce immune tolerance through an IL-27-based circuit. IL-27 is an IL-12 family cytokines that is synthesized by liver pDCs, and acts back on them via a STAT3 signaling pathway to induce their expression of PD-L1 (34). When these cells are used as stimulators in a mixed leukocyte culture, the IL-27 enhanced PD-L1 expression promotes the expansion of FoxP3+ CD4+ T-reg cells (Figure 1).
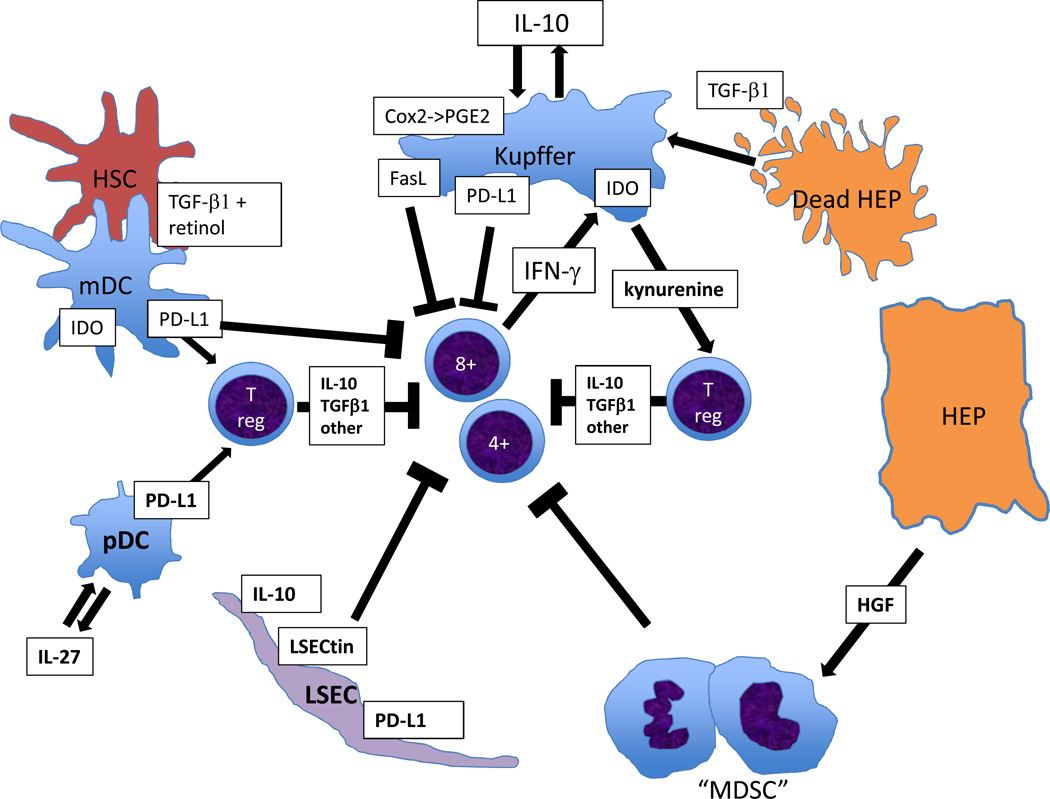
Figure 1.
Immunosuppressive circuits mediated mainly through liver myeloid cells. Kupffer cells may endocytose fragments of damaged hepatocytes, which convey TGF-β1 and cause the Kupffer cells to secrete IL-10. This acts in an autocrine manner to lead to Kupffer cells inducing a variety of immunosuppressive mechanism, both suppressing effector T cells and promoting T-reg cells. One of these, IDO, also directly responds to IFN-γ from activated effector T cells, creating a negative feedback loop. Healthy hepatocytes secrete HGF, which promotes MDSCs, while hepatic stellate cells secrete retinol and TGF-β1, promoting T-reg cell activation in the presence of DCs. Thus T cell immunity is suppressed both directly by diverse non-parenchymal cells, and also indirectly through mechanisms that activate T-reg cells.
Tolerance-inducing liver mDCs are synchronized with other tolerance mechanisms. Thus, in mouse models, liver mDCs express PD-L1, and this drives the activation and expansion of classic FoxP3+ CD25+ CD4+ T-reg cells, which in turn suppress liver allograft rejection (35). Dendritic cells may also promote T-reg development through their expression of IDO (indoleamine 2,3-dioxygenase), an enzyme that catabolizes tryptophan and generates an immunosuppressive product, kynurenine. In HCV patients, both the circulating level of kynurenine, and the capacity of blood monocyte-derived mDCs to express IDO correlated with disease activity (36). What controls IDO expression in DCs? In diverse models, including rat liver allografts, both IDO message and IDO enzymatic activity are potentiated by IFN-γ (37). This establishes a circuit with inflammatory T cells secreting IFN-γ, inducing IDO, and promoting T-reg cells (38) (Figure 1). Such a feedback loop would suppress both the effector functions of inflammatory cells, and their potential to cause tissue injury.
In addition to mDCs and pDCs, the mouse liver contains other subsets, including DCs that express CD8-α and are specialized to present antigen to CD8+ T cells, and a subset termed “NK-DC” of uncertain lineage affinities (39, 40). The role of these cell types in human liver immunology and immune tolerance has not been explored, but they are mentioned here for completeness.
Kupffer Cells as Agents of Liver Tolerance
Kupffer cells, resident macrophages of the hepatic sinusoids, show multiple immunosuppressive mechanisms that predispose the liver to immune tolerance. Thus, during the induction of liver transplantation tolerance, Kupffer cells can be recovered from the liver that express high levels of Fas Ligand (FasL, CD95L) and will kill CD8+ T cells that recognize them; and the adoptive transfer of such Kupffer cells can prolong liver allograft survival (41). The elevated FasL expression can be suppressed by treating experimental animals with Gadolinium chloride (42), and such Kupffer cell blockade also impairs liver allograft survival.
Other cell surface molecules expressed on Kupffer cells may participate in tolerance. These cells also express B7-H1 (PD-L1), a ligand that engages the PD-1 receptor on activated T cells, resulting in clonal exhaustion. In a study of Kupffer cells in hepatocellular cancer, secretion of IL-10 by the cancer cells induced PD-L1 on the Kupffer cells, creating an immunosuppressive circuit (43). Kupffer cells also secrete immunosuppressive cytokines, including IL-10 and Transforming Growth Factor-β1 (TGF-β1). There is evidence both from the animal model of liver injury induced by Concanavalin A, and from infection of Kupffer cells with Yellow Fever Virus, that IL-10 acts to limit tissue injury (44, 45). Kupffer cells also respond to TGF-β1 on apoptotic cell fragments by secreting IL-10 (46). The integration of a Kupffer cell response to apoptotic cell fragments that acts via TGF-β1 and IL-10 to induce PD-L1 may be a mechanism to promote both reduced liver inflammation, and tolerance of adaptive immune mechanisms (Figure 1).
Kupffer cells also impose immune tolerance through their expression of IDO, which is induced by IFN-γ, providing immediate local immunosuppressive feedback through the creation of a milieu depleted of Tryptophan (47). Kupffer cell activation also induces the enzyme cyclooxygenase-2, and participates in the synthesis of immunosuppressive prostaglandin-E2, (PGE-2) (48). Local intrahepatic expression of the enzyme Arginase causes a depletion of L-arginine, and this also increases PGE-2 synthesis (49).
Kupffer cells may be immunosuppressive as a result of their continuous exposure to LPS from the intestine, which results in the down-regulation of TLR4 signaling pathways, so called “LPS tolerance” that acts in part by the induction of IRAK-M (50). LPS tolerance also affects LSECs (51), and may be a general mechanism of innate immune suppression in the liver. In contrast to Kupffer cells, newly-recruited blood monocytes readily assume a pro-inflammatory phenotype in the liver (reviewed in (52)). Whether such cells are subsequently rendered immunosuppressive under the ongoing influence of LPS is unknown.
iMATES and myeloid suppressor cells
Immune responsiveness in the liver may also be controlled by other myeloid cells subsets. Local aggregations of such myeloid cells, which have been termed iMATES (for “intrahepatic Myeloid cell Aggregates for T cell clonal Expansion), promote the proliferation of CD8+ T cells in the apparent absence of local antigen, providing a local amplification step that enhances the delivery of CTL against liver pathogens, and may promote viral clearance (53). The myeloid cells in these aggregates have many properties of blood monocytes, including expression of CD11b, and are accompanied by monocyte-derived DCs. Two problems now arise concerning these cells. One is to relate them to incoming, inflammatory monocytes, and test whether they are of the same, or a distinct lineage from that which gives rise to the bone marrow-derived subset of Kupffer cells; for example, are they from the same stock as sessile Kupffer cells? (54). The second is to determine whether such aggregates are a liver-specific phenomenon, or whether transient myeloid cell aggregates act to amplify primed CTL locally in other tissues.
While less-mature myeloid cells in iMATES enhance immunity, other less-mature myeloid cells may act as myeloid-derived suppressor cells (MDSC). These cells have received much interest from the communities that study HIV (55) and the stromal infiltrate in cancers (56), since they appear to suppress effective immunity and their removal might restore it. Murine models of hepatocellular cancer recruit MDSC (57). In humans, expression of CD33 along with CD11b and low levels of HLA-DR broadly define these cells, which may also express either CD14 or CD15 (58). A subset of human CD11b+, CD33+ CD14-negative MDSC responds to Hepatocyte Growth Factor, while in mice the injection of HGF results in the expansion of hepatic CD11b+, Gr-1+ MDSC via a STAT3-dependent signaling pathway (59) (Figure 1). Both monocytic, and granulocytic cells can act as myeloid-derived suppressor cells. In the liver a subset of CD11b+ Gr-1+ (and therefore granulocytic) cells suppressed T cell activation in an MLR; these cells were increased in abundance in HBV-transgenic mice (60). This is a rapidly developing research area, and the relationships of MDSCs to other known myeloid cells, such as neutrophils, are still being worked out (56).
Alternative antigen-presenting cells
The capacity of liver cells to present antigen and engage with T cells is not limited to DCs. Hepatocytes themselves, and the full range of non-parenchymal cells, are documented antigen-presenting cells. Thus, hepatocytes express MHC class I and can cause the primary activation of CD8+ T cells in vitro; however this activation is abortive and results in limited proliferation, followed by premature death of the T cells, as discussed above.
Among non-parenchymal cells, liver sinusoidal endothelial cells (LSECs) have the strongest credentials as antigen-presenting cells (61). During liver inflammation, LSECs lose their fenestrations over several days, become thicker, and since the fenestrations promote lymphocyte trafficking, they also are less permissive for extravasation (62). These cells express a variety of Scavenger receptors and other cell surface molecules specialized for antigen uptake, and they express both MHC class I and class II molecules, together with co-stimulatory molecules. However, when these cells are isolated and used to activate either CD8+ or CD4+ T cells, the result is a strong bias towards immune tolerance. Thus, LSECs may promote functional inactivation in CD8+ T cells, and bias CD4+ T cells towards the T-reg fate (63, 64). The mechanism of T cell suppression seems to depend in part on a cell surface proteins LSECtin (LSEC lectin), since lack of this molecule resulted in enhanced T cell-dependent liver injury, while administration of recombinant, soluble LSECtin suppressed injury (65). In an experimental situation, these cells can cross-present a cellular antigen from hepatocytes (66) or from tumor cells (67). Therefore, it is likely that they are continuously sampling the liver environment in vivo, presenting antigens, and maintaining tolerance by causing T cell inactivation.
Hepatic stellate cells are perivascular cells that have many roles. They store vitamin A in prominent cytoplasmic lipid droplets; they regulate the flow of blood through the sinusoids; and they undergo trans-differentiation into myofibroblasts during liver fibrosis. Immunologically, they have two well-documented roles. First, they secrete diverse chemokines, which may be important in the recruitment of inflammatory cells to the liver; and second they can present antigen and activate T cells, particularly CD1-dependent NK-T cells (68). However, the fate of classical T cells activated by HSCs may be more complex. HSCs manifest immunosuppressive activities in the context of T cell immunity. Thus, mouse HSCs co-transplanted with allogeneic pancreatic islets promoted graft acceptance, mediated by PD-L1 (69); human HSCs similarly suppressed T cell activation via PD-L1 (70). HSCs exposed to IFN-γ can activate and expand T-reg cells in an IL-2-dependent manner, but apparently independent of PD-L1 (71). In contrast, a recent study showed that HSCs alone did not activate CD4+ T cells, yet in the presence of both DCs and TGF-β1, they promoted differentiation of T-reg cells. This process was driven by retinoic acid metabolism, ascribing to the HSCs not the role of APCs, but more likely that of critical bystanders in the liver DC-induced activation of T-reg (72). A similar indirect mechanism of human HSC-mediated immunosuppression involved promoting the differentiation of human peripheral blood monocytes into MDSCs (73).
Regulatory T cells
Expression of the Forkhead box P3 (FoxP3) transcription factor programs CD4+ T cells to adopt the regulatory T cell (T-reg) fate, and they will then suppress the immune responses of both CD4+ and CD8+ T cells (74). Such T-reg typically express CD25 and CLTA4, and may mediate suppression either through the secretion of immunosuppressive cytokines such as IL-10 and TGF-β1, or through a contact-dependent mechanism that induces a specific gene program in the suppressed T cell (75). Regulatory T cells are strongly implicated in liver transplantation tolerance. Thus, in mouse models, T-reg cells are abundant in tolerated liver allografts and their depletion causes loss of tolerance (76). In rat liver allografts, IL-10 was important in tolerance of the graft, and also promoted the abundance of T-reg cells (77). In human subjects, stable liver allografts contain such cells, and they were observed to increase after the resolution of a rejection episode (78). Graft-derived T-reg cells appeared in the peripheral blood of liver transplant patients (79), offering one possible mechanism by which the graft could impose systemic allospecific tolerance on the recipient (80). Conversely, during acute liver transplant rejection, T-reg cells were reduced in peripheral blood and appeared to localize to the liver (81).
How are T-reg induced in the context of a liver allograft? One possibility is that intrahepatic APCs drive this differentiation pathway. Candidate cells include liver DCs, Kupffer cells, LSECs and HSCs. Isolated murine HSCs could be primed by IFN-γ to express APC function, but the predominant effect was to increase the abundance of T-reg cells. Furthermore, administration of HSCs by adoptive transfer increased the frequency of T-reg in vivo (82). Similarly, murine LSECs have been reported to bias CD4+ T cells towards an immunoregulatory fate, and although these cells lacked the classical FoxP3 marker they were nevertheless immunosuppressive (64).
Liver endothelial cells might also have an important role in recruiting T-reg from the blood. Thus, T-reg isolated from inflamed human liver expressed the chemokine receptors CXCR3 and CCR4. In a flow assay across primary human liver endothelial cells, CXCR3 was important in promoting adhesion and extravasation, but CCR4 was not, due probably to the lack of its known ligands CCL17 and CCL22 (83). However, in inflamed liver DCs secreted these two cytokines, giving them a potential role in T-reg recruitment via CCR4 (84).
Tolerance and immune subversion in viral hepatitis
Viral infections of the liver run the gamut from acute hepatitis leading to either resolution or death, as in Yellow Fever Virus and Hepatitis A Virus (HAV) infection, to chronic hepatitis that may either cause no severe disease, or lead to fibrosis, cirrhosis and liver cancer, such as HCV and HBV. The immunological consequences of acute versus chronic hepatitis were first identified in mouse models (12) but soon confirmed in human HCV infection (15). In chronic non-resolving infections, the outcome is that CD8+ T cells display the exhausted phenotype, with PD-1, Tim3 and Lag3 on the cell surface. One possible explanation for this effect is the vagaries of hepatic antigen presentation, as described above; but an alternative is active immune subversion by virus-encoded proteins.
To subvert immunity, HCV disables innate immune sensing. The NS3/4 protease cleaves the adapter protein MAVS (IPS-1), which transmits signals from the cytoplasmic RNA sensors of the RIG-1 family (85), and also cleaves the TRIF adapter protein downstream of TLR-3 (86), thus disabling both pathways of viral RNA sensing. HAV, another flavivirus, also encodes proteases that cleave MAVS (87); yet HAV is usually an acute, self-resolving infection while HCV is more often chronic. While these flaviviruses have evolved mechanisms to disable RNA signaling, another acute flavivirus, West Nile Virus, activates RIG-1-like viral RNA receptors, and initiates signaling via IRF3 and IRF7, leading to the synthesis of IFN-α/β (88). Thus, while a subset of these viruses disable RNA sensing, this does not map neatly to the viruses that commonly evade immune defenses and establish chronicity.
Hepatotropic viruses have evolved mechanisms to disable both innate and adaptive immunity. Thus, while HCV-encoded proteases attack intracellular innate sensing pathways, HCV core protein has its own direct effects on both Kupffer cells and T cells. In T cells HCV core directly suppresses the T cell response (89, 90). In Kupffer cells, HCV core acts on TLR2 to induce a subset of inflammatory cytokines including TNF-α and IL-1-α/β, but strongly suppresses the antiviral IFN-α/β responses and also the up-regulation of the immunosuppressive ligand PD-L1, and the cell surface cytotoxic ligand, TRAIL (91). In addition, HCV NS5A protein acts via TLR4 to induce the expression of cytokines, including IL-10 (92). These latter effects suggest the subversion by HCV of endogenous immunoregulatory pathways. Thus, TRAIL-R deficient mice showed enhanced anti-viral immunity due to IFN-α/β, which argues that TRAIL-dependent signals are negatively regulating the Type-1 IFN response (93). Furthermore, IFN-α/β strongly suppresses IL-1-β in human monocytes (94). Conversely, in monkey cells IL-1 suppressed the antiviral activity of IFN-α/β (95). Overall, these data suggest that the IFN-α/β and IL-1α/β mechanisms of innate immunity are mutually antagonistic, and that HCV has evolved multiple mechanisms to tip the balance against the IFN-α/β response. The price may be liver inflammation driven by TNF-α, IL-1-α/β and possibly also by TRAIL.
The Trade-off between Immunity and Tissue Injury
Immune responses in the liver seem to have evolved to strike a balance between virus eradication and immunopathology. Thus, the introduction of non-tolerant CD8+ T cells into HBV transgenic mice caused both transient hepatitis, and suppression of the viral transgene. Analysis of the mechanism of suppression implicated IFN-γ secreted by the CD8+ T cells, but not their cytotoxic activity (96, 97). It has been argued that in HBV the frequency of infected hepatocytes is very high, and the cytotoxic elimination of infected cells would surely cause liver damage incompatible with survival. This argument interprets the suppression of viral transcription by IFN-γ (also known as “intracellular cure”), rather than CTL-mediated killing of all infected hepatocytes, as a well-balanced response that limits tissue damage. However, the need for cytokine-mediated anti-viral activity may also be a side effect of the finite precursor frequency of anti-viral CTL (98). While each cytotoxic CD8+ T cell can kill a series of target cells, its capacity is not infinite. The number of potential target cells in a mouse liver is larger than the total number of T cells by several orders of magnitude, so that even if the frequency of CTL was very high there would not be enough CTL to kill all the hepatocytes. IFN-γ mediated antiviral effects offer the advantage of economy, since a modest number of antigen-specific CD8+ T cells can create an IFN-γ rich environment. However, the trade-off is that such mechanisms are more effective in suppressing virus infection than in eliminating viral DNA. The price of a non-destructive anti-viral response is therefore failure to completely eliminate the virus.
Conclusion
In the liver, powerful interlocking mechanisms predispose the immune response towards tolerance. Thus, multiple liver cell types can present antigen to T cells in ways that predispose to tolerance, “exhaustion” or apoptosis. Liver DCs also show this bias, while Kupffer cells express ligands and cytokines that kill T cells, render them “exhausted”, and at the same time limit liver injury both in experimental models, and in infections. In HBV infection, the suppression of active immunity may be a necessary adaptation to prohibit a level of cytotoxic immunity that could destroy the liver. Novel food-derived molecules and PAMPs derived from the intestinal microbiota impinge on the liver via the hepatic portal vein. Normally, the coincidence of antigens and PAMPs triggers an immune response, but in fact the non-self antigens in food do not pose a threat, and neither do the healthy microbiota. Therefore it is reasonable to expect that the threshold for engaging immune defenses will be optimized against this background noise. A hot area for current research is how the many populations of immunogenic and suppressive myeloid cells in the liver are related, and how they interact in health and disease to sometimes generate immunity in the context of prevailing liver tolerance.
Acknowledgements
This work was supported by the NIAID, NIDDK and the UW Department of Pathology.
Literature cited
- 1.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Calne RY, White HJ, Binns RM, Herbertson BM, Millard PR, Pena J, Salaman JR, et al. Immunosuppressive effects of the orthotopically transplanted porcine liver. Transplant Proc. 1969;1:321–324. [PubMed] [Google Scholar]
- 3.Calne RY. Immunological tolerance--the liver effect. Immunol Rev. 2000;174:280–282. doi: 10.1034/j.1600-0528.2002.017419.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertolino P, Bowen DG, McCaughan GW, Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J Immunol. 2001;166:5430–5438. doi: 10.4049/jimmunol.166.9.5430. [DOI] [PubMed] [Google Scholar]
- 5.Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203:437–447. doi: 10.1084/jem.20051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, Fung JJ, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- 10.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 13.Mays LE, Wang L, Lin J, Bell P, Crawford A, Wherry EJ, Wilson JM. AAV8 Induces Tolerance in Murine Muscle as a Result of Poor APC Transduction, T Cell Exhaustion, and Minimal MHCI Upregulation on Target Cells. Mol Ther. 2013 doi: 10.1038/mt.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller M, Spangenberg HC, Kersting N, Altay T, Blum HE, Klenerman P, Thimme R, et al. Virus-specific CD4+ T cell responses in chronic HCV infection in blood and liver identified by antigen-specific upregulation of CD154. Journal of Hepatology. 2010;52:800–811. doi: 10.1016/j.jhep.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Shoukry NH, Sidney J, Sette A, Walker CM. Conserved hierarchy of helper T cell responses in a chimpanzee during primary and secondary hepatitis C virus infections. J Immunol. 2004;172:483–492. doi: 10.4049/jimmunol.172.1.483. [DOI] [PubMed] [Google Scholar]
- 18.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 19.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha B, Choudhary MC, Sarin SK. Expression of inhibitory markers is increased on effector memory T cells during hepatitis C virus/HIV coinfection as compared to hepatitis C virus or HIV monoinfection. AIDS. 2013;27:2191–2200. doi: 10.1097/QAD.0b013e32836285e4. [DOI] [PubMed] [Google Scholar]
- 21.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz M, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Livingstone AM. Dendritic cells: the immune information management experts. Nat Immunol. 2004;5:564–566. doi: 10.1038/ni0604-564. [DOI] [PubMed] [Google Scholar]
- 24.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–454. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 25.Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, Langhans B, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez VM, Hon H, Ibegbu C, Knechtle SJ, Kirk AD, Grakoui A. Hepatic enrichment and activation of myeloid dendritic cells during chronic hepatitis C virus infection. Hepatology. 2012;56:2071–2081. doi: 10.1002/hep.25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly A, Fahey R, Fletcher JM, Keogh C, Carroll AG, Siddachari R, Geoghegan J, et al. CD141(+) myeloid dendritic cells are enriched in healthy human liver. J Hepatol. 2014;60:135–142. doi: 10.1016/j.jhep.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshio S, Kanto T, Kuroda S, Matsubara T, Higashitani K, Kakita N, Ishida H, et al. Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-lambda in response to hepatitis C virus. Hepatology. 2013;57:1705–1715. doi: 10.1002/hep.26182. [DOI] [PubMed] [Google Scholar]
- 31.Hsu W, Shu SA, Gershwin E, Lian ZX. The current immune function of hepatic dendritic cells. Cell Mol Immunol. 2007;4:321–328. [PubMed] [Google Scholar]
- 32.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 33.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Bakthavatsalam R, Meng Z, Li Z, Li W, Perkins JD, Reyes J. PD-L1 signal on liver dendritic cells is critical for Foxp3(+)CD4(+)CD25(+) Treg and liver tolerance induction in mice. Transplant Proc. 2013;45:1853–1855. doi: 10.1016/j.transproceed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Higashitani K, Kanto T, Kuroda S, Yoshio S, Matsubara T, Kakita N, Oze T, et al. Association of enhanced activity of indoleamine 2,3-dioxygenase in dendritic cells with the induction of regulatory T cells in chronic hepatitis C infection. J Gastroenterol. 2013;48:660–670. doi: 10.1007/s00535-012-0667-z. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Gong ZJ, Wang ZW, Li T, Zhang JY, Sun HC, Liu S, et al. IDO-competent-DCs induced by IFN-gamma attenuate acute rejection in rat liver transplantation. J Clin Immunol. 2012;32:837–847. doi: 10.1007/s10875-012-9681-4. [DOI] [PubMed] [Google Scholar]
- 38.Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009;114:3235–3243. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 39.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 40.Terme M, Mignot G, Ullrich E, Bonmort M, Minard-Colin V, Jacquet A, Schultze JL, et al. The dendritic cell-like functions of IFN-producing killer dendritic cells reside in the CD11b+ subset and are licensed by tumor cells. Cancer Research. 2009;69:6590–6597. doi: 10.1158/0008-5472.CAN-08-4473. [DOI] [PubMed] [Google Scholar]
- 41.Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9:489–497. doi: 10.1053/jlts.2003.50091. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J, Peng Y, et al. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14:823–836. doi: 10.1002/lt.21450. [DOI] [PubMed] [Google Scholar]
- 43.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Research. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 45.Woodson SE, Freiberg AN, Holbrook MR. Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology. 2011;412:188–195. doi: 10.1016/j.virol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-beta. Hepatology. 2011;53:306–316. doi: 10.1002/hep.24029. [DOI] [PubMed] [Google Scholar]
- 47.Yan ML, Wang YD, Tian YF, Lai ZD, Yan LN. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World journal of gastroenterology : WJG. 2010;16:636–640. doi: 10.3748/wjg.v16.i5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez RV, Swanson C, Morgan M, Erickson K, Hubbard NE, German JB. Portal venous transfusion up-regulates Kupffer cell cyclooxygenase activity: a mechanism of immunosuppression in organ transplantation. Transplantation. 1997;64:135–139. doi: 10.1097/00007890-199707150-00023. [DOI] [PubMed] [Google Scholar]
- 49.Callery MP, Mangino MJ, Flye MW. Arginine-specific suppression of mixed lymphocyte culture reactivity by Kupffer cells--a basis of portal venous tolerance. Transplantation. 1991;51:1076–1080. doi: 10.1097/00007890-199105000-00028. [DOI] [PubMed] [Google Scholar]
- 50.Liu ZJ, Yan LN, Li XH, Xu FL, Chen XF, You HB, Gong JP. Up-regulation of IRAK-M is essential for endotoxin tolerance induced by a low dose of lipopolysaccharide in Kupffer cells. J Surg Res. 2008;150:34–39. doi: 10.1016/j.jss.2007.12.759. [DOI] [PubMed] [Google Scholar]
- 51.Uhrig A, Banafsche R, Kremer M, Hegenbarth S, Hamann A, Neurath M, Gerken G, et al. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J Leukoc Biol. 2005;77:626–633. doi: 10.1189/jlb.0604332. [DOI] [PubMed] [Google Scholar]
- 52.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 53.Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, Schildberg FA, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- 54.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macatangay BJ, Landay AL, Rinaldo CR. MDSC: a new player in HIV immunopathogenesis. AIDS. 2012;26:1567–1569. doi: 10.1097/QAD.0b013e328355e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol. 2013;23:171–182. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–1013. doi: 10.1016/j.jhep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen PA, Ko JS, Storkus WJ, Spencer CD, Bradley JM, Gorman JE, McCurry DB, et al. Myeloid-derived suppressor cells adhere to physiologic STAT3- vs STAT5-dependent hematopoietic programming, establishing diverse tumor-mediated mechanisms of immunologic escape. Immunol Invest. 2012;41:680–710. doi: 10.3109/08820139.2012.703745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, Sytwu HK. Multipotent Human Mesenchymal Stromal Cells Mediate Expansion of Myeloid-Derived Suppressor Cells via Hepatocyte Growth Factor/c-Met and STAT3. Stem Cell Reports. 2013;1:139–151. doi: 10.1016/j.stemcr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol. 2011;166:134–142. doi: 10.1111/j.1365-2249.2011.04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, Djandji D, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 62.Warren A, Bertolino P, Benseler V, Fraser R, McCaughan GW, Le Couteur DG. Marked changes of the hepatic sinusoid in a transgenic mouse model of acute immune-mediated hepatitis. J Hepatol. 2007;46:239–246. doi: 10.1016/j.jhep.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Knolle PA, Limmer A. Control of immune responses by savenger liver endothelial cells. Swiss medical weekly. 2003;133:501–506. doi: 10.4414/smw.2003.10261. [DOI] [PubMed] [Google Scholar]
- 64.Kruse N, Neumann K, Schrage A, Derkow K, Schott E, Erben U, Kuhl A, et al. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3- regulatory T cells suppressing autoimmune hepatitis. Hepatology. 2009;50:1904–1913. doi: 10.1002/hep.23191. [DOI] [PubMed] [Google Scholar]
- 65.Tang L, Yang J, Liu W, Tang X, Chen J, Zhao D, Wang M, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137:1498–1508. e1491–e1495. doi: 10.1053/j.gastro.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology. 2011;54:1379–1387. doi: 10.1002/hep.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berg M, Wingender G, Djandji D, Hegenbarth S, Momburg F, Hammerling G, Limmer A, et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur J Immunol. 2006;36:2960–2970. doi: 10.1002/eji.200636033. [DOI] [PubMed] [Google Scholar]
- 68.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 70.Charles R, Chou HS, Wang L, Fung JJ, Lu L, Qian S. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation. 2013;96:17–24. doi: 10.1097/TP.0b013e318294caae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang HR, Chou HS, Gu X, Wang L, Brown KE, Fung JJ, Lu L, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, Grakoui A. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hochst B, Schildberg FA, Sauerborn P, Gabel YA, Gevensleben H, Goltz D, Heukamp LC, et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol. 2013;59:528–535. doi: 10.1016/j.jhep.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 74.Thompson C, Powrie F. Regulatory T cells. Current opinion in pharmacology. 2004;4:408–414. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Sukiennicki TL, Fowell DJ. Distinct molecular program imposed on CD4+ T cell targets by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:6952–6961. doi: 10.4049/jimmunol.177.10.6952. [DOI] [PubMed] [Google Scholar]
- 76.Li W, Carper K, Zheng XX, Kuhr CS, Reyes JD, Liang Y, Perkins DL, et al. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006;38:3205–3206. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 77.Yang ZF, Ngai P, Lau CK, Ho DW, Tam KH, Lam CT, Poon RT, et al. Induction of long-term liver allograft survival by delayed immunosuppression is dependent on interleukin-10. Liver Transpl. 2007;13:571–578. doi: 10.1002/lt.21091. [DOI] [PubMed] [Google Scholar]
- 78.Demirkiran A, Baan CC, Kok A, Metselaar HJ, Tilanus HW, van der Laan LJ. Intrahepatic detection of FOXP3 gene expression after liver transplantation using minimally invasive aspiration biopsy. Transplantation. 2007;83:819–823. doi: 10.1097/01.tp.0000258597.97468.88. [DOI] [PubMed] [Google Scholar]
- 79.Demirkiran A, Bosma BM, Kok A, Baan CC, Metselaar HJ, Ijzermans JN, Tilanus HW, et al. Allosuppressive donor CD4+CD25+ regulatory T cells detach from the graft and circulate in recipients after liver transplantation. J Immunol. 2007;178:6066–6072. doi: 10.4049/jimmunol.178.10.6066. [DOI] [PubMed] [Google Scholar]
- 80.Flye MW, Duffy BF, Phelan DL, Ratner LE, Mohanakumar T. Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitized patient. Transplantation. 1990;50:1051–1054. [PubMed] [Google Scholar]
- 81.Stenard F, Nguyen C, Cox K, Kambham N, Umetsu DT, Krams SM, Esquivel CO, et al. Decreases in circulating CD4+CD25hiFOXP3+ cells and increases in intragraft FOXP3+ cells accompany allograft rejection in pediatric liver allograft recipients. Pediatr Transplant. 2009;13:70–80. doi: 10.1111/j.1399-3046.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 82.Jiang G, Yang HR, Wang L, Wildey GM, Fung J, Qian S, Lu L. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, Shetty S, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 84.Riezu-Boj JI, Larrea E, Aldabe R, Guembe L, Casares N, Galeano E, Echeverria I, et al. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422–431. doi: 10.1016/j.jhep.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Loo YM, Owen DM, Li K, Erickson AK, Johnson CL, Fish PM, Carney DS, et al. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreon JC, Ferreon AC, Li K, Lemon SM. Molecular determinants of TRIF proteolysis mediated by the hepatitis C virus NS3/4A protease. J Biol Chem. 2005;280:20483–20492. doi: 10.1074/jbc.M500422200. [DOI] [PubMed] [Google Scholar]
- 87.Qu L, Feng Z, Yamane D, Liang Y, Lanford RE, Li K, Lemon SM. Disruption of TLR3 signaling due to cleavage of TRIF by the hepatitis A virus protease-polymerase processing intermediate, 3CD. PLoS pathogens. 2011;7:e1002169. doi: 10.1371/journal.ppat.1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013;9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hahn CS, Cho YG, Kang BS, Lester IM, Hahn YS. The HCV core protein acts as a positive regulator of fas-mediated apoptosis in a human lymphoblastoid T cell line. Virology. 2000;276:127–137. doi: 10.1006/viro.2000.0541. [DOI] [PubMed] [Google Scholar]
- 90.Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- 91.Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305–314. doi: 10.1053/j.gastro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Sene D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pene V, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. doi: 10.1371/journal.ppat.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 94.de Paus RA, van Wengen A, Schmidt I, Visser M, Verdegaal EM, van Dissel JT, van de Vosse E. Inhibition of the type I immune responses of human monocytes by IFN-alpha and IFN-beta. Cytokine. 2013;61:645–655. doi: 10.1016/j.cyto.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Takahara T, Fukuyama Y, Saito S, Ogino T, Miyajima N, Kohase M. Il-1, EGF, and HGF suppress the antiviral activity of interferon in primary monkey hepatic parenchymal cells. Jpn J Infect Dis. 1999;52:45–48. [PubMed] [Google Scholar]
- 96.Guidotti LG, Chisari FV. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 97.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 98.Rehermann B, Chang KM, McHutchison JG, Kokka R, Houghton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]