Abstract
The field of ballistocardiography seems to be enjoying a recent resurgence, most notably through the development of novel technologies and signal processing methods for measurement and analysis. After the method almost vanished in the late 80’s and 90’s, it is reasonable to wonder what is different this time, and if the technique has now more chances of becoming what its pioneer always wanted – a widespread clinical tool. This paper is an effort to compare and contrast this novel wave of research (notably in the context of the authors’ own work). It also suggests that the new approaches have several key differences with past embodiments that place them in a good position to address some specific issues such as cardiac resynchronization therapy device optimization or congestive heart failure monitoring. This optimism is largely fed by the recent technological advances enabling the measurement of the BCG unobtrusively, frequently, at home or in a hospital, and by a re-focus on monitoring and trending applications.
Index Terms: Ballistocardiogram, cardiovascular monitoring, non-invasive monitoring, hemodynamics
I. Introduction
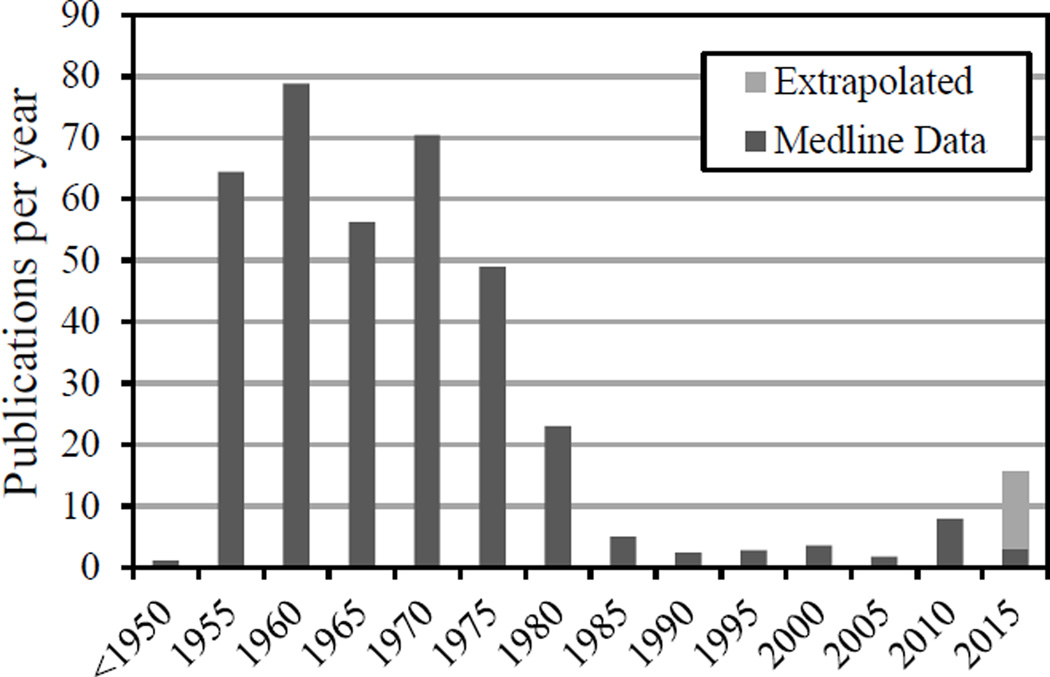
Ballistocardiography is a non-invasive method based on the measurement of the body motion generated by the ejection of the blood at each cardiac cycle. It is one of the many methods relying on detection of cardiac and cardiovascular-related mechanical motions, such as phonocardiography, apexcardiography, seismocardiography, kinetocardiography to list just a few. Ballistocardiography (BCG), originally discovered in the late 19th century [1], has been the focus of intense research in 1940’s through the early 80’s, a period after which the method faded away (see Figure 1). This disappearance can be traced to a few general factors: 1) a lack of standard measurement techniques, with various methods leading to subtly different signals [2]; 2) a lack of understanding of the exact physiologic origin of the BCG waveform, as well as clear guidelines for interpretation of the results, leading to circumspection from the medical community; 3) a primary focus on clinical diagnostic (e.g., myocardial infarction, angina pectoris, coronary heart disease [3, 4]), which typically requires a high level of specificity and reliability that the BCG had not reached [5]; 4) the dawn of ultrasound and echocardiography techniques, which rapidly overtook BCG and related techniques for non-invasive cardiac and hemodynamic diagnostic.
Fig. 1.
Publication rate of BCG-related research over the past 100 years (source: Medline. Search keywords: ballistocardiogram and derivative, excluding specifically EEG, MRI and fMRI papers)
In the last decade or so, however, the general field of ballistocardiography has seen a surprising revival (see Figure 1). In light of the many shortcomings of the method listed above, it is thus legitimate to ask whether this comeback is based on solid grounds. This paper, without being an exhaustive review of current research, is pointing at some significant differences (as well as some similarities) in today’s approaches, which the authors believe should warrant the BCG a second chance.
II. Definition and Physiological Relevance
Imperative to the discussion of the physiologic origin of the ballistocardiogram is the clarification of what constitutes a ballistocardiogram in the first place. As mentioned in the introduction, mechanical motions due to cardiac and hemodynamic events have been recorded from multiple locations, with multiple types of sensors (position, velocity, and acceleration), leading to a confusing number of techniques and signals, sometimes related, sometimes not. This multitude of methods has certainly contributed to blurring the field in the past, and care should be taken not to repeat this situation.
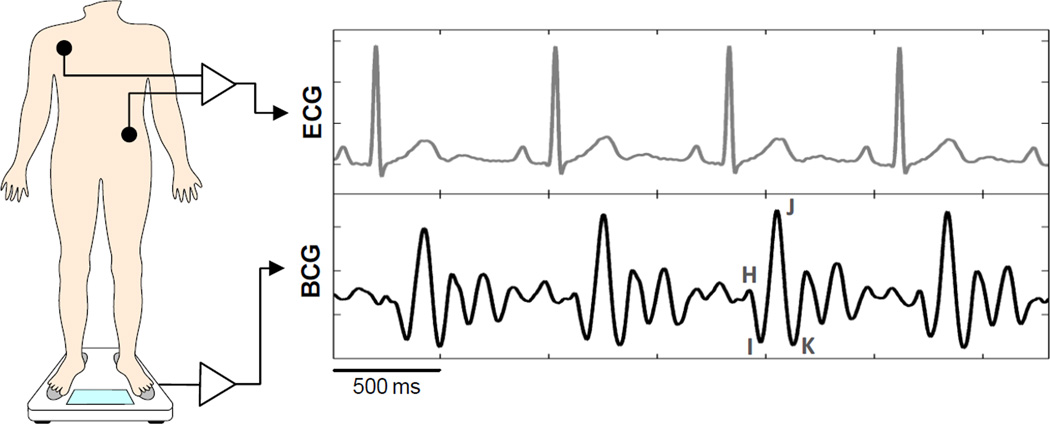
The ballistocardiogram is defined as the reaction (displacement, velocity or acceleration) of the whole body resulting from cardiac ejection of blood. Consequently, it is an integration of multiple forces related to movements of blood inside the heart, inside the arteries (primarily the aorta), and movement of the heart itself. It is inherently a 3D signal, although most measurement techniques focus on the longitudinal, head-to-toe component. Its interpretation has been rendered more difficult by the fact that the signal is dependent on the measurement method [2]. Early on, an effort was made to standardize the measurement techniques and signal labeling in order to help comparison and dissemination of data [2]. In the case of the classic, Starr-based longitudinal BCG, there is a general agreement that the early peak (H, Figure 2) is related to the motion of the heart early in systole, and that the main IJK complex is related to the ventricular ejection and aortic flow [6, 7]. There is less agreement on the later waves. While the BCGs of healthy people can be rather well interpreted in light of physiologic events, BCGs of patients with cardiovascular diseases tend to be more difficult to interpret because of the complex interplay of the various internal forces. As a result, interpretation of abnormal BCG has been mostly based on experimental data, and largely qualitative [8]. Since the early work on interpretation, research was aimed at refining the understanding of the signals, using various models and transfer functions (e.g., [9]), but it did not fundamentally improve the situation. Modern imaging and simulation tools, however, may offer interesting new approaches.
Fig. 2.
Example of BCG waveforms acquired by a modified weighing scale (see Inan, et al. [17]).
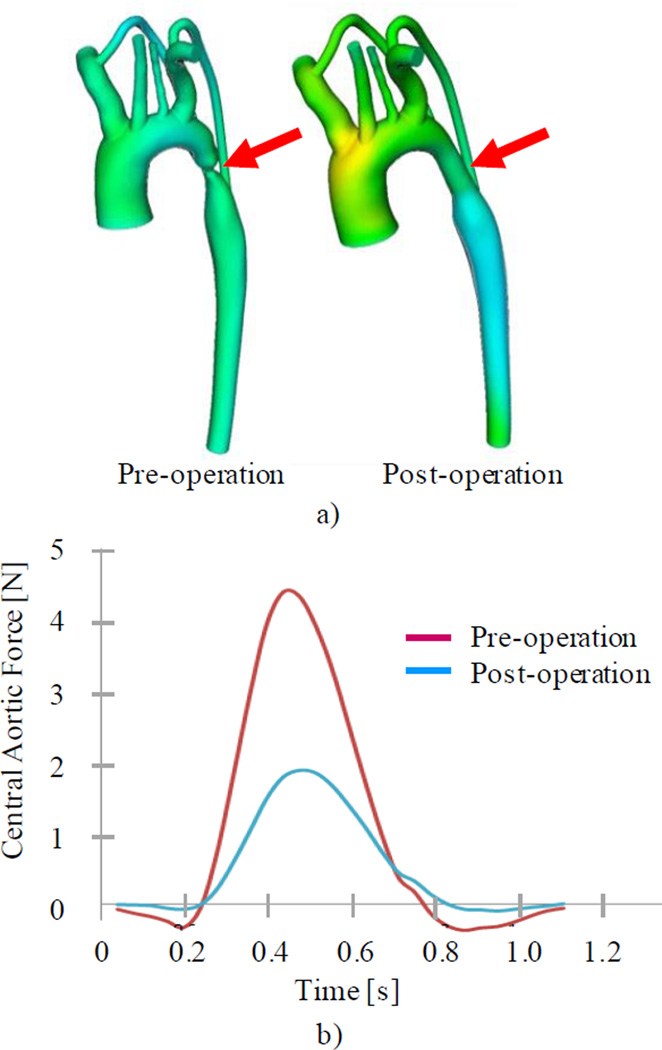
In collaboration with the Cardiovascular Biomechanics Research Laboratory at Stanford University, we started using Computational Fluid Dynamics (CFD) to quantitatively relate BCG signals to hemodynamics. Using Computer Tomography (CT) models of aortas (where it is believed most of the force related to J wave is generated), we computed the forces at the fluid-solid interface [10]. These forces would be transferred to the whole body through the tight coupling of the aorta to the spine. An example of simulation for a case of aortic coarctation is presented in Figure 3. Of particular relevance is the two-fold decrease in generated force (projection over the longitudinal axis), and the magnitude of the force post-operation – approximately 2 N – similar to normal, measured BCGs. This model is still limited, but points to a novel research direction that can potentially augment the understanding of the BCG signals. We are now looking to extend this model to include lower limbs (in order to provide adequate simulation of pulse wave reflections), and a realistic coupling to body tissues, which will help modeling the mass-spring-damper response of the whole body.
Fig. 3.
a) Coarctation model from CT scans (red arrows show site of coarctation), and b) CFD simulations of force resulting from fluid-solid interaction in the longitudinal direction, pre- and post-operation. The two-fold reduction in force is consistent with experimental data from [11].
III. Measurement Methods
For the larger part of the active period of BCG research, the measurements were dominated by three types of systems – the Starr BCG (high-frequency BCG), the Nickerson BCG (ultra-low frequency BCG), and the Dock BCG (direct-body BCG). Characteristics of these methods can be found in Scarborough [2]. Each of these systems, although standardized, generated different BCG morphologies. This lack of consistency in signal certainly contributed too to the low penetration of BCG into the clinic, as interpretation was slightly different. Signal processing was also rudimentary, only making use of computer methods starting in the 70’s. It is thus fair to say that the major developments in the field of BCG in the past decade have come mostly from the technology side. Novel sensing modalities, such as static charge-sensitive beds [12], piezoelectric films on chairs and beds [13, 14], force-plates [15], have considerably simplified the measurement of BCG signals. Inspired by Williams [16], our group has pioneered the use of modified weighing scales and developed several methods for the acquisition of high fidelity standing or sitting BCGs [17–19].
These technological developments have made the BCG much more accessible and enabled its use in applications previously impossible (e.g. monitoring at home). However, it should be noted that many of these approaches lead to non-standard BCG (in the definition of Scarborough) due to the sensing modality, direction and location, or the coupling to the subject. For instance, bed-based systems measuring the dorso-ventral BCG (different from the more traditional longitudinal BCG [20]), may also sense components related to local heart motion (as seen in seismocardiogram or apexcardiogram). Cross-axis coupling through the mattress or sensor may further complicate the signal [14]. This may not impact all applications, but must be acknowledged, for the signals may be misinterpreted.
IV. Applications
A. Diagnostics versus monitoring
As highlighted in the introduction, a majority of the BCG work in the early days was focused on the diagnosis [3, 4], or even prognosis [21, 22] of cardiovascular diseases. Such applications typically require a high sensitivity and specificity which has not been achieved with BCG, owing largely to its complex origin and confounding factors. Age for instance, has been shown to reduce the diagnostic value for coronary heart disease in older people, as changes in the BCG observed with aging in healthy, asymptomatic people tend to be similar to those due to coronary disease [23, 7].
Of particular interest are studies involving monitoring changes in the BCG over time or following a stress (exercise [24], drugs [25]). While the integral aspect of the BCG precludes a specific anatomical diagnosis, the signal remains very sensitive to disturbances of factors impacting cardiac dynamics [20]. For instance, Mandelbaum followed post-myocardial infarction patients over 18 months and found the serial BCG a valuable indicator of prognosis for recovery [22]. As a relative measure, much of the inter-patient variability in the BCG is indeed removed. The impact of the measurement system is also reduced. We would argue that these types of trending approaches, paired with an evaluation of primary features in the BCG (such as J or IJ amplitude, broadly accepted as reflecting ventricular ejection), might offer a realistic goal. Monitoring of cardiac ejection in diagnosed heart failure patients, during exercise stress or cardiac resynchronization therapy optimization are good examples of such applications, as illustrated below. The current availability of unobtrusive sensors also open the door to many non-contact, heart rate-based applications [14, 15], relaxing greatly constrains on fidelity and interpretation of the BCG.
B. Example application – CRT device optimization
Cardiac resynchronization therapy (CRT) has been developed to treat for abnormal delays in contraction between the two ventricles, typically occurring in case of advanced heart failure. Using a biventricular pacemaker, the timing between the two ventricles and the atria can be controlled. However, the atrio-ventricular (AV) and inter-ventricular (VV) delays corresponding to an optimal cardiac function (or cardiac ejection) will vary from patient to patient. This optimization has relied so far mostly on the experience of the cardiologist, and echocardiography measurements. It has been shown recently though that even echocardiographic data, thought to be the non-invasive ‘gold-standard’, does not lead to reliable tuning [26].
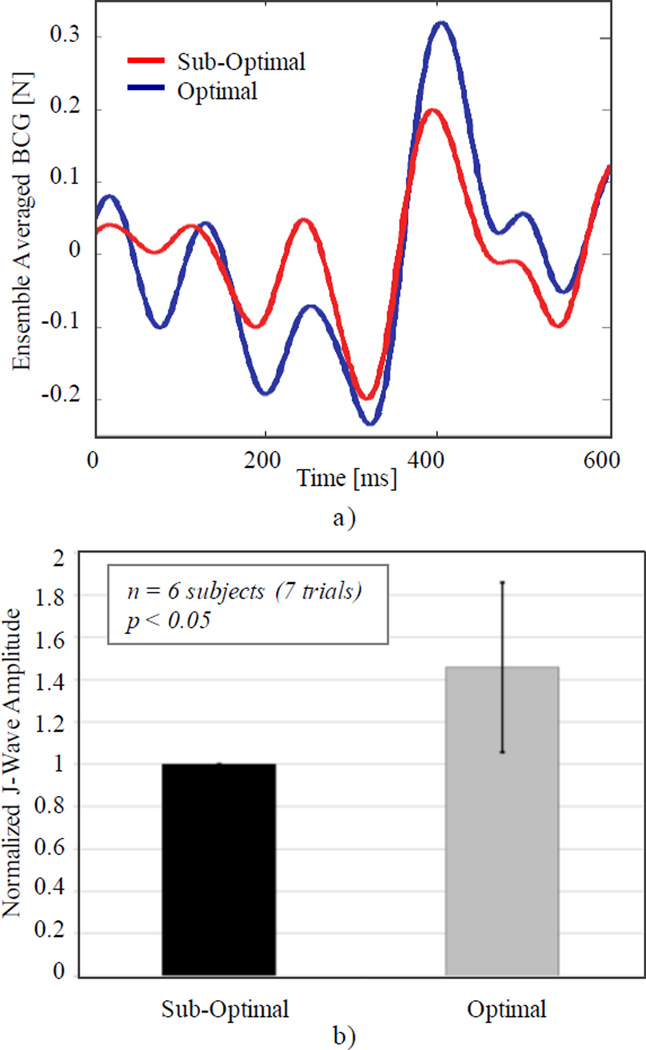
We thus explored the use of BCG to guide this optimization. BCG is a well-accepted measure of ejection force (especially its IJ component), and the differential measurement pre- and post-tuning provides a strong immunity to confounding variables and a weak dependence on absolute values. For these preliminary experiments, we used a sitting BCG configuration, in which the same scale as described in [17] was placed between the seat and the patient. Recordings were carried out throughout the normal optimization session (under IRB-approved protocol #10342), and data was analyzed according to [17]. Figure 4 shows the relative change in J-wave amplitude for optimal VV delays (as previously established by the cardiologist) and sub-optimal positive VV delays (ranging from +50 to +200 ms depending on the patient). The higher J-wave amplitudes for optimal delays would indicate an increased cardiac ejection compared to suboptimal settings. Even with a small number of subjects, statistical significance was reached, demonstrating the strong potential of BCG measurements in well-controlled, differential or longitudinal applications.
Fig. 4.
Optimization of inter-ventricular delays (VV) in CRT: a) ensemble averaged sitting BCG waveforms for optimal and sub-optimal delays; b) comparison of J-wave amplitudes (related to cardiac ejection) for optimal VV delays (based on prior optimization) and sub-optimal positive delays (normalized, mean±std, paired t-test).
V. CONCLUSION
Despite the elapsed time, the BCG of today seems to face many of the same challenges that brought it down in the 70’s: a multitude of measurement methods, a lack of standardization, and a lack of deep understanding of the physiological basis. In addition, the researchers must fight the somewhat negative image that its previous demise left in the medical field. So is BCG worth revisiting? We argue that a positive answer lies in the choice of applications of the BCG. Modern technology enable the use of BCG in places not reachable previously – the cardiologist’s office, the home. But more importantly, a case is made for applications requiring basic analysis of the signal (as for heart rate, or cardiac ejection), and based on serial measurements. CRT device optimization was given as an illustration of one of these applications.
In parallel, further research in the modeling and signal interpretation enabled by modern computing should be pursued, and one day may enable the BCG to be the diagnostic tool long-sought after in the early years.
Acknowledgment
The authors thank Dr. R. Hardwin Mead, M.D., for his support in collecting the CRT data, Drs. A. Figueroa and C. Taylor from the Cardiovascular Biomechanics Lab for their help with the modeling data. The modeling was supported by the National Institutes of Health (U54 GM072970) and the National Science Foundation (0205741).
Contributor Information
Laurent Giovangrandi, Email: giovan@stanford.edu, Electrical Engineering Department, Stanford University, Stanford, CA 94305 USA..
Omer T. Inan, Electrical Engineering Department, Stanford University, Stanford, CA 94305 USA..
Richard M. Wiard, Bioengineering Department, Stanford University, Stanford, CA 94305 USA..
Mozziyar Etemadi, Division of Pediatric Surgery, University of California San Francisco, San Francisco, CA, USA..
Gregory T.A. Kovacs, Electrical Engineering Department and Department of Medicine, Stanford University, Stanford, CA 94305 USA..
References
- 1.Gordon JW. On certain molar movements of the human body produced by the circulation of the blood. J. Anat. Phys. 1877;11:533–536. [PMC free article] [PubMed] [Google Scholar]
- 2.Scarborough WR. Proposals for Ballistocardiographic Nomenclature and Conventions: Revised and Extended: Report of Committee on Ballistocardiographic Terminology. Circulation. 1956;14:435–450. doi: 10.1161/01.cir.14.3.435. [DOI] [PubMed] [Google Scholar]
- 3.Starr I, Wood FC. Studies with the ballistocardiograph in acute cardiac infarction and chronic angina pectoris. Am. Heart J. 1943;25:81–101. [Google Scholar]
- 4.Mandelbaum H, Mandelbaum RA. Studies utilizing the portable electromagnetic ballistocardiograph. I. Abnormal HIJK patterns in hypertensive and coronary artery heart disease. Circulation. 1951;3(5):663–673. doi: 10.1161/01.cir.3.5.663. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham W, Sutton GC, Rondinelli R, Sutton DC. Interpretation of the velocity measurement ballistocardiogram. Am. Heart J. 1953;46(3):341–347. doi: 10.1016/0002-8703(53)90325-1. [DOI] [PubMed] [Google Scholar]
- 6.Noordergraaf A, Heynekamp ChE. Genesis of the human longitudinal ballistocardiogram from the changing blood distribution. Am. J. Cardiol. 1958;2:748. doi: 10.1016/0002-9149(58)90272-8. [DOI] [PubMed] [Google Scholar]
- 7.Gubner RS, Rodstein M, Ungerleider HE. Ballistocardiography: An Appraisal of Technic, Physiologic Principles, and Clinical Value. Circulation. 1953;7:268–286. doi: 10.1161/01.cir.7.2.268. [DOI] [PubMed] [Google Scholar]
- 8.Starr I. Progress towards a Physiological Cardiology. Ann. Int. Med. 1965;63(6):1079–1105. doi: 10.7326/0003-4819-63-6-1079. [DOI] [PubMed] [Google Scholar]
- 9.Elzinga G, Westerhof N, van den Bos G, Verdouw P. Transfer of Cardiovascular Forces Through the Body. Med. Bio. Eng. and Comp. 1974;12:322–327. doi: 10.1007/BF02477799. [DOI] [PubMed] [Google Scholar]
- 10.Wiard RM, Kim HJ, Figueroa C, Kovacs GTA, Taylor CA, Giovangrandi L. Estimation of central aortic forces in the ballistocardiogram under rest and exercise conditions. Conf. Proc. IEEE Eng. Med. Biol. Soc. (EMBC) 2009;1:2831–2834. doi: 10.1109/IEMBS.2009.5333577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickerson JL, Humphreys GH, Deterling RA, Fleming TC, Mathers LJA. Diagnosis of Coarctation of the Aorta with the Aid of the Low Frequency, Critically Damped Ballistocardiograph. Circulation. 1950;1:1032–1036. [Google Scholar]
- 12.Alihanka J, Vaahtoranta K, Saarikivi I. A new method for long-term monitoring of the ballistocardiogram, heart rate, and respiration. Am. J. Physiol. 1981;240(5):R384–R392. doi: 10.1152/ajpregu.1981.240.5.R384. [DOI] [PubMed] [Google Scholar]
- 13.Alametsä J, Värri A, Koivuluoma M, Barna L. 2nd OpenECG Workshop "Integration of the ECG into the EHR & Interoperability of ECG Device Systems". Berlin, Germany: 2004. Apr, The Potential of EMFi Sensors in Heart Activity Monitoring; pp. 1–3. [Google Scholar]
- 14.Bruser C, Stadlthanner K, de Waele S, Leonhardt S. Adaptive Beat-to-Beat Heart Rate Estimation in Ballistocardiograms. IEEE TITB. 2011 doi: 10.1109/TITB.2011.2128337. in press. [DOI] [PubMed] [Google Scholar]
- 15.Chung GS, Lee JS, Hwang SH, Lim YK, Jeong D-U, Park KS. Wakefulness estimation only using ballistocardiogram: Nonintrusive method for sleep monitoring. Conf. Proc. IEEE Eng. Med. Biol. Soc. (EMBC) 2010:2459–2462. doi: 10.1109/IEMBS.2010.5626544. [DOI] [PubMed] [Google Scholar]
- 16.Williams J. Bridge Circuits: Marrying Gain and Balance. Linear Technology, Application Note. 1990;43:8–9. [Google Scholar]
- 17.Inan OT, Etemadi M, Wiard RM, Giovangrandi L, Kovacs GTA. Robust Ballistocardiogram Acquisition for Home Monitoring. Physiological Measurements. 2009;30:169–185. doi: 10.1088/0967-3334/30/2/005. [DOI] [PubMed] [Google Scholar]
- 18.Inan OT, Etemadi M, Wiard RM, Kovacs GTA, Giovangrandi L. Novel methods for estimating the ballistocardiogram signal using a simultaneously acquired electrocardiogram. Conf. Proc. IEEE Eng. Med. Biol. Soc. (EMBC) 2009;1:5344–5347. doi: 10.1109/IEMBS.2009.5333709. [DOI] [PubMed] [Google Scholar]
- 19.Wiard RM, Inan OT, Argyres B, Etemadi M, Kovacs GTA, Giovangrandi L. Automatic Detection of Motion Artifacts in the Ballistocardiogram Measured on a Modified Bathroom Scale. Med. Bio. Eng. and Comp. 2011;49(2):213–220. doi: 10.1007/s11517-010-0722-y. [DOI] [PubMed] [Google Scholar]
- 20.Soames RW, Atha J. Three-dimensional ballistocardiographic responses to changes of posture. Clin. Phys. Physiol. Meas. 1982;3(3):169–177. doi: 10.1088/0143-0815/3/3/001. [DOI] [PubMed] [Google Scholar]
- 21.Starr I. Prognostic Value of Ballistocardiograms. JAMA. 1964;187(7):511–517. [PubMed] [Google Scholar]
- 22.Mandelbaum H, Mandelbaum RA. Studies utilizing the portable electromagnetic ballistocardiograph. IV. The clinical significance of serial ballistocardiograms following acute myocardial infarction. Circulation. 1953;7(6):910–915. doi: 10.1161/01.cir.7.6.910. [DOI] [PubMed] [Google Scholar]
- 23.Goedhard WJA. Ballistocardiography: Past, Present and Future. Bibl. Cardiol. 1979;37:27–45. [PubMed] [Google Scholar]
- 24.Mackinson DH. Changes in the Ballistocardiogram after Exercise in Normal and Abnormal. Circulation. 1950;2:186–196. doi: 10.1161/01.cir.2.2.186. [DOI] [PubMed] [Google Scholar]
- 25.Mandelbaum H, Mandelbaum RA. Studies utilizing the portable electromagnetic ballistocardiograph. II. The Ballistocardiogram as a Means of Determining Nicotine Sensitivity. Circulation. 1952;5(6):885–891. doi: 10.1161/01.cir.5.6.885. [DOI] [PubMed] [Google Scholar]
- 26.Turcott RG, Witteles RM, Wang PJ, Vagelos RH, Fowler MB, Ashley EA. Measurement Precision in the Optimization of Cardiac Resynchronization Therapy. Circulation: Heart Failure. 2010;3:395–404. doi: 10.1161/CIRCHEARTFAILURE.109.900076. [DOI] [PMC free article] [PubMed] [Google Scholar]