Abstract
Introduction
The role of surgery in addition to chemotherapy and radiation for stage IIIA non-small cell lung cancer (NSCLC) remains controversial. Since there is limited data on the benefit from surgery in this setting, we evaluated the use of combined modality therapy nationally, and explored the outcomes with and without the addition of surgery.
Methods
Patient variables and treatment-related outcomes were abstracted for patients with clinical stage IIIA NSCLC from the National Cancer Database. Patients receiving chemotherapy and radiation (CR) were compared to those undergoing chemotherapy, radiation, and surgery in any sequence (CRS).
Results
Between 1998 and 2010, 61339 patients underwent combined modality treatment for clinical stage IIIA NSCLC. Of these, 51979 (84.7%) received CR while 9360 (15.3%) underwent CRS. Patients in the CRS group were younger, more likely females and Caucasians, had smaller tumors and lower Charlson comorbidity scores. The 30-day surgical mortality was 200/8993 (2.2%). The median overall survival favored the CRS group in both unmatched (32.4 months vs. 15.7 months, p<.001) and matched analysis based on patient characteristics (34.3 months vs. 18.4months, p<.001).
Conclusion
There is significant heterogeneity in the treatment of stage IIIA NSCLC in the United States. Patients selected for surgery in addition to chemoradiation therapy appear to have better long-term survival.
Keywords: non-small cell lung cancer, surgery, outcomes
INTRODUCTION
In the United States more than 220,000 new patients are diagnosed with lung cancer each year.(1) Among those with newly diagnosed non-small cell lung cancer (NSCLC), it is estimated that 27% of patients present with stage III disease, for which the 5-year overall survival (OS) is typically less than 20%.(2, 3)
The most common treatment modality for stage IIIA NSCLC is a combination of chemotherapy and radiation, with studies suggesting a median survival of 16 to 28 months when both are administered concurrently.(4, 5) While concurrent chemoradiation has been associated with increased toxicity relative to sequential treatment, it does provide a survival advantage.(6, 7) Surgical resection, in addition to chemotherapy and radiation, has been selectively offered to patients with stage IIIA lung cancer and single-center studies report good outcomes with median survival up to 43 months and 5-year survival of 33%.(8–11)
Over the last decade, randomized trials have aimed to address the role of surgery in addition to chemotherapy and radiation in stage III NSCLC. In the INT-0139 trial, patients with stage IIIA NSCLC underwent concurrent induction chemoradiation therapy. Patients were then randomly assigned to the surgical group or the chemoradiation group where they continued radiotherapy. There was no difference in OS between the treatment arms, though there was an improvement in progression-free survival in favor of surgery.(12) In a European study, patients with stage IIIA NSCLC were administered induction chemotherapy and responding patients were subsequently randomly assigned to surgery or radiotherapy. Again, no difference in OS was seen between the treatment arms.(13)
Given the variable data from clinical trials, with randomized trials not showing a survival advantage for patients having received surgery and smaller institutional studies suggesting good long-term survival with the addition of surgery to chemoradiation therapy, the algorithms used by institutions vary widely. Additionally, the penetrance and efficacy of surgical resection still remain inadequately understood. The National Cancer Data Base (NCDB), a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, is a nationwide oncology outcomes database for more than 1,500 Commission-accredited cancer programs. About 70 percent of all newly diagnosed cases of cancer in the US are captured at the institutional level and reported to the NCDB.(14, 15) We aimed to study the actual practice patterns of treatment for stage IIIA NSCLC in the United States and to understand the efficacy of surgical resection in conjunction with chemotherapy and radiation in this population using the NCDB.
METHODS
Using deidentified patient information from the NCDB participant user file, we abstracted patients with clinical stage IIIA NSCLC who received treatment between 1998 and 2010 with either a combination of chemotherapy and radiation therapy in any sequence (CR group) or a combination of chemotherapy, radiation, and surgery in any sequence (CRS group). Patients who did not receive either one of these 2 treatment plans (CR or CRS) were excluded. Patients who received only palliative treatment (as coded in the database) were excluded. The study was exempted by the institutional review board.
For each patient, information on patient-related variables, tumor-related variables, treatment, and short-, and long-term outcomes was obtained. Using information on race, income, and the population size of the area from which a patient presented, we formed dichotomized groups in which a patient was either Caucasian or not Caucasian, had an annual income less than or greater than $35000, and presented from a rural location (regional population less than 250000) or an urban location respectively. The Charlson/Deyo score was used as a measure of comorbidity in the database. It was categorized as 0, 1, or ≥2. The NCDB combined those with scores of 2 or greater into one group as very few patients had scores greater than 2. Treatment facilities were classified as community cancer programs, comprehensive community cancer programs, and academic/research centers. Last known vital status and the time between diagnosis and the follow-up date were used to determine survival. We initially contrasted patients receiving chemotherapy and radiation (CR group) to those who received surgery in addition to chemotherapy and radiation therapy in any sequence (CRS group) in an unmatched comparison. Patients in the CR group were then matched to those in the CRS group using a propensity score based technique. The propensity score was the probability of receiving surgery during the study period, estimated using a logistic regression model including age, gender, race, income, rural versus urban status, year of diagnosis, Charlson/Deyo score, tumor size, and type of facility where treatment was administered. These variables were selected from univariate analyses comparing the CR and CRS groups. Patients for whom the propensity scores matched to the third decimal place were matched in 1:1 fashion. Automated matching was performed using the Fuzzy extension command in SPSS (SPSS 21.0 for Windows, SPSS Inc, Chicago, IL).(16) Recognizing that neoadjuvant chemotherapy and radiation followed by surgery, henceforth referred to as trimodality therapy, is the de facto standard for CRS in the United States, we performed a secondary analysis (unmatched and matched) restricting CRS patients only to those who received neoadjuvant treatment. (Figure 1)
Figure 1.
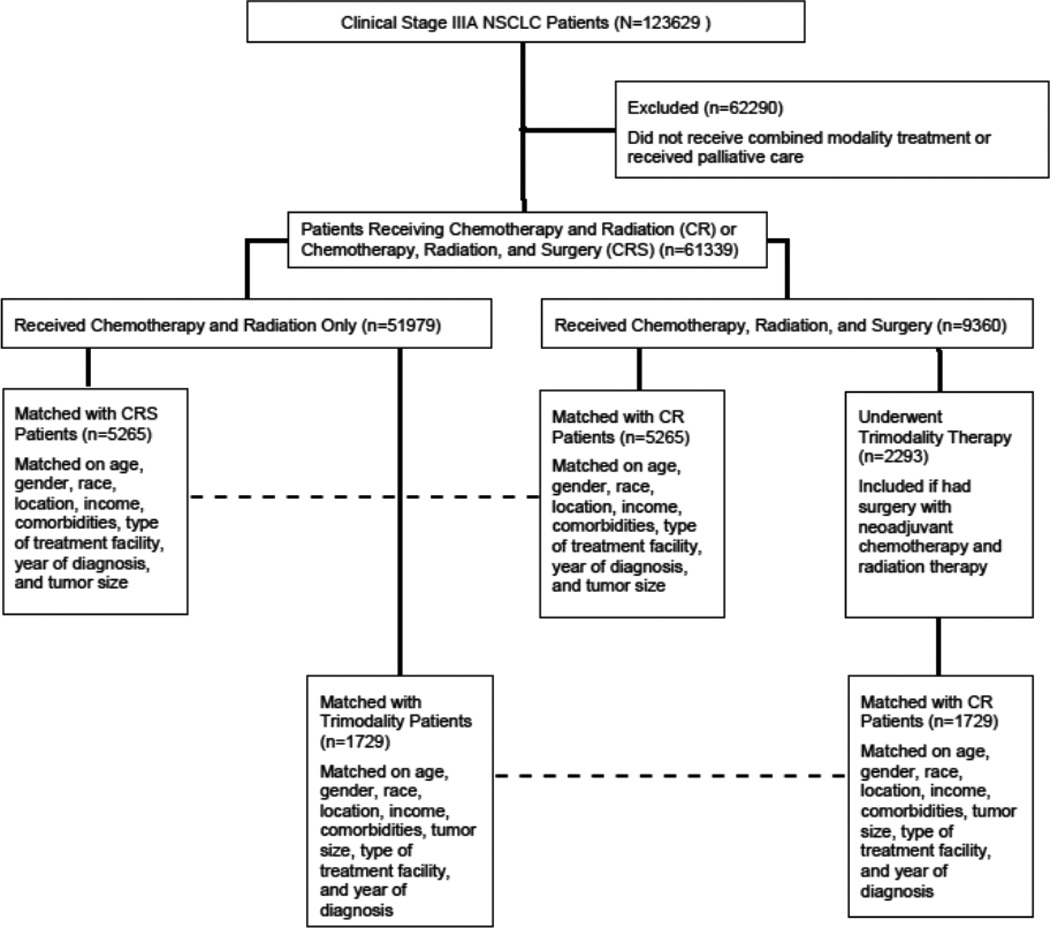
Consort Diagram showing schema of study subject selection and analysis.
All analyses were performed using SPSS 21.0. Descriptive statistics were expressed as mean ± standard deviation unless otherwise specified. Independent samples t tests and one-way ANOVA were used to compare continuous variables. Chi-square tests were used to compare categorical data. Overall survival was estimated by the Kaplan-Meier method. P-values less than 0.05 were considered statistically significant.
RESULTS
Between 1998 and 2010, 123629 patients were diagnosed with clinical stage IIIA NSCLC at 1588 institutions. Of these 61339 (49.6%) were treated using combined modality therapy, with 51979 (84.7%) receiving chemotherapy and radiation (CR) and 9360 (15.3%) undergoing surgical resection in addition to chemotherapy and radiation (CRS). Of the CRS group, 3811/6635 (57.4%) had pathologically confirmed N2 disease. For the entire cohort of patients receiving combined modality treatment, the mean age was 65.5 ± 10.1 years and 35167/61339 (57.3%) were males. Most patients were treated at either community comprehensive cancer programs (32654/61339, 53.2%) or academic cancer centers (17038/61339, 27.8%),
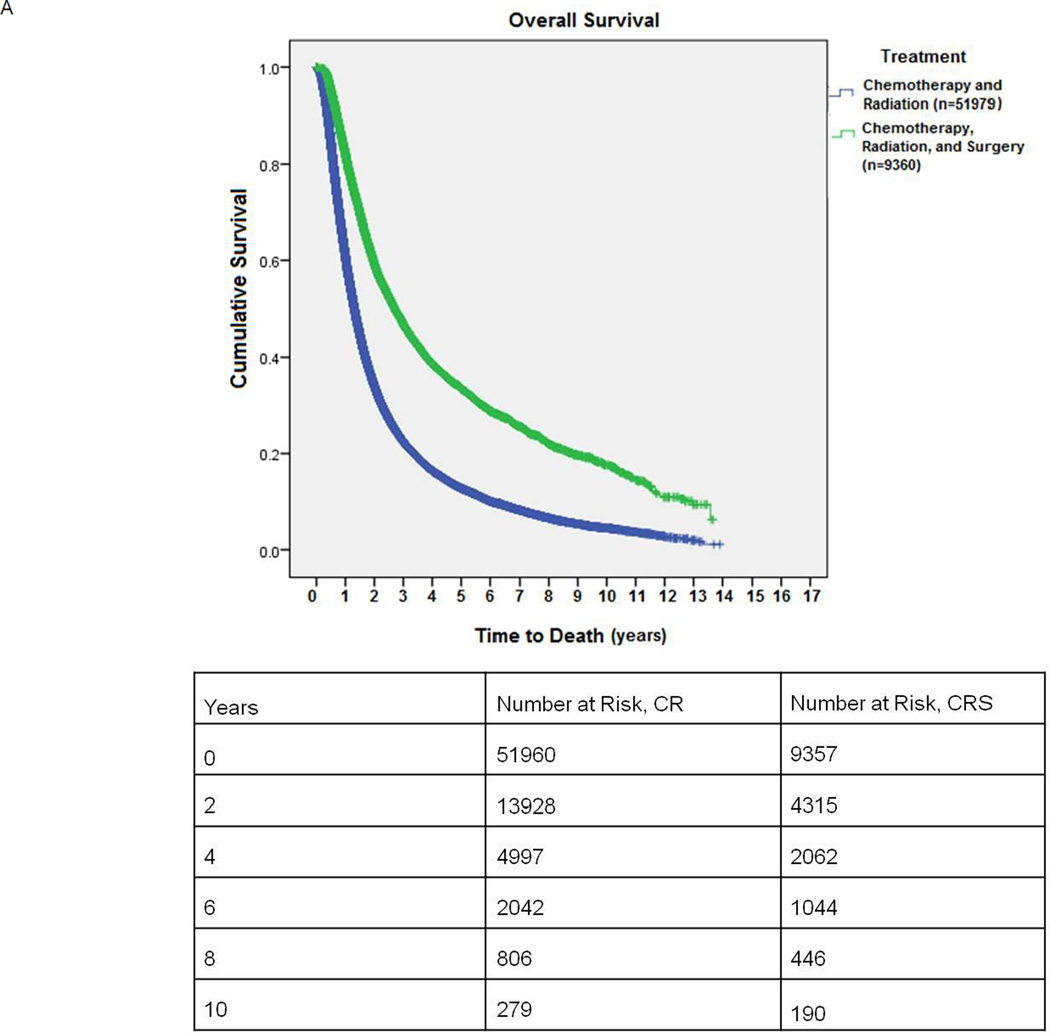
Patients in the CRS group were younger, and were more likely to be females and Caucasians. (Table 1) Surgical patients also had higher incomes and traveled farther for treatment than patients in the CR only group. Patients who underwent CRS had smaller tumors and lower Charlson comorbidity scores. (Table 1) Between 1998 and 2004, 4078/28464 (14.3%) patients received CRS for definitive treatment, while this proportion increased to 5282/32875 (16.1%, p<.001) from 2005 to 2010. Additionally, the difference in mean survival of patients diagnosed between 1998–2004 (30.57±.2 months) and those between 2005–2010 (29.33±.2 months), while statistically significant (p<.001), was deemed clinically insignificant. In the CR arm, 9710/38166 (25.5%) patients received a cumulative radiation dose of less than 50 Gray (Gy) and 17353/38166 (45.4%) less than 60 Gy. In the surgical arm, 2692/3875 (69.5%) received preoperative chemotherapy. For patients receiving preoperative radiation in the surgical arm, mean radiation dose was 51.02 Gy. Mean postoperative hospital stay was 6.8 ± 8.4 days and the 30-day surgical mortality was 200/8993 (2.2%). Median survival for unmatched patients receiving CR versus CRS was 15.7 months vs. 32.4 months, respectively, (p<0.001). (Figure 2A)
Table 1.
Baseline characteristics, treatment-related variables, and long-term outcomes in all patients with clinical stage IIIA NSCLC who received combination therapy. This table shows an unmatched comparison. The CR group refers to patients who received chemotherapy and radiation in any sequence. The CRS group refers to patients who underwent chemotherapy, radiation therapy, and surgery in any sequence.
| Chemotherapy and Radiation (CR) (n=51979) |
Chemotherapy, Radiation and Surgery (CRS) (n=9360) |
P value |
|
|---|---|---|---|
| Age (years) | 66.3 ± 10.0 | 61.3 ± 9.9 | <.001 |
| Male Gender | 30133 (58.0%) | 5034 (53.8%) | <.001 |
| Caucasian | 44935 (87.1%) | 8281 (89.2%) | <.001 |
| Urban Location | 32182 (65.2%) | 6162 (70.2%) | <.001 |
| Income >$35000/year | 30876 (62.4%) | 6184 (69.9%) | <.001 |
| Charlson/Deyo Score (available n=35717, CR, n=6713, CRS) | 0 23774 (66.6%) 1 8669 (24.3%) 2 3274 (9.2%) |
0 4497 (67.0%) 1 1765 (26.3%) 2 451 (6.7%) |
<.001 |
| Distance traveled for treatment (miles) | 37.5 ± 387.0 | 92.2 ± 775.4 | <.001 |
| Tumor Size (mm) | 48.1 ± 39.5 | 43.0 ± 33.5 | <.001 |
| Facility Reporting Case | Community Cancer Program: 9464 (18.2%) Comprehensive Community Cancer Program: 28183 (54.2%) Academic/Research Program: 13537 (26.0%) Other: 795 (1.5%) |
Community Cancer Program: 1211 (12.9%) Comprehensive Community Cancer Program: 4471 (47.8%) Academic/Research Program: 3501 (37.4%) Other: 177 (1.9%) |
<.001 |
| Chemotherapy type | Single agent: 4193 (9.1%) Multiagent: 42082 (90.9%) |
Single agent: 413 (5.0%) Multiagent: 7849 (95.0%) |
|
| Chemotherapy-Surgery sequence (available n=4535) | Before surgery: 2185 (48.2%) After surgery: 1843 (40.6%) Before and after surgery: 507 (11.2%) |
||
| Cumulative Radiation Dose (cGy) | 5914.1 ± 3831.8 (26.6% Missing) | 5295.0 ± 2901.8 (27.6% Missing) | <.001 |
| Cumulative radiation dose (cGy) (available n=38166) | ≤4000: 5678 (14.9%) 4001–5000: 4032 (10.6%) 5001–6000: 7643 (20.0%) >6000: 20813 (54.5%) |
||
| Cumulative radiation dose (cGy) (n=6774) | ≤3500: 344 (5.1%) 3501–4500: 1971 (29.1%) >4500: 4459 (65.8%) |
||
| Radiation-Surgery sequence (available n=9044) | Before surgery: 4749 (52.5%) After surgery: 4140 (45.8%) Before and after surgery: 155 (1.7%) |
||
| Type of operation | Lobectomy: 6284 (67.1%) Pneumonectomy: 1517 (16.2%) Other: 1559 (16.7%) |
||
| Median Survival (months) | 15.7 ± 0.1 | 32.4 ± 0.6 | <.001 |
Figure 2.
A: Kaplan-Meier survival of patients undergoing combination chemotherapy and radiation (CR) versus chemotherapy, radiation, and surgery (CRS). This is an unmatched comparison.
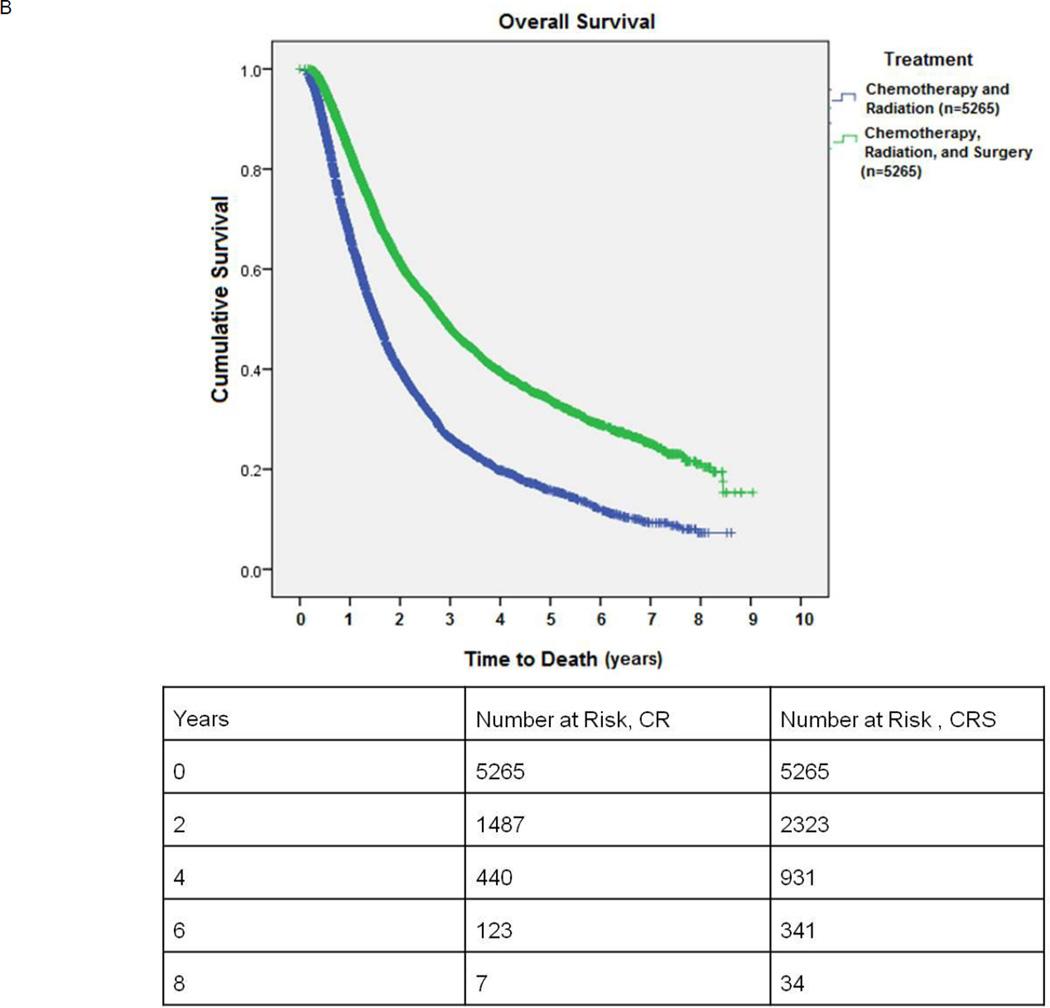
B: Kaplan-Meier survival of patients undergoing combination chemotherapy and radiation (CR) versus chemotherapy, radiation, and surgery (CRS): propensity score matched comparison.
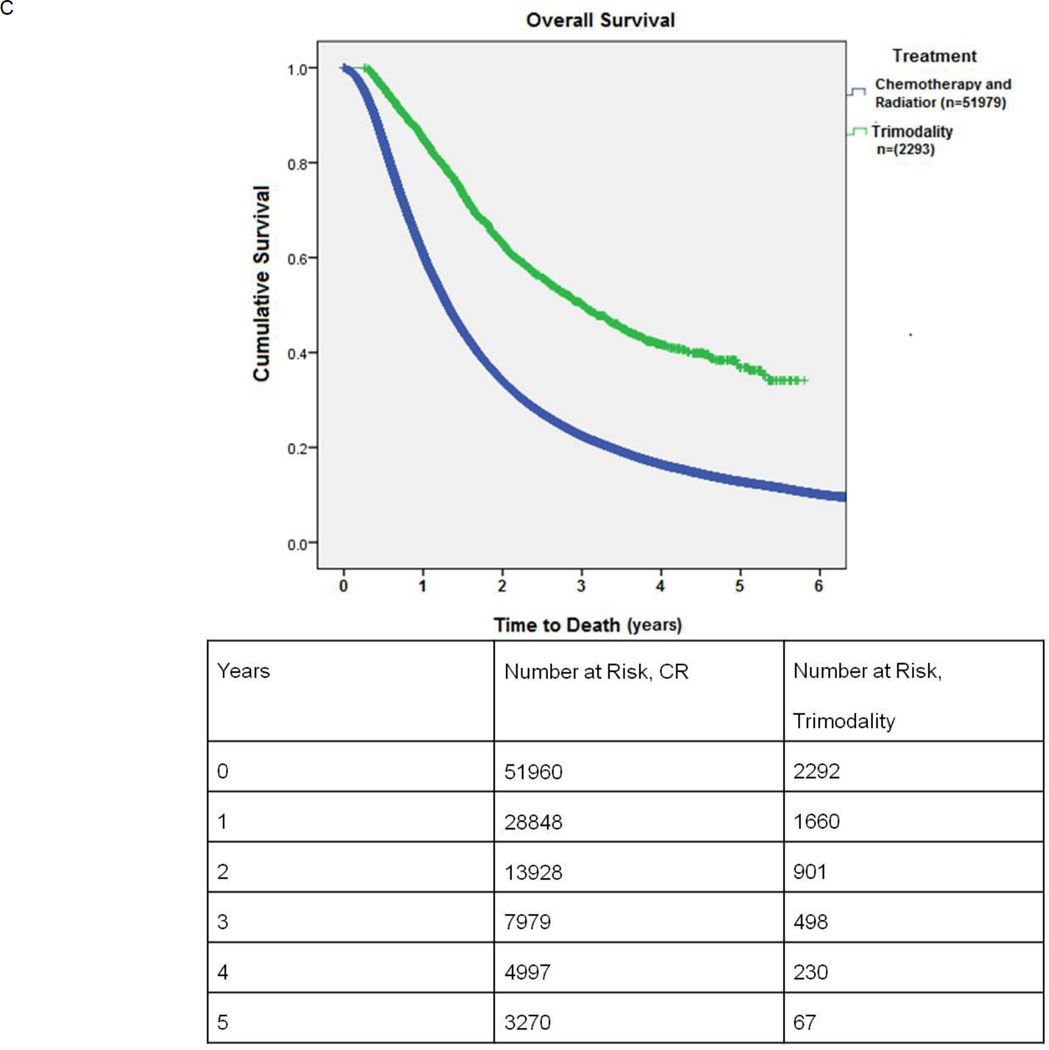
C: Kaplan-Meier survival of patients undergoing combination chemotherapy and radiation (CR) versus trimodality therapy (neoadjuvant chemoradiation followed by surgery): This is an unmatched comparison.
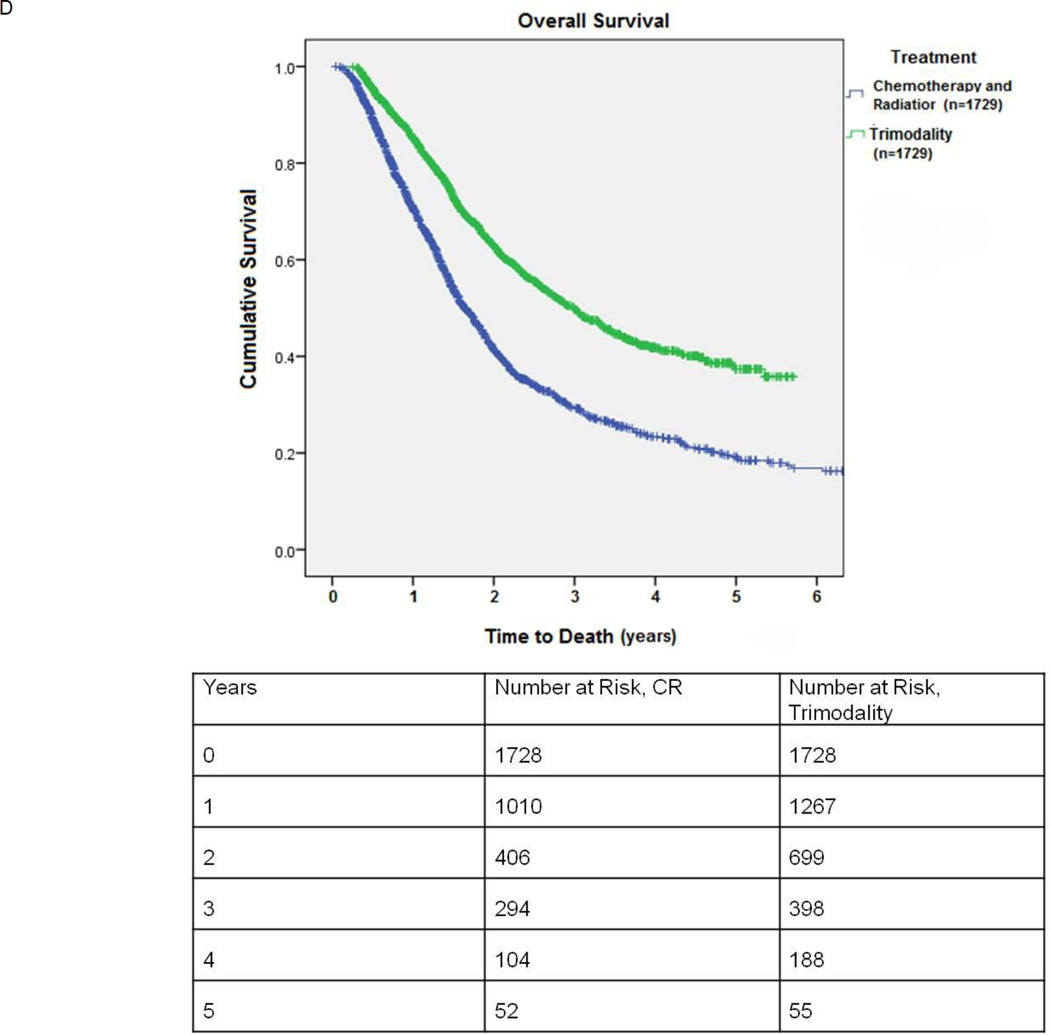
D: Kaplan-Meier survival of patients undergoing combination chemotherapy and radiation (CR) versus trimodality therapy (neoadjuvant chemoradiation followed by surgery): propensity score matched comparison.
Propensity score matching between the CR and CRS groups yielded 5265 matched pairs. These groups were comparable in age, gender, race, location (rural versus urban), income, comorbidities, treatment facility, and year of diagnosis. (Table 2) Tumors in the CR group were slightly larger than those in the CRS group (43.7 mm vs. 42.5 mm, p=0.01) though the 1.2 mm difference was not considered clinically meaningful. In the CR arm, 1017/4963 (20.5%) patients received a cumulative radiation dose of less than 50Gy and, 1977/4963 (39.8%) less than 60 Gy. In the surgical arm, 2112/3619 (58.3%) received preoperative chemotherapy. For the CRS group, the mean postoperative hospital stay was 6.9 ± 8.4 days and 234/5265 (4.4%) patients experienced unplanned readmissions after surgery. The 30-day surgical mortality was 114/5265 (2.2%). Median survival for matched patients receiving CR versus CRS was 18.4 months vs. 34.3 months, respectively (p<0.001). (Figure 2B)
Table 2.
Baseline characteristics, treatment-related variables, and long-term outcomes in propensity score matched patients with clinical stage IIIA NSCLC who received combination therapy. The CR group refers to patients who received chemotherapy and radiation in any sequence. The CRS refers to patients who underwent chemotherapy, radiation therapy, and surgery in any sequence.
| Chemotherapy and Radiation (CR) (n=5265) |
Chemotherapy, Radiation and Surgery (CRS) (n=5265) |
P value |
|
|---|---|---|---|
| Age (years) | 62.5 ± 9.9 | 62.5 ± 9.3 | .856 |
| Male Gender | 2790 (53.0%) | 2773 (52.7%) | .755 |
| Caucasian | 4653 (88.4%) | 4652 (88.4%) | 1.0 |
| Urban Location | 3660 (69.5%) | 3660 (69.5%) | 1.0 |
| Income >$35000/year | 3634 (69.0%) | 3607 (68.5%) | .585 |
| Charlson/Deyo Score | 0 3435 (65.2%) 1 1458 (27.7%) 2 372 (7.1%) |
0 3496 (65.8%) 1 1394 (26.5%) 2 375 (7.1%) |
.371 |
| Tumor Size (mm) | 43.7 ± 24.2 | 42.5 ± 26.6 | .01 |
| Facility Reporting Case | Community Cancer Program: 726 (13.8%) Comprehensive Community Cancer Program: 2658 (50.5%) Academic/Research Program: 1881(35.7%) |
Community Cancer Program: 755 (14.3%) Comprehensive Community Cancer Program: 2649 (50.3%) Academic/Research Program: 1861(35.3%) |
.708 |
| Distance traveled for treatment (miles) | 49.0 ± 520.5 | 93.1 ± 787.5 | .001 |
| Chemotherapy Type | Single agent: 354 (7.4%) Multiagent: 4424 (92.6%) |
Single agent: 216 (4.6%) Multiagent: 4472 (95.4%) |
|
| Chemotherapy-Surgery Sequence (available n=3619) | Before surgery: 1724 (47.6%) After surgery: 1507 (41.6%) Before and after surgery: 388 (10.7%) |
||
| Cumulative Radiation Dose (cGy) | 6005.0 ± 2893.2 (5.8% Missing) | 5339.5 ± 2707.4 (9.5% Missing) | <.001 |
| Cumulative Radiation Dose (cGy) (n=4963) | ≤4000: 561 (11.3%) 4001–5000: 456 (9.2%) 5001–6000: 960 (19.3%) >6000: 2986 (60.2%) |
||
| Cumulative Radiation Dose (cGy) (n=4770) | ≤3500: 206 (4.3%) 3501–4500: 1276 (26.8%) >4500: 3288 (68.9%) |
||
| Radiation-Surgery Sequence (n=5265) | Before surgery: 2676 (50.8%) After surgery: 2494 (47.4%) Before and after surgery: 95 (1.8%) |
||
| Type of operation | Lobectomy: 3708 (70.4%) Pneumonectomy: 682 (13.0%) Other: 875 (16.6%) |
||
| Median Survival (months) | 18.4 ± 0.3 | 34.3 ± 0.8 | <.001 |
In the entire cohort 2293 patients underwent trimodality therapy with neoadjuvant chemotherapy and radiation followed by surgical resection. When compared to the 51979 patients who received CR only, patients in the trimodality group tended to be younger, were more likely to be females, had smaller tumors and lower Charlson comorbidity scores (Table 3) For the trimodality group, the 30-day surgical mortality was 84/2291 (3.7%); 8.5% of patients died after pneumonectomy. Median survival for unmatched patients receiving CR versus CRS with neoadjuvant chemotherapy and radiation was 15.7 months vs. 36.0 months, respectively (p<0.001) (Figure 2C)
Table 3.
Baseline characteristics, treatment-related variables, and long-term outcomes in patients with clinical stage IIIA NSCLC who received combination therapy with chemotherapy and radiation versus those who received trimodality therapy. This table shows an unmatched comparison. The CR group refers to patients who received chemotherapy and radiation in any sequence. The trimodality group refers to patients who underwent preoperative chemotherapy and radiation therapy.
| Chemotherapy and Radiation (CR) (n=51979) |
Preoperative Chemotherapy and Radiation (Trimodality) (n=2293) |
P value |
|
|---|---|---|---|
| Age (years) | 66.3 ± 10.0 | 60.8 ± 9.8 | <.001 |
| Male Gender | 30133 (58.0%) | 1166 (50.9%) | <.001 |
| Caucasian | 44935 (87.1%) | 2029 (89.2%) | .003 |
| Urban Location | 32182 (65.2%) | 1506 (70.9%) | <.001 |
| Income >$35000/year | 30876 (62.4%) | 1552 (72.2%) | <.001 |
| Charlson/Deyo Score(n=35717 CR, n=2293 Trimodality) | 0 23774 (66.6%) 1 8669 (24.3%) 2 3274 (9.2%) |
0 1546 (67.4%) 1 607 (26.5%) 2 140 (6.1%) |
<.001 |
| Distance traveled for treatment (miles) | 37.5 ± 387.0 | 111.8 ± 876.5 | <.001 |
| Tumor Size (mm) | 48.1 ± 39.5 | 44.6 ± 30.1 | <.001 |
| Facility Reporting Case | Community Cancer Program: 9464 (18.2%) Comprehensive Community Cancer Program: 28183 (54.2%) Academic/Research Program: 13537 (26.0%) Other: 795 (1.5%) |
Community Cancer Program: 260 (11.3%) Comprehensive Community Cancer Program: 1120 (48.8%) Academic/Research Program: 892 (38.9%) Other: 21 (0.9%) |
<.001 |
| Chemotherapy Type | Single agent: 4193 (9.1%) Multiagent: 42082 (90.9%) |
Single agent: 83 (4.1%) Multiagent: 1940 (95.9%) |
|
| Chemotherapy-Surgery Sequence (available n=2293) | Before surgery: 1912 (83.4%) Before and After: 381 (16.6%) |
||
| Cumulative radiation dose (cGy) | 5914.1 ± 3831.8 (26.6% Missing) | 5249.1 ± 2339.9 (10.3% Missing) | <.001 |
| Cumulative radiation dose (cGy) (n=2059) | ≤3500: 37 (1.8%) 3501–4500: 744 (36.1%) >4500: 1278 (62.1%) |
||
| Cumulative radiation dose (cGy) (n=38166) | ≤4000: 5678 (14.9%) 4001–5000: 4032 (10.6%) 5001–6000: 7643 (20.0%) >6000: 20813 (54.5%) |
||
| Radiation Surgery Sequence | Before surgery: 2220 (96.8%) Before and after surgery: 73 (3.2%) |
||
| Type of operation | Lobectomy: 1816 (79.2%) Pneumonectomy: 342 (14.9%) Other: 135 (5.9%) |
||
| Median Survival (months) | 15.7 ± 0.1 | 36.0 ± 1.5 | <.001 |
Propensity score matching between the CR only and trimodality groups yielded 1729 matched pairs. These groups were comparable in age, gender, race, location, income, comorbidities, tumor size, distance traveled for treatment, and type of treatment facility.(Table 4) In the CR arm, 266/1625 (16.4%) patients received a cumulative radiation dose of less than 50Gy and 583/1625 (35.9%) less than 60 Gy. For the trimodality group, the mean postoperative hospital stay was 7.5 ± 9.9 days. The 30-day surgical mortality was 67/1727 (3.9%). Median survival for matched patients receiving CR versus CRS with neoadjuvant chemotherapy and radiation was 19.7 months vs. 35.9 months, respectively, (p<0.001). (Figure 2D) The 5-year survival for patients receiving trimodality treatment was 37.4%, compared to 19.2% for CR.
Table 4.
Baseline characteristics, treatment-related variables, and long-term outcomes in propensity score matched patients with clinical stage IIIA NSCLC who received combination therapy with chemotherapy and radiation versus those who received trimodality therapy. The CR group refers to patients who received chemotherapy and radiation in any sequence. The trimodality group refers to patients who underwent preoperative chemotherapy and radiation therapy.
| Chemotherapy and Radiation (CR) (n=1729) |
Preoperative Chemotherapy and Radiation (Trimodality) (n=1729) |
P value |
|
|---|---|---|---|
| Age (years) | 61.9 ± 9.9 | 61.7 ± 9.4 | .686 |
| Male Gender | 921 (53.3%) | 886 (51.2%) | .247 |
| Caucasian | 1527 (88.3%) | 1534 (88.7%) | .749 |
| Urban Location | 1232 (71.3%) | 1212 (70.1%) | .478 |
| Income >$35000/year | 1241 (71.8%) | 1232 (71.3%) | .763 |
| Charlson/Deyo Score | 0 1170 (67.7%) 1 459 (26.5%) 2 100 (5.8%) |
0 1164(67.3%) 1 455 (26.3%) 2 110 (6.4%) |
.775 |
| Distance traveled for treatment (miles) | 78.0 ± 716.0 | 115.3 ± 902.0 | .178 |
| Tumor Size (mm) | 44.9 ± 24.7 | 44.9 ± 31.6 | .996 |
| Facility Reporting Case | Community Cancer Program: 189 (10.9%) Comprehensive Community Cancer Program: 904 (52.3%) Academic/Research Program: 636 (36.8%) |
Community Cancer Program: 213 (12.3%) Comprehensive Community Cancer Program: 896 (51.8%) Academic/Research Program: 620 (35.9%) |
.433 |
| Chemotherapy Type | Single agent: 98 (6.2%) Multiagent: 1487 (93.8%) |
Single agent: 61 (4.0%) Multiagent: 1477 (96.0%) |
|
| Chemotherapy-Surgery Sequence (available n=1729) | Before surgery: 1448 (83.7%) Before and after surgery: 281 (16.3%) |
||
| Cumulative radiation dose (cGy) | 6263.4 ± 3798.9 (6.1% Missing) | 5279.0± 2606.8 (9.8% Missing) | <.001 |
| Cumulative radiation dose (cGy) (n=1560) | ≤3500: 25 (1.6%) 3501–4500: 561 (36.0%) >4500: 974 (62.4%) |
||
| Cumulative radiation dose (cGy) (n=1625) | ≤4000: 156 (9.6%) 4001–5000: 110 (6.8%) 5001–6000: 317 (19.5%) >6000: 1042 (64.1%) |
||
| Radiation-Surgery Sequence | Radiation before surgery: 1674 (96.8%) Radiation before and after surgery: 55 (3.2%) |
||
| Type of operation | Lobectomy: 1372 (79.4%) Pneumonectomy: 257 (14.9%) Other: 100 (5.8%) |
||
| Median Survival (months) | 19.7 ± 0.6 | 35.9 ± 1.6 | <.001 |
Given the large proportion of patients receiving radiation therapy (RT) doses less than 60 Gy, the effect of RT dose on OS was explored. For CR patients in the unmatched cohort of 51979 patients, OS for those with RT dose ≥ 60 Gy was 19.2 months compared to 15.7 months for the overall cohort. In the matched cohort of 5265 patients, OS in the CR group improved from 18.4 months to 20.5 months with ≥ 60Gy RT. In the CR cohort matched to trimodality therapy patients, OS improved from 19.7 months to 22.3 months with ≥ 60Gy RT. However, in all comparisons of CRS vs. CR, OS was significantly greater for CRS irrespective of RT dose (data not shown).
Of all clinical stage IIIA patients in this study, about 90% had N2 disease while the rest included other subgroups of stage IIIA disease, such as T3N1. We performed a subgroup analysis of this subset (N2) and found no difference in findings from the analyses of the larger cohort of all stage IIIA patients (data not shown). Similarly, matched comparisons in the N2 subgroup analyses between the CR and CRS arms did not differ from findings in the larger group.
DISCUSSION
Our study demonstrates that there is significant heterogeneity in treatment of clinical stage IIIA NSCLC in the United States, and selected patients who undergo surgical resection, in addition to chemotherapy and radiation, appear to have better long-term OS. Within the framework of published guidelines by bodies such as the National Comprehensive Cancer Network, decision-making in stage III NSCLC is often dependent upon institutional preferences, assessment of tumor burden, and the patient’s physiologic reserve. A population-based Canadian study noted that while 12% of patients with stage III NSCLC underwent primary surgery, radiation therapy was part of the initial treatment for 78% and chemotherapy in 31% of patients only.(17) The utilization of chemotherapy was especially lower than predicted. Similarly, we found that 15.3% of patients targeted for definitive treatment underwent a surgical resection and preoperative systemic therapy was utilized in only 69.5% of patients. Other large population-based studies have found a similar lack of uniformity in treatment of stage III NSCLC, with up to 28% of patients receiving palliative care and combination therapy being utilized in 26–47% of patients only.(17–19) Poor performance status of patients has not been shown to solely account for these wide variations in treatment.(17)
We noted that urban dwellers, Caucasian patients, and those with higher income levels were more likely to receive combination chemotherapy, radiation, and surgery. The relationship between socioeconomic status (SES), race, and treatment for lung cancer has been previously studied and a meta-analysis concluded that patients living in more socioeconomically deprived circumstances are less likely to receive any type of treatment, especially surgery, and chemotherapy.(20) Specifically, the association between likelihood of surgery and SES has been investigated in patients with stage I and II NSCLC where surgical resection is standard of care. One such study describing a cohort of patients treated between 1991 and 1999 noted that black race and lower SES were associated with a lower likelihood of surgery.(21) Our findings from a more contemporary period (1998–2010) are quite similar in patients treated for stage III NSCLC. We also noted that treatment at academic cancer centers was associated with trimodality therapy including surgery. Other authors have described a closer relationship between higher-volume centers and surgery for NSCLC.(21) The NCDB does not provide details of hospital volume directly, however it is likely that academic cancer centers have higher case volumes compared to other institutions.
The role of surgery in stage IIIA NSCLC is controversial. Though the new American College of Chest Physicians guidelines suggest that surgery may play a role in specific stage IIIA patients, publications based upon comprehensive reviews do not make any firm statements about the efficacy of surgery (22). The ACCP suggests that in patients with discrete N2 involvement by NSCLC identified preoperatively (IIIA), either definitive chemoradiation therapy or induction therapy followed by surgery be considered over either surgery or radiation alone. Several single-center cohort studies highlight good outcomes when surgery is performed in conjunction with chemoradiation therapy. These series demonstrate median OS ranging from 33–61months with 5-year survival of up to 43%.(8–11, 23–25) Four randomized trials have attempted to study the efficacy of surgery for stage IIIA disease.(12, 13, 26, 27) The Medical Research Council Lung Cancer Working Party trial attempted to enroll 350 patients with stage IIIA disease not amenable to primary surgery and randomize them to definitive radiotherapy or chemotherapy followed by resection. The trial closed due to poor accrual, with only 48 patients randomized in 3 years.(26) RTOG 89-01 treated patients with confirmed N2 disease with induction chemotherapy, and randomized them to either surgery or radiation therapy, followed by consolidation chemotherapy for both arms. The trial accrued 75 patients toward a goal of 224 patients. No significant difference was noted in the median survival time between the surgical and radiation arms. (19.4 vs. 17.4 months)(27)
Among the studies that met enrollment goals, the INT-0139 trial treated patients with positive N2 nodes with concurrent induction chemotherapy plus radiotherapy.(12) If no progression occurred, patients in the surgical group underwent resection and those in the chemoradiation group continued radiotherapy. A total of 396 patients were evaluated with no difference noted in the OS between the 2 groups (23.6 versus 22.2 months). In an exploratory analysis, OS was improved for patients who underwent lobectomy (33.6 months), but not pneumonectomy (18.9 months), versus chemotherapy plus radiotherapy. This study is criticized for an unusually high mortality rate of 26% following pneumonectomy which may have diluted any OS advantage in the surgical arm. The 30-day mortality rate after pneumonectomy following induction chemoradiation in our study was 8.5%, which is line with the 3–8% operative mortality in several prior reports.(28–30) Finally, in the European Organization for Research and Treatment of Cancer-Lung Cancer Group study patients with stage IIIA-N2 NSCLC were given three cycles of induction chemotherapy. Responding patients were randomized to surgical resection or radiotherapy. Median survival for patients assigned to surgery versus radiotherapy was 16.4 versus 17.5 months respectively.(13) Surprisingly, nearly 55% of surgical patients in this trial underwent a pneumonectomy and the median survival after pneumonectomy was 13.4 months compared to 25.4 months after a lobectomy. Less than 15% of patients in the trimodality group in our study underwent a pneumonectomy.
An important finding in our study is the wide variability in the application of individual treatment modalities. For definitive therapy with CR, doses of 60 Gy or higher are recommended based on prospective data demonstrating inferior survival at lower doses.(31) The recommended dose for preoperative radiotherapy is 45 Gy or higher, though no such dose response has been suggested in this setting (22) In the overall unmatched cohort, the mean radiation dose for CR was 59.14 Gy with 25% of patients receiving a cumulative dose of less than 50 Gy and 45% of patients receiving less than 60 Gy. The implication for such a large proportion of patients with potentially curable stage IIIA NSCLC being treated with suboptimal doses is significant, and will be explored further in a separate analysis. In the CRS cohort, the mean radiation dose was 52.95 Gy, and 35% of patients received a cumulative dose of less than 45 Gy. Similarly, nearly 10% of patients in the CR cohort and 5% in the CRS cohort were treated with single agent chemotherapy although dual agent chemotherapy is the standard of care. More than 15% of surgical patients underwent sublobar lung resection despite lobectomy (or pneumonectomy) being widely considered to be the appropriate operation for stage IIIA disease. Even rigorously conducted clinical trials demonstrate a degree of non-adherence to protocol. Investigators in the INT-0139 trial administered radiotherapy per protocol or with acceptable variation to 96% patients in the trimodality group and 79% in the chemoradiation only group.(12) In the same study 95% of patients in the trimodality arm and 92% in the chemoradiation arm received appropriate chemotherapy per protocol, and only 2% of patients underwent a sublobar resection. The higher levels of deviation from recommended guidelines in this cohort are likely due to a variety of reasons, not all of which imply a lack of adherence. For an individual patient, issues of comorbidity and patient preferences may preclude optimal treatment. However, it seems equally likely given the large numbers of patients for which less than optimal treatment was delivered that lack of a multidisciplinary team approach, low patient volume, and lack of awareness of recommended treatment options may contribute as well.
Our study has some strengths and limitations when compared to prior publications. It includes information from a national database that reflects actual practice patterns for all environments where patients with lung cancer receive care. Thus the findings are more likely to be generalizable to the population when compared to trials conducted with strict entry criteria at major cancer centers. The relatively large sample size available for primary and secondary analyses is another advantage compared with prospective studies where subset analysis may be underpowered. However, our retrospective analysis may miss significant selection bias in treatment allocation, such that early disease may have been preferentially allocated to the CRS treatment group. We attempted to overcome this by propensity score matching patients based upon available variables associated with treatment allocation to surgery but the process potentially misses important variables not recorded in the database. We attempted to control for tumor burden by matching on size of the lung mass but detailed information about the size and number of lymph nodes involved is unavailable. The accuracy of individual observations in large databases is arguably lower than that in closely monitored clinical trials however the general trends of perioperative outcomes and long-term survival we observed is similar to prior cohort studies. Additionally, while all of the included patients were clinical stage IIIA, most of the pathologic staging information was missing. Pathologic stage data was only available for 7% of the patients in the CR arm and about 36% of patients in the CRS arm had missing pathologic staging data. This missing pathologic staging information adds potential bias as this may have led to under-staged patients in the CR group. The magnitude of mediastinal disease is important in consideration of surgery; however a comparison of the degree of nodal involvement was not possible because nodal information was available for less than 17% of patients in the CR group and missing for a significant proportion of the surgical group.
We conclude that there is significant variability in treatment of patients with clinical stage IIIA NSCLC in the US and patients selected for surgery in addition to chemotherapy and radiation appear to show better long-term survival relative to chemotherapy and radiation alone. We recommend that patients with stage IIIA NSCLC should be discussed at a multidisciplinary meeting that includes a medical oncologist, radiation oncologist, and thoracic surgeon.
Acknowledgments
Grant support
Varun Puri -NIH K07CA178120, K12CA167540-02 (Paul Calabresi award)
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Waqar S, Subramanian J, et al. Prognostic significance of tumor size in patients with stage III non-small-cell lung cancer: a surveillance, epidemiology, and end results (SEER) survey from 1998 to 2003. J Thorac Oncol. 2012;7:1479–1484. doi: 10.1097/JTO.0b013e318267d032. [DOI] [PubMed] [Google Scholar]
- 3.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 4.Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol. 2013;31:1029–1038. doi: 10.1200/JCO.2012.44.5064. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. ASCO Meeting Abstracts. 2013;31:7501. [Google Scholar]
- 6.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg. 2008;86:1632–1638. doi: 10.1016/j.athoracsur.2008.07.042. discussion 1638–1639. [DOI] [PubMed] [Google Scholar]
- 9.Friedel G, Budach W, Dippon J, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol. 2010;28:942–948. doi: 10.1200/JCO.2008.21.7810. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelillo RM, Trodella L, Ciresa M, et al. Multimodality treatment of stage III non-small cell lung cancer: analysis of a phase II trial using preoperative cisplatin and gemcitabine with concurrent radiotherapy. J Thorac Oncol. 2009;4:1517–1523. doi: 10.1097/JTO.0b013e3181b9e860. [DOI] [PubMed] [Google Scholar]
- 11.Vora SA, Daly BD, Blaszkowsky L, et al. High dose radiation therapy and chemotherapy as induction treatment for stage III nonsmall cell lung carcinoma. Cancer. 2000;89:1946–1952. doi: 10.1002/1097-0142(20001101)89:9<1946::aid-cncr10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 14.Keller D, Guilfoyle C, Sariego J. Geographical influence on racial disparity in breast cancer presentation in the United States. Am Surg. 2011;77:933–936. [PubMed] [Google Scholar]
- 15.Raigani S, Ammori J, Kim J, Hardacre JM. Trends in the Treatment of Resectable Pancreatic Adenocarcinoma. J Gastrointest Surg. 2013 doi: 10.1007/s11605-013-2335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan J, Rosenfeldt F, Chaudhuri K, Marasco S. Cardiac surgery in patients with a history of malignancy: increased complication rate but similar mortality. Heart Lung Circ. 2012;21:255–259. doi: 10.1016/j.hlc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Vinod SK, Wai E, Alexander C, Tyldesley S, Murray N. Stage III non-small-cell lung cancer: population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol. 2012;7:1155–1163. doi: 10.1097/JTO.0b013e31824fea07. [DOI] [PubMed] [Google Scholar]
- 18.Vinod SK, O'Connell DL, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol. 2008;3:871–879. doi: 10.1097/JTO.0b013e31818020c3. [DOI] [PubMed] [Google Scholar]
- 19.Little AG, Gay EG, Gaspar LE, Stewart AK. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007;57:253–260. doi: 10.1016/j.lungcan.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Forrest LF, Adams J, Wareham H, Rubin G, White M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med. 2013;10:e1001376. doi: 10.1371/journal.pmed.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lathan CS, Neville BA, Earle CC. Racial composition of hospitals: effects on surgery for early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:4347–4352. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–e340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 23.Caglar HB, Baldini EH, Othus M, et al. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer. 2009;115:4156–4166. doi: 10.1002/cncr.24492. [DOI] [PubMed] [Google Scholar]
- 24.Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;134:188–193. doi: 10.1016/j.jtcvs.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 25.Kim AW, Liptay MJ, Bonomi P, et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg. 2011;92:233–241. doi: 10.1016/j.athoracsur.2011.03.001. discussion 241–233. [DOI] [PubMed] [Google Scholar]
- 26.Stephens RJ, Girling DJ, Hopwood P, Thatcher N Medical Research Council Lung Cancer Working P. A randomised controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer. 2005;49:395–400. doi: 10.1016/j.lungcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 28.Stamatis G, Djuric D, Eberhardt W, et al. Postoperative morbidity and mortality after induction chemoradiotherapy for locally advanced lung cancer: an analysis of 350 operated patients. Eur J Cardiothorac Surg. 2002;22:292–297. doi: 10.1016/s1010-7940(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 29.Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg. 2010;139:1424–1430. doi: 10.1016/j.jtcvs.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 30.Steger V, Spengler W, Hetzel J, et al. Pneumonectomy: calculable or non-tolerable risk factor in trimodal therapy for Stage III non-small-cell lung cancer? Eur J Cardiothorac Surg. 2012;41:880–885. doi: 10.1093/ejcts/ezr160. discussion 885. [DOI] [PubMed] [Google Scholar]
- 31.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]