Abstract
Objective:
Loading of temporomandibular tissues during mandibular distraction may cause changes in condylar growth and cartilage thickness. This study examines the effects of distraction on the condyle in a large animal model by explicitly measuring growth and in vivo loading.
Design:
Unilateral mandibular distraction was carried out on twenty growing minipigs divided into three groups. One group underwent distraction but not consolidation, whereas the other two groups were allowed a period of consolidation of either one or two weeks. Animals received fluorochrome and 5-bromo-2'-deoxyuridine (BrdU) labeling and masticatory strain was measured from the condylar neck. Condylar strain was also recorded in an age-matched sample of eight animals that received no distraction surgery. Immunohistochemical procedures were used to identify dividing prechondroblasts and histological analysis was used to measure mineral apposition rate, count dividing cells, and measure the thickness of condylar cartilage.
Results:
Strain magnitude, particularly compressive strain, was much larger on the non-distraction side compared to the distraction side condyle. Compared to normal loading levels, the distraction side condyle was underloaded whereas the condyle on the intact side was overloaded. Mineral apposition and cartilage thickness were greater on the distraction side condyle compared to the opposite side. Differences between the sides were most pronounced in the group with no consolidation and became progressively reduced with consolidation time.
Conclusions:
Increased mineralization and cartilage thickness on the distraction side condyle is associated with reduced, not increased loading, perhaps because of disruption of the distraction side masseter muscle.
Keywords: Distraction osteogenesis, condyle, mineral apposition, loading, minipig
1. Introduction
In the last decade distraction osteogenesis (DO) of the mandible has become a commonplace treatment option for children with micrognathia. A number of studies on animals have examined the effects of this procedure on the structures of the temporomandibular joint1-11. Except at extreme rates and/or distraction distances, mandibular osteodistraction seems not to have permanent deleterious consequences for the TMJ3,12,13. However, even a temporary change would affect the process of cartilage growth and endochondral ossification of the condyle. Because the mandible primarily grows in length at the condylar cartilage, and because mandibular osteodistraction is intended to compensate for deficient growth, it is important to ask what effect this procedure has on the condyle in growing individuals.
Previous studies have generated contradictory results. Reorientation and suppression of growth at the condyle following distraction osteogenesis have been documented in a rat model5-7, and thinning of the articular cartilage was found in a dog model1. On the other hand, increased cartilage thickness on the distracted side of a pig model12,13 and a rabbit model8 suggested an increased growth rate. In another study using growing sheep, there was a short-term increase in cartilage thickness and osteoblastic activity on the distraction side compared to controls2. All authors ascribed the condylar changes to a presumed elevated compressive load at the TMJ, but considered the compressive loads to be either deleterious5-7 or stimulatory2,8,12,13. Of these studies, only Liu et al.7, using rats, measured growth directly. The other studies on larger animals assessed condylar growth indirectly rather than explicitly. If distraction osteogenesis is confirmed to be associated with changes in growth, then two questions arise. First, does the process of distraction increase compressive loading at the TMJ, as all previous authors have assumed? Second, do compressive TMJ loads increase or decrease growth at the condyle?
Previously, we quantified distraction loads on the condyle in minipigs by measuring bone strain (a proxy for force) on the lateral surface of the condylar neck while the distraction device was being activated14. A total of 2 mm of appliance activation produced barely measurable strains on the condyle (4-9 fold lower in magnitude than masticatory strain), suggesting that, at least in pigs, activation of the distractor itself does not overload the tissue structures of the TMJ in an immediate sense. This is not surprising given that the intervening soft tissues (disc and ligaments) would first deform and reduce the force transmitted to the condyle. However, a static force imposed on the TMJ with several millimeters of distraction would likely produce higher strains on the condyle. In their finite element model of unilateral mandibular distraction in a human, Kofod et al.15 predicted asymmetrical loads, with the distraction condyle experiencing higher stresses and strains than the non-distraction side. The forces transferred to the TMJ increased more rapidly with progressive distraction on the distraction side compared to the non-distraction side, but still fell short of TMJ forces predicted during maximal biting. It is difficult to model dynamic masticatory forces and efforts to understand the effects of distraction on the loading of the TMJ have focused largely on the forces that are transmitted through the appliance itself. Changes in the functional loading of the TMJ following distraction have been neglected, although they may have important consequences for the growth and general health of the TMJ structures.
Previously, we measured functional strains on the condylar neck immediately following appliance placement, but prior to distraction, and found that the distraction side condyle was underloaded16. At the time, we did not know if these changes in condylar loading were transient. In the present study we measured the rate of condylar growth and in vivo masticatory strains at three time points: after five days of distraction, and after one and two weeks of consolidation.
2. Materials and Methods
2.1. Animal Procedures
Twenty-eight Hanford minipigs (Sinclair Research Farms, Columbia, MO) of both sexes were used in this study. The animals were between 3-6 months of age and weighed between 6-25kg. All procedures were approved by the University of Washington Animal Care and Use Committee. Under aseptic conditions, a Synthes® distraction appliance (Monument, CO) was attached to the right side of the mandible and a mandibular osteotomy was performed at this location, as described previously for our acute studies16,17. This surgical procedure was performed on a total of twenty animals, (14 male, 6 female). Antibiotics (amikacin, trimethoprim/sulfadiazine, and cephalexin) and analgesics (buprenorphine and fentanyl) were administered prior to, and on a daily basis during the recovery period. Infections at the exit site of the distractor rod were controlled with a topical powder containing neomycin sulfate and tetracaine hydrochloride. All animals eagerly ate pig chow softened with water the day after the surgery. Starting on the day after surgery, the distraction appliance was activated by turning the distractor rod a total of two turns, resulting in about 1.0 mm of distraction. All animals had their mandibles distracted for five days. The distraction period was followed by a period of consolidation that varied from 0-1 days in Group 1, to 6-8 days in Group 2, and 14-15 days in Group 3. Animals were given an intravenous injection of calcein (12.5 mg/kg dissolved in saline and neutralized to a PH of 7.4) four days prior to the terminal procedure. The day before the terminal procedure the animals received an IV injection of alizarin complexone (same dose and dilution as calcein) and 5-bromo-2'-deoxyuridine (BrdU) (40 mg/kg dissolved in saline).
A terminal procedure was carried out following the consolidation period (or the distraction period in Group 1). This same procedure was also carried out on eight control pigs (5 females and 3 males). The control animals were fed a diet of pig chow that was not softened with water. During the terminal procedure the animals were anesthetized by mask with isoflurane and nitrous oxide. A small U-shaped incision was made along the lower border of the posterior part of the zygomatic arch and down the superior part of the posterior border of the ramus. The lateral surface of the condylar neck was exposed and prepared for strain gauge adhesion18. A rosette strain gauge (Vishay Micro-Measurements SK-06-030WR-120, Raleigh NC) was glued to the condylar neck just below the joint capsule, and the incision was sutured closed around the lead wires. This procedure was carried out on both the distraction and non-distraction side condylar necks. Fine wire EMG electrodes were inserted bilaterally into the masseter and temporalis muscles. A local anesthetic, lidocaine, was applied to the incision, and buprenorphine and Ketorolac were administered via intramuscular injection. The gas anesthesia was removed and the animals were allowed to recover briefly and were fed their normal diet of unsoftened pig chow.
Animals were unrestrained and ate enthusiastically for approximately 15 minutes while strain and EMG data were recorded at 500 Hz using the MP100 System and Acqknowledge software (BIOPAC Systems Inc., Goleta CA). Following data recording, the pigs were re-anesthetized. Stimulating electrodes were placed bilaterally in the masseter muscles. With the teeth in occlusion, trains of supra-maximal tetani were elicited while condylar strains were recorded. Following this procedure animals were euthanized and perfused with heparinized saline and the tissue preservative Prefer (Anatech Ltd., Battle Creek MI). Histology was not performed on the control pigs, which had not received labels. The condyles were removed and sagittally sectioned with a Stryker saw. The medial halves were decalcified for BrdU and histological analysis and the lateral halves were stored in 70% ethanol for mineralization analysis. The decalcified specimens were embedded in paraffin and sagittally sectioned at 7-10 μm on a microtome. The undecalcified sections were embedded in plastic resin and sagittally sectioned at 50-70 μm on a Leica SP1600 saw microtome. Decalcified sections (following BrdU immunohistochemistry or staining with hematoxylin and eosin) and undecalcified sections were coverslipped with mounting media and viewed with a Nikon Eclipse E400 microscope in transmission light mode or in fluorescent mode (fluorochrome labels). Images were captured to computer and morphometrically analyzed (see below) using the imaging software MetaVue™ (Universal Imaging Corp., Downington PA). Specimens were analyzed only if good sections were available from both sides. All measurements were made with the investigator blinded to the specimen side and identity.
2.2. Condylar strain analysis
The EMG data were used to identify appropriate chewing sequences for analysis (e.g. 10-20 consecutive chews at 2-3 Hz). In most cases EMG quality was sufficient to identify the side of chewing19. Strain analysis began by first selecting voltage peaks, which occurred during the power stroke of each chewing cycle. Each peak was then subtracted from baseline voltages and converted to microstrain. Principal strain magnitudes (maximum and minimum principal strain, or tension and compression, respectively) and orientations were calculated from the three peak strain values using standard algorithms. The peak principal strains for each power stroke were identified as those coinciding with the maximum shear strain (maximum − minimum principal strain, Tech Note 515, Measurements Group Inc.), following the procedure of Hylander and Johnson19. The orientation of the strain was calculated as an angle measured from the occlusal plane to the minimum (compressive) principal strain.
2.3. BrdU immunohistochemistry and analysis
Slides were deparaffinized, rehydrated through a graded series of ethanol solutions, and then washed in phosphate buffered saline (PBS). Sections were then incubated 20 minutes in a trypsin solution. Steps were taken to prevent non-specific binding by incubating sections in 2% H2O2 for 10 minutes and avidin and biotin (Avidin and Biotin Kit, Vector Labs, Burlingame CA) for 15 minutes each. Sections were then exposed sequentially to (1) the mouse monoclonal antibody to BrdU (Vector Labs), overnight at 4°C (diluted 1:50 in PBS); (2) the secondary antibody, biotinylated horse antimouse IgG (Vector Labs), 30 min at room temperature; (3) the tertiary antibody, streptavidin horseradish peroxidase (ABC Reagent, Vector Labs), 30 min at room temperature; (4) 3,3'-diaminobenzidine-4HCl (3 mg/ml in 0.05 Tris-HCl, pH 7.6, Vector Labs) containing 0.02% hydrogen peroxide with nickel enhancement of oxidized DAB, 2.5 min at room temperature.
Slides were examined for the presence of BrdU positive cells in the proliferative zone of cartilage. Images were acquired at three regions (anterior, middle and posterior) of the condylar cartilage for analysis. The anterior and posterior counts were made 500 μm from the reflection of the condylar disc within the capsule space, while the middle region was defined as halfway between the two reflection points. Cells positive for BrdU staining within a 500 μm × 500 μm area were counted using MetaVue Software.
2.4. Cartilage thickness
Decalcified sections of the mandibular condyle were stained with hematoxylin and eosin. Because the junction between the fibrous articular layer and the cartilage is gradual and because the fibrous layer was relatively uniform, cartilage thickness was measured as the linear distance between the outer edge of the fibrous layer and the bony front of endochondral ossification. Cartilage thickness measurements were made at the midpoint of the articular region of the condyle (i.e. halfway between the reflections of the articular capsule), and perpendicular to the articular surface. One section from each condyle was measured.
2.5. Mineralization
Overlapping images from each undecalcified section were joined together in Adobe Photoshop in order to reconstruct the surface of the condyle. A grid of radiating lines was superimposed on the articular region of each condyle image (Figure 1). Mineralization was defined as the linear distance between the two fluorochrome labels and was measured from the seven locations where the grid lines intersected the condylar surface (Figure 1). The mineral apposition rate (MAR) was arrived at by dividing mineralization at the seven locations by the number of full days (three) that elapsed between administration of the two labels. Measurements were taken from three sections per condyle and averaged for each location.
Figure 1.
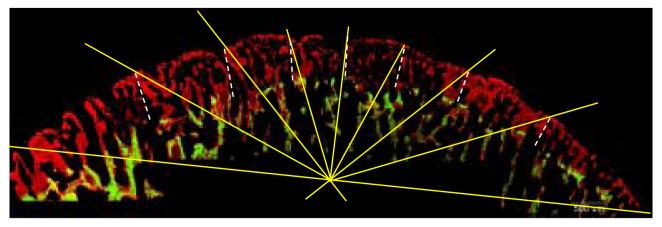
Undecalcified sagittal section of a pig condyle shown under UV light. Calcein labeling (green) was followed by alizarin complexone (red). The radiating lines of the superimposed grid lie at 22.5°, 45°, 67.5°, 90°, 112.5°, 135° and 157.5° to the horizontal line that extends through the anterior (right side) and posterior (left side) limits of the articular surface. Mineralization was defined as the distance between the end of the green label to the end of the red label at these seven locations, as represented by the dashed white lines.
2.6. Statistical analysis
For the strain and mineralization data, two sample t-tests and paired t-tests were used for comparisons between the distraction and non-distraction groups. Comparisons with control data were made using ANOVA followed by the Dunnett C post-hoc test, which does not assume homogeneity of variance. The BrdU data were analyzed using paired t-tests for differences in cell counts between sides, and with ANOVA for differences between consolidation groups. To test for differences in the number of dividing cells in the three different regions of each condyle (anterior, middle, posterior), BrdU-positive counts were transformed into a rank for each condylar site, and statistical comparisons between sites were done using the nonparametric ANOVA equivalent (Friedman test).
3. Results
The average amount of distraction achieved was 4.2 mm, slightly less than the 5.0 mm goal. Visual inspection of the electromyograms recorded during the terminal procedure indicated the normal alternating-side pattern of mastication typical of pigs20. However, in some instances the EMG was not of sufficient quality to establish chewing side. For these experiments all chewing cycles were combined.
3.1. Strain
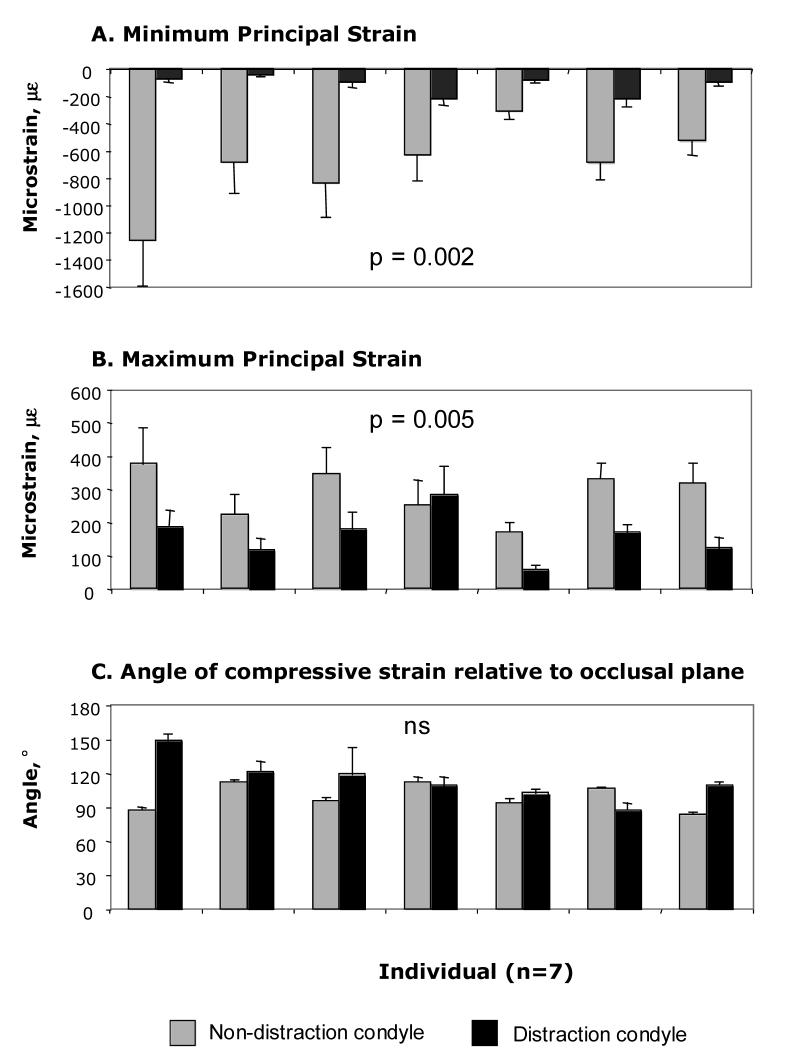
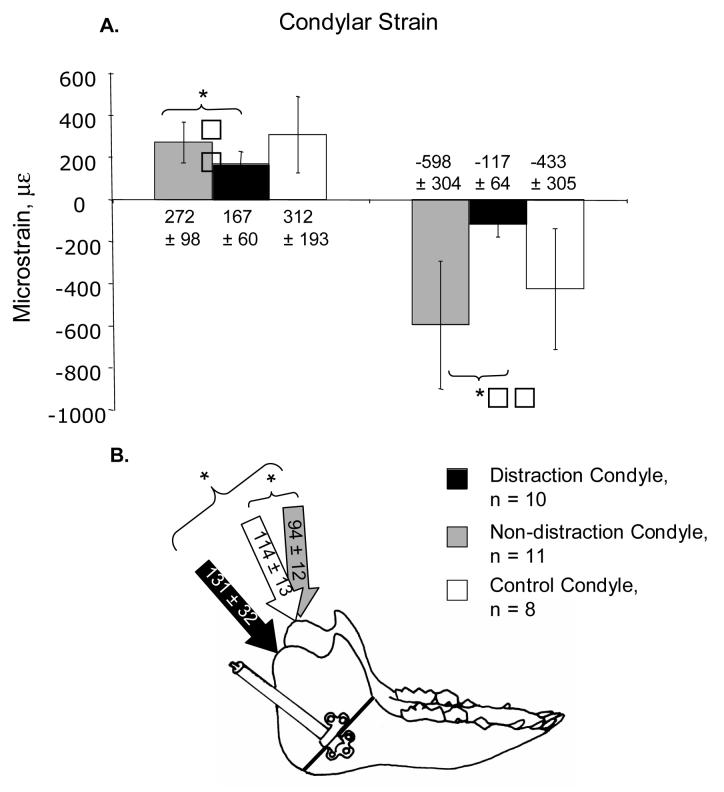
Strain data were not available for the entire sample because of instrumentation failure during the recording period. Within the experimental group, strain data were obtained from eleven individuals (“unpaired sample”), but only seven (“paired sample”) had masticatory strain data from both condyles (Table 1, Figure 2). No significant differences in strain were related to whether the animals chewed on the distraction or non-distraction sides, so chewing-side data were combined. For both the unpaired and paired total samples, the peak principal strains during mastication were significantly larger in magnitude on the non-distraction condyle compared to the distraction condyle (Table 1). When analyzed by consolidation group, peak principal strains were again larger on the non-distraction side, although not always significantly, due in part to the small sample sizes in some groups. In general, compressive strain was oriented dorsocaudally (94-131°, where 0° and 180° are the occlusal plane and 90° is orthogonal to the occlusal plane). Strain orientation usually showed higher angles on the distraction side, but the difference was significant only in the unpaired sample (Table 1). Notably, the difference in strain orientation between sides was highly significant in the group with no consolidation, less so in the 1 week consolidation group, and non-significant in the group allowed to consolidate for two weeks (Table 1). There were no significant differences in principal strains in within-side comparisons between the three consolidation groups. However, the orientation of compressive strain on the distraction side condyle was significantly greater in the group with no consolidation compared to the other groups (p = 0.001, ANOVA, Dunnett C). Specifically, compressive strain was strongly dorsocaudal in orientation immediately following distraction (164°±17), but became progressively more vertical and more like the non-distraction side with consolidation (120°±2 and 102°±9). In contrast, compressive strain orientation on the non-distraction side was close to vertical in all three groups (albeit a sample of one individual in one group hampers statistical comparisons).
Table 1.
Masticatory strains at the condylar neck
| Maximum Principal Strain (με) |
Minimum Principal Strain (με) |
Strain orientation (degrees) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Non- Distraction |
Distraction | P value | Non- Distraction |
Distraction | P value | Non- Distraction |
Distraction | P value |
| Paired | |||||||||
| Total | 288±74 (n=7) |
159±70 (n=7) |
0.005 | −707±295 (n=7) |
−120±70 (n=7) |
0.003 | 99±12 (n=7) |
114±19 (n=7) |
NS |
| Unpaired | |||||||||
| Total | 272±98 (n=11) |
167±60 (n=10) |
0.01 | −598±304 (n=11) |
−117±64 (n=10) |
0.001 | 94±12 (n=11) |
131±32 (n=10) |
0.005 |
| Consolidation | |||||||||
| None | 377 (n=1) |
186±19 (n=4) |
0.001* | −1260 (n=1) |
−101±53 (n=4) |
0.001* | 88 (n=1) |
164±17 (n=4) |
0.003* |
| 1 week | 218±75 (n=5) |
149±43 (n=2) |
NS | −483±273 (n=5) |
−71±37 (n=2) |
NS | 91±14 (n=5) |
120±2 (n=2) |
0.05 |
| 2 weeks | 305±103 (n=5) |
158±94 (n=4) |
NS | −580±172 (n=5) |
−155±74 (n=4) |
0.005 | 98±11 (n=5) |
102±9 (n=4) |
NS |
because n=1 in the non-distraction group, 1 sample t-tests were done using 95% confidence limits of the distraction side values.
Figure 2.
Summary of strain during mastication in the seven individuals with data from both the distraction and non-distraction side condyles (paired data). Minimum principal strain = compression and maximum principal strain = tension. Results of paired t-tests between distraction and non-distraction sides are shown as p values.
Because the non-distraction side condyle is not an independent control for the distraction side, the total unpaired data were compared with data from the control sample (Figure 3). This three-way comparison indicated that the distraction and non-distraction condyles were different from each other in principal strains, but neither group differed significantly from the control group in these parameters (Figure 3). Compressive strain was more orthogonally oriented in the non-distraction side condyle than in the control condyle. The orientation of compressive strain in the distraction side condyle was more variable than in the other groups.
Figure 3.
Comparison of masticatory strain in the distraction and non-distraction side condyles (total, unpaired data) with the non-experimental controls. A. Maximum and minimum principal strains (tension and compression, respectively), B. Orientation of minimum principal strain in the three groups. Mean ± standard deviation.
Only five experimental animals had strain data available from both condyles during masseter stimulation (Table 2). Strain magnitudes produced by ipsilateral stimulation (e.g. distraction side strain with distraction side stimulation and non-distraction side strain with non-distraction side stimulation) produced consistently higher but non significant differences compared to masticatory strain. On the non-distraction side masseter stimulation produced the same strain orientation as did mastication (91° vs. 94°), but on the distraction side the orientation was significantly more caudal (131°) during mastication than during masseter stimulation (75°).
Table 2.
Masticatory versus stimulation strains at the condylar neck
| Non Distraction side | Distraction side | |||||
|---|---|---|---|---|---|---|
| Chewing | Stimulation | P value |
Chewing | Stimulation | P value |
|
|
Maximum Principal Strain (με) |
272±98 (n=11) |
383±251 (n=5) |
NS | 167±60 (n=11) |
123±74 (n=5) |
NS |
|
Minimum Principal Strain (με) |
−598±304 (n=11) |
−913±676 (n=5) |
NS | −117±64 (n=11) |
−263±226 (n=5) |
NS |
| Strain Orientation, ° | 94±12 (n=11) |
91±23 (n=5) |
NS | 131±32 (n=11) |
75±13 (n=5) |
0.005 |
3.2. Morphology and cartilage thickness
The mandibular condyles and discs were examined post-mortem and appeared generally healthy. However, the distraction side condyle frequently appeared shortened in the anteroposterior direction compared to the contralateral condyle. In sagittal section, the distraction condyle was typically more “humped” in the middle region under the intermediate band of the disc, as shown in Figure 4. The articular cartilage was significantly thicker on the distraction side than the non-distraction side (p = 0.02, paired t-test) (Table 3). When broken down by groups, the disparity in thickness between the two sides was greatest in the group that had no consolidation (p = 0.03, paired t-test), but did not reach significance in the groups with one and two weeks consolidation.
Figure 4.
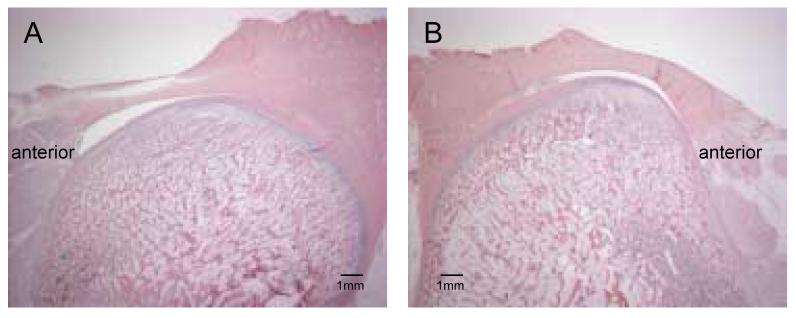
Decalcified sagittal sections stained with hematoxylin and eosin. A: non-distraction condyle, B: distraction condyle. Note shape differences between the two sides. 4.0 mm of distraction, 1 week of consolidation.
Table 3.
Cartilage Thickness, μm
| Sample | N | Non- Distraction |
Distraction | P value | % Difference |
|---|---|---|---|---|---|
| Total | 18 | 754± 257 | 875± 273 | 0.019 | + 14% |
| Consolidation | |||||
| None | 5 | 679± 298 | 979± 420 | 0.033 | + 30% |
| 1 week | 8 | 770± 227 | 821± 209 | NS | + 6% |
| 2 weeks | 5 | 802± 302 | 856± 213 | NS | + 6% |
3.3. Mineralization
On the distraction side, the newly mineralizing bone in the most superficial part of the ossification zone (i.e. the second label, alizarin complexone) had a more irregular woven appearance than on the non-distraction side (Figure 5). The non-distraction side condyle had a smoother contour and a thin layer of subchondral cortical bone. In contrast, the distraction side condyle tended to have multiple involutions and lacked a distinct cortex, sometimes making it difficult to define the edge of the mineralizing zone. Measurements were made to the more distinct edge, rather than the more superficial and fainter edge (see Figure 5), as this could be background fluorescence of unmineralized osteoid. Thus the measurement may have underestimated distraction side mineralization rate.
Figure 5.
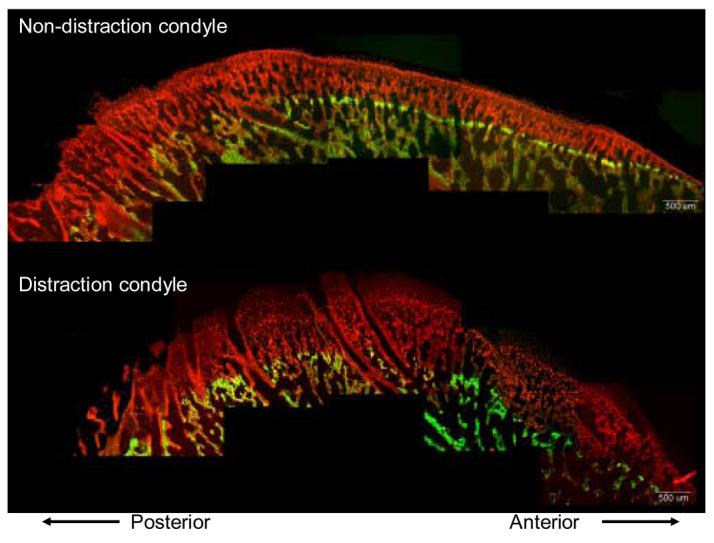
Undecalcified sagittal sections under epifluorescent illumination. Note that the distraction condyle exhibits more exuberant growth than the non-distraction condyle, as indicated by the greater distance from the end of the green (calcein) to the end of the red (alizarin) label. The distraction side condyle has a poorly defined cortex. 5.6 mm of distraction, no consolidation
The average mineral apposition rate was 16% higher on the distraction condyle than on the non-distraction condyle, a difference that was highly significant (p= 0.004 paired t-test, Table 4). When examined by group, MAR was significantly greater on the distraction side compared to the non-distraction side in those individuals with no consolidation and in those with one week of consolidation (Table 4. However, the group with two weeks of consolidation showed no side difference in MAR. Individuals in this group were equally likely to exhibit greater MAR on the non-distraction side as the distraction side. A within-condyle comparison between the three consolidation groups (ANOVA) showed no difference for the non-distraction side, but borderline significance (p = 0.06) for the distraction side.
Table 4.
Mineralization rate, μm/day
| Sample | n | Non- Distraction |
Distraction | p value | % Difference |
|---|---|---|---|---|---|
| Paired | |||||
| Total | 15 | 173±59 | 206± 66 | 0.004 | + 16% |
| Consolidation | |||||
| None | 4 | 207± 50 | 271± 57 | 0.006 | + 24% |
| 1 week | 7 | 148± 52 | 186± 26 | 0.025 | + 20% |
| 2 weeks | 4 | 181± 75 | 177± 91 | 0.801 | − 2% |
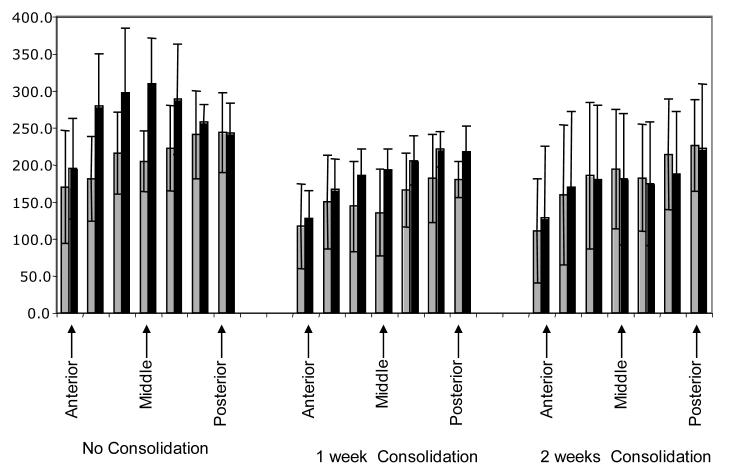
There were regional differences in the MAR that varied with consolidation group. In the group with no consolidation, the greater mineralization on the distraction side was most marked in the middle region of the articular surface (Figure 6). In the group with one week of consolidation, the disparity in MAR between the two sides was only slightly more marked in the central than the posterior articular region. In the two week consolidation group, the central and posterior regions actually had slightly lower MAR on the distraction than the non-distraction condyle.
Figure 6.
Mineral apposition rates from the seven measurement locations of the condyle. Note that the greatest difference between the two sides is in the central region of the condyle in the group with no consolidation, but that this difference diminishes and even reverses with consolidation.
3.4. Dividing cells
Only a limited sample of individuals (n=8) was judged to have good immunohistochemical reaction on both condyles. The number of dividing cells was remarkably homogeneous (Table 5) and there were no significant side differences in total cell counts or in cell counts for each location (paired t-tests). Furthermore, within a given side and location there were no differences between consolidation groups (ANOVA). Regionally, the distraction side condyle appeared to have the most dividing cells in anterior location, followed by the middle and then posterior locations, whereas the non-distraction condyle had the most dividing cells in the middle location, with equal numbers anteriorly and posteriorly. However, these regional differences were not significant for either side (Friedman test).
Table 5.
Counts of cells labeled with BrdU by side and location (500 μm2 area).
| Total | Anterior | Middle | Posterior | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | N | Non- Distraction |
Distraction | Non- Distraction |
Distraction | Non- Distraction |
Distraction | Non- Distraction |
Distraction |
| Total | 8 | 55±30 | 60±28 | 17±10 | 21±9 | 22±15 | 23±14 | 17±13 | 16±10 |
| Consolidation | |||||||||
| None | 3 | 57±15 | 61±35 | 21±9 | 26±9 | 28±9 | 23±18 | 9±4 | 12±9 |
| 1 week | 4 | 57±44 | 61±31 | 13±9 | 18±10 | 22±18 | 22±16 | 22±17 | 21±9 |
| 2 weeks | 1 | 44 | 46 | 20 | 15 | 18 | 16 | 19 | 8 |
4. Discussion and conclusions
Gross degenerative changes were not observed in the mandibular condyles following unilateral distraction, perhaps because we used a moderate distraction rate of 1mm/day for a short duration (5 days). However, the articular cartilage was thicker on the distraction side compared to the non-distraction side, as has been found previously in pigs and other large animal models2,8,12,13. The disparity in articular cartilage thickness between the two sides was greatest in the group that underwent distraction but no consolidation, suggesting that this thickening lessens over time. Cell division may contribute to cartilage thickness, but the absence of differences in BrdU labeling between sides suggests that other factors, such as mesenchymal cell differentiation, matrix accumulation, or cell hypertrophy may be more important contributions than replication. Notably, there were pronounced differences in the rate of mineralization between the two sides, which suggests that the major effect involved the matrix or hypertrophy. The distraction side condyle was mineralizing faster (especially considering the likely underestimation of MAR), suggesting increased endochondral ossification followed the thickening of articular cartilage. These findings of increased growth and ossification on the distraction side support previous observations on pigs12,13, sheep2, and rabbits8, rather than the reverse outcome in rats5-7 and dogs1. These different results may relate to species-specific anatomy and mechanics, or to more extreme distraction rates or distances in studies showing deleterious effects.
The mineralization increase on the distraction side condyle was site-specific, showing acceleration in the central articular region compared to the unoperated side. The result was a more convex but anteroposteriorly shortened condyle than normal. In their minipig study, Thurmüller et al. also reported that the anteroposterior diameter of the distraction side condyles decreased and became more convex compared to controls and to the non-distraction side13. Interestingly, simply detaching the masseter muscle can lead to increases in condylar convexity. In rats that had their masseter muscle unilaterally detached, the operated side condyle became higher and narrower than the unoperated side, an effect that was especially marked in the short term and in animals that had their masseters surgically reattached21.
Our measurements of masticatory strain following the 5-day distraction period, and after one and two weeks of consolidation, indicate that highly asymmetrical loading of the two condyles, both in terms of magnitude and orientation, is maintained throughout the entire period. A control comparison revealed that a combination of underloading of the distraction-side condyle and overloading of the non-distraction side condyle are both probably involved in the striking differences between the two sides. Notably, the principal compressive strain, as measured by the strain gauges, became more caudally directed on the distraction side but more upright (orthogonal) on the non-distraction side. This strain orientation difference was even more extreme immediately following distractor placement and prior to distraction or consolidation16. This suggests that functional unloading of the condyle, rather than overloading during distraction, is sufficient for the morphological and growth changes that have been observed.
It is possible that following surgery the animals changed their chewing patterns so as to “favor” the non-operated side. However, all of the animals had returned to their normal alternating pattern of mastication (cf. ref16). More importantly, for each condyle the strain magnitude did not differ according to chewing side. A better explanation for the asymmetrical strain measurements is disruption of the masseter muscle during distractor placement. In order to place the distractor over the osteotomy site at the ramal-corpus junction the anterior portion of the masseter had to be detached from the mandible. During wound closure the masseter was generally sutured back to the soft tissues comprising the pterygomandibular sling on the inferior border of the ramus. Postmortem inspection revealed that while the masseter always reattached itself to the mandible, the new attachment was above the inferior border of the mandible and the masseter itself appeared smaller than the contralateral muscle. In their rat study, Liu et al. found a significant and sustained decrease in the weight of the masseter on the distraction side in both growing and maturing animals5,6. Our data from masseter stimulation demonstrate that supramaximal contractions on the distraction side produced low strains that were similar in magnitude to those measured during mastication, strongly suggesting that the low masticatory strains were due to incapacitation of the distraction side masseter rather than behavior changes during feeding. Furthermore, the difference between mastication and stimulation strain orientation on the distraction side suggests that in contrast to the normal and the non-distraction side condition, the masseter is not the dominant source of condylar loading during mastication.
Findings by other authors could also be interpreted as the result of underloading, rather than overloading, of the distraction side condyle. A decrease in subchondral bone density in the mandibular condyle following distraction has been noted in dogs10,11 and rats5,9. Such decreases in the number and thickness of trabeculae and increases in trabecular spacing were likened by the authors to disuse or aging, i.e. unloading phenomena. Similarly, Liu et al. found decreased condyle size and density in rats, which they attributed to disruption and stretching of the masseter5,6. While we did not measure bone density, rapidly growing woven bone such as we observed in the subchondral zone of the distraction side condyle is consistent with other reports of reduced bone quality and quantity. None of these changes in condylar morphology is consistent with increased compression. Therefore, we suggest a different interpretation. Contrary to the universal assumption that distraction causes compressive loading at the TMJ, our data imply that functional unloading, rather than overloading, accompanies distraction, at least when the masseter is detached. This unloading in turn accounts for the exuberant growth of the distraction condyle. The association of increased growth and unloading is also supported by the changes in strain orientation. In the non-distraction condyle, loading is roughly vertical and growth is predominantly caudal, whereas in the distraction side condyle loading is more caudal and growth is more vertical. In both cases, growth occurs in the direction orthogonal to loading, as if loading depressed growth rate.
Although the unloaded distraction-side condyle had thicker cartilage and mineralized faster, our data do not show that the increased condylar growth actually elongated the mandible, augmenting the distraction itself. First, we did not measure mandibular length directly. Second, the observed growth acceleration was sort lived, lasting only about one week into the consolidation period. Third and most importantly, the increased growth was primarily in the vertical direction and would have contributed more to the height than the length of the mandible.
Nevertheless, our results generally support the concept that unloading the condyle in a given direction will lead to increased growth in that direction. This concept thus explains why forward displacement of the condyle, at least in rats, is followed by increased condylar growth in the presumably underloaded posterior direction22-24.
Acknowledgments
We thank Frank Starr and Hannah Hook for their assistance with animal procedures and care, Patricia Emry for her histological preparations, and Holly Hicks for immunohistochemistry work. This research was funded by NIH/NIDCR awards DE 14336 and T32 DE07023.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCormick SU, McCarthy JG, Grayson BH, Staffenberg D, McCormick SA. Effect of mandibular distraction on the temporomandibular joint: Part 1, Canine study. J Craniofac Surg. 1995;6:358–63. doi: 10.1097/00001665-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Karaharju-Suvanto T, Peltonen J, Ranta R, Laitinen O, Kahri A. The effect of gradual distraction of the mandible on the sheep temporomandibular joint. Int J Oral Maxillofac Surg. 1996;25:152–6. doi: 10.1016/s0901-5027(96)80063-4. [DOI] [PubMed] [Google Scholar]
- 3.Kruse-Lösler B, Meyer U, Flören C, Joos U. Influence of distraction rates on the temporomandibular joint position and cartilage morphology in a rabbit model of mandibular lengthening. J Oral Maxillofac Surg. 2001;59:1452–9. doi: 10.1053/joms.2001.28281. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZJ, King GJ, Herring SW. Growth and remodeling of the condyle following mandibular osteodistraction in rat. Am J Oral and Maxillofac Surg. 2003;61:918–27. doi: 10.1016/s0278-2391(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZJ, King GJ, Herring SW. Alterations of morphology and microdensity in the condyle after mandibular osteodistraction in the rat. J Oral Maxillofac Surg. 2003;61:918–27. doi: 10.1016/s0278-2391(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu ZJ, King GJ, Herring SW. Why do we fail to achieve predicted lengthening in mandibular osteodistraction? Observations on condylar morphology and microdensity in growing and maturing rats. In: Davidovitch Z, Mah J, editors. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Harvard Soc. Adv. Orthodontics; Bangkok: 2004. pp. 39–51. [Google Scholar]
- 7.Liu ZJ, King GJ, Herring SW. Condylar mineralization following mandibular distraction in rats. J Dent Res. 2006 Jul;85:653–7. doi: 10.1177/154405910608500714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SG, Ha JW, Park JC. Histological changes in the temporomandibular joint in rabbits depending on the extent of mandibular lengthening by osteodistraction. Br J Oral Maxillofac Surg. 2004 Dec;42:559–65. doi: 10.1016/j.bjoms.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Shibazaki R, Maki K, Tachikawa T, Shibasaki Y, Hinton RJ, Carlson DS, et al. Changes in parathyroid hormone-related protein and 3-dimensional trabecular bone structure of the mandibular condyle following mandibular distraction osteogenesis in growing rats. J Oral Maxillofac Surg. 2005 Apr;63:505–12. doi: 10.1016/j.joms.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Sant'Anna EF, Gomez DF, Sumner DR, Williams JM, Figueroa AA, Ostric SA, et al. Micro-computed tomography evaluation of the glenoid fossa and mandibular condyle bone after bilateral vertical ramus mandibular distraction in a canine model. J Craniofac Surg. 2006 May;17:611–9. doi: 10.1097/00001665-200605000-00041. [DOI] [PubMed] [Google Scholar]
- 11.Sant'Anna EF, Gomez DF, Polley JW, Sumner RD, Williams JM, Figueroa AA, et al. Histological evaluation of the temporomandibular joint after bilateral vertical ramus mandibular distraction in a canine model. J Craniofac Surg. 2007;18:155–62. doi: 10.1097/01.scs.0000248653.07663.fd. [DOI] [PubMed] [Google Scholar]
- 12.Thurmüller P, Troulis MJ, Rosenberg A, Chuang S-K, Kaban LB. Microscopic changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2006;64:249–58. doi: 10.1016/j.joms.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Thurmüller P, Troulis MJ, Rosenberg A, Kaban LB. Changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2002;60:1327–33. doi: 10.1053/joms.2002.35733. [DOI] [PubMed] [Google Scholar]
- 14.Rafferty KL, Sun Z, Egbert MA, Herring SW. Bony strains in response to distractor activation. In: Davidovitch Z, Mah J, editors. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Harvard Soc. Adv. Orthodontics; Bangkok: 2004. pp. 59–62. [Google Scholar]
- 15.Kofod T, Cattaneo PM, Dalstra M, Melsen B. Three-dimensional finite element analysis of the mandible and temporomandibular joint during vertical ramus elongation by distraction osteogenesis. J Craniofac Surg. 2005 Jul;16:586–93. doi: 10.1097/01.scs.0000157305.60505.b5. [DOI] [PubMed] [Google Scholar]
- 16.Rafferty KL, Sun Z, Egbert MA, Baird EE, Herring SW. Mandibular mechanics following osteotomy and appliance placement. II. Bone strain on the body and condylar neck. J Oral Maxillofac Surg. 2006;64:620–7. doi: 10.1016/j.joms.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, Rafferty KL, Egbert MA, Herring SW. Mandibular mechanics following osteotomy and appliance placement. I. Postoperative mobility of the osteotomy site. J Oral Maxillofac Surg. 2006;64:610–9. doi: 10.1016/j.joms.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafferty KL, Herring SW, Artese F. Three-dimensional loading and growth of the zygomatic arch. J Exptl Bio. 2000;203:2093–3004. doi: 10.1242/jeb.203.14.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hylander WL, Johnson KR. The relationship between masseter force and masseter electromyogram during mastication in the monkey Macaca fascicularis. Arch Oral Biol. 1989;34:713–22. doi: 10.1016/0003-9969(89)90078-2. [DOI] [PubMed] [Google Scholar]
- 20.Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morph. 1973;141:427–60. doi: 10.1002/jmor.1051410405. [DOI] [PubMed] [Google Scholar]
- 21.Ghafari J, Heeley JD. Condylar adaptation to muscle alteration in the rat. Angle Orthod. 1982;52(1):26–37. doi: 10.1043/0003-3219(1982)052<0026:CATMAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Tsolakis AI, Spyropoulos MN, Katsavrias E, Alexandridis K. Effects of altered mandibular function on mandibular growth after condylectomy. Eur J Orthod. 1997;19:9–19. doi: 10.1093/ejo/19.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Rabie AB, Xiong H, Hagg U. Forward mandibular positioning enhances condylar adaptation in adult rats. Eur J Orthod. 2004;26:353–8. doi: 10.1093/ejo/26.4.353. [DOI] [PubMed] [Google Scholar]
- 24.Tang GH, Rabie ABM. Runx2 regulates endochondral ossification in condyle during mandibular advancement. J Dent Res. 2005;84:166–71. doi: 10.1177/154405910508400211. [DOI] [PubMed] [Google Scholar]