Abstract
L-012, a luminol-based chemiluminescent (CL) probe, is widely used in vitro and in vivo to detect NADPH oxidase (Nox)-derived superoxide (O2·−) and identify Nox inhibitors. Yet understanding of the free radical chemistry of L-012 probe is still lacking. We report that peroxidase and H2O2 induce superoxide dismutase (SOD)-sensitive, L-012-derived CL in the presence of oxygen. O2·− alone does not react with L-012 to emit luminescence. Self-generated O2·− during oxidation of L-012 and luminol-analogs artifactually induce CL inhibitable by SOD. These aspects make assays based on luminol analogs less than ideal for specific detection and identification of O2·− and NOX inhibitors.
Keywords: Superoxide radical anion, luminescence, NADPH oxidase, redox cycling, L-012, luminol, redox probe
Introduction
L-012 (8-amino-5-chloro-7-phenyl-pyrido[3,4-d]pyridazine-1,4(2H,3H)dione) (Fig. 1A) is a luminol-based molecule that has been reported to produce much stronger chemiluminescence (CL) than other CL probes (lucigenin, luminol, and MCLA) (1,2). Thus, L-012 is rapidly emerging as a popular CL probe for measuring superoxide (O2·−) and other reactive oxygen species (ROS) derived particularly from NADPH oxidases (Nox) (3–6). In an earlier report, investigators concluded that unlike other CL probes, L-012 is not subject to redox cycling and is, therefore, reliable for detecting Nox-derived O2·− inhibitors in high throughput screening (HTS) assays (7). More recently, L-012 was used to noninvasively image ROS and reactive nitrogen species (RNS) in living mice under pro-inflammatory conditions (8,9). Despite its increasing use in vitro and in vivo, rigorous understanding of the free radical chemistry of L-012 has been lacking. Screening of small-molecule inhibitors of Nox, using a medium throughput L-012-based assay, revealed a high rate of false positives that included inhibitors of myeloperoxidase (Lambeth JD, unpublished data). Clearly, the current situation with regard to L-012 use in free radical biology is murky and confusing. The objectives of the present study are to assess the mechanism(s) by which chemiluminescence arises from ROS-induced oxidation of L-012, and to determine its suitability as a potential CL probe in O2·− detection and HTS assays for Nox inhibitors. Results from the present study indicate that both the peroxidase activity and L-012-derived O2·− are responsible for the overall luminescent signal intensity. Implications of the present findings are discussed with regard to the use of L-012 and other CL probes in HTS-based identification of Nox inhibitors.
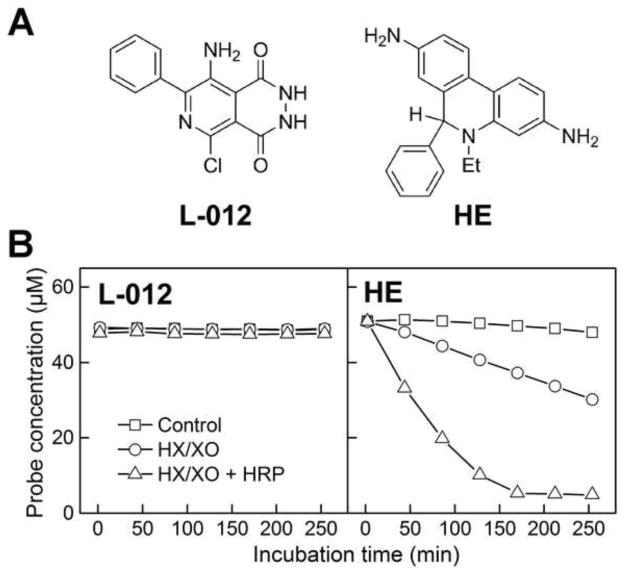
Figure 1.
Superoxide radical anion and HRP-dependent kinetics of L-012 and HE consumption. (a) Chemical structures of L-012 and HE probes. (b) The concentration of L-012 and HE was monitored by HPLC. Reaction mixtures contained L-012 (50 μM) or HE (50 μM) in phosphate buffer (pH 7.4, 50 mM) containing dtpa (100 μM), HX (200 μM) and XO (1 mU/ml). Where indicated, HRP (1 mU/ml) was present. Samples were placed in an HPLC autosampler thermostated at 25°C and repeatedly injected over a 4 h incubation time.
Materials and Methods
Chemicals
L-012 probe was from Wako Chemicals USA, Inc. and hydroethidine was from Invitrogen. Horseradish peroxidase (Type VI) and all other reagents were from Sigma-Aldrich. L-012 stock solution (20 mM) was prepared in water and HE stock solution (20 mM) was prepared in DMSO.
All reactions were carried out in aqueous phosphate buffer (50 mM) containing dtpa (0.1 mM). The flux of O2·− generated from xanthine oxidase in the presence of hypoxanthine was determined based on the rate of reduction of ferricytochrome c, as described previously (10). 1 mU/ml of xanthine oxidase corresponded to 0.35 μM/min of O2·−.
Luminescence studies
Plate reader-based luminescence measurements were performed in white 96-well plates using Beckman-Coulter DTX880 multimode plate reader, with temperature set at 37 °C. For the investigation of the effect of oxygen and sequential addition of reactants, Perkin Elmer LS 55 luminescence spectrometer was used. The solution was placed in a 3 mL fluorescence cell equipped with magnetic stirrer and thermostated at 37 °C. To test the effect of oxygenation/deoxygenation the screw cap, gas-tight fluorescence cell (Starna) was used, with continuous passing of appropriate gas (O2 for oxygenation and Ar for deoxygenation) through the solution.
HPLC measurements
HPLC analysis of HE oxidation was performed as recently reported (10) using Agilent 1100 HPLC system and C18 column (Phenomenex, Kinetex C18, 100 mm × 4.6 mm, 2.6 μm). Similar setup was used for L-012 detection, but gradient elution was based on an increase in acetonitrile fraction from 10% to 60% over 5 min and L-012 probe was monitored using absorption detector set at 400 nm.
Results
Superoxide-dependent oxidation of L-012 and HE: The effect of peroxidase
To optimize the experimental conditions for detecting O2·− and peroxidase-mediated L-012-derived chemiluminescence, we initially used the reaction conditions established for the fluorescent dye hydroethidine (HE). Figure 1 shows the relative rates of oxidation of L-012 and HE in the presence of O2·− alone and in combination with horseradish peroxidase (HRP). The parent compounds and products were analyzed by HPLC. As compared to HE, which was significantly consumed in the presence of O2·− (0.35 μM/min) with the concomitant formation of a diagnostic product, 2-hydroxyethidium (11), there was no O2·−-induced consumption or formation of product from L-012. Surprisingly, even under conditions generating 0.35 μM/min flux of O2·− in the presence of low concentration of HRP (1 mU/ml), L-012 consumption was negligible (Fig. 1b). In contrast, under the same conditions, HE oxidation was nearly 100% complete (Fig. 1b) with the formation additional oxidation products including ethidium cation and dimers (12). These findings indicate that, in contrast to HE, L-012 does not react with O2·− and that, unlike HE, L-012 is a relatively weak substrate for peroxidase. Thus, substantially higher levels of oxidant fluxes and probe concentrations are required to detect luminescence from L-012.
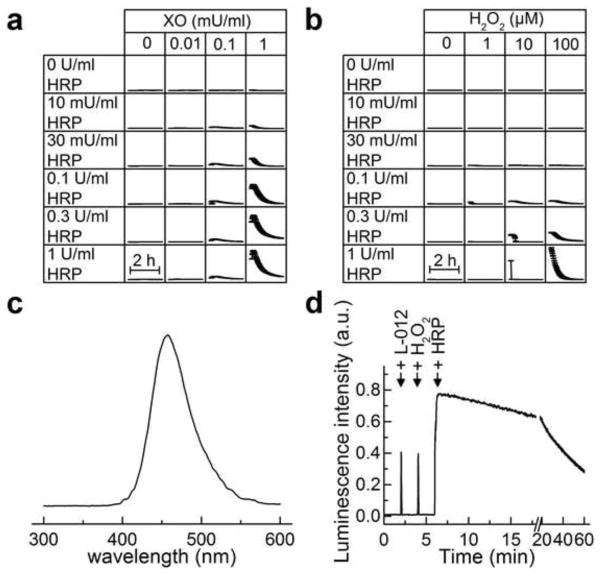
To this end, we monitored the kinetics of L-012-derived luminescence using a 96-well plate under varying O2·− and H2O2 fluxes as controlled by xanthine oxidase (XO) levels and different concentrations of HRP (Fig. 2a). As shown, high concentrations of XO and HRP were required to observe the luminescence signal. Similar luminescence kinetic profiles were observed from L-012 in the presence of HRP and H2O2 (Fig. 2b). The luminescence spectrum obtained during aerobic oxidation of L-012 by HRP/H2O2 or HX/XO/HRP is shown in Figure 2c. From measuring the dynamics of luminescence, we optimized the conditions under which the steady-state luminescence is maintained for 10–15 min. Figure 2d shows the changes in the luminescence intensity during successive addition of L-012, H2O2, and HRP to incubations in phosphate buffer at 37 °C. In subsequent experiments, we used these conditions for determining the effects of additives on L-012-derived luminescence in HX/XO and H2O2 plus HRP systems.
Figure 2.
Comparison of the kinetics of luminescence formed from L-012. (a) Incubations contained L-012 (50 μM) in phosphate buffer (pH 7.4, 50 mM) containing HX (200 μM), dtpa (100μM), and XO ± HRP at concentrations indicated. (b) Incubations contained L-012 (50 μM) in the presence of different concentrations of HRP and H2O2 (at the indicated concentrations) in phosphate buffer (pH 7.4, 50 mM) containing dtpa (100 μM). Reactions were carried out in a 96-well plate thermostated at 37°C and the luminescence monitored with time for 2 h. The data points represent the mean values of three replicates and the error bars represent standard deviation. (c) Luminescence spectrum recorded during oxidation of L-012 in H2O2/HRP system. (d) Changes in luminescence intensity during consecutive addition of reactants as follows: L-012 (100 μM), H2O2 (50 μM) and HRP (0.1 U/ml).
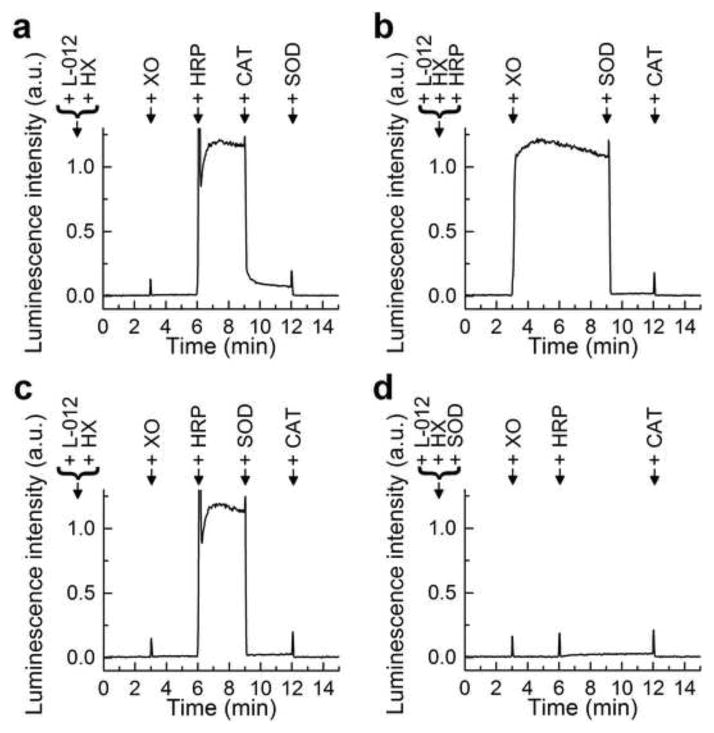
As shown in Figure 3a, when added sequentially, the presence of both O2·−/H2O2-generating system (HX + XO) and HRP are required for L-012-derived luminescence. The addition of catalase (CAT) inhibited HX/XO/HRP/L-012 luminescence by over 90%, indicating the requirement of H2O2 generated from HX/XO (Fig. 3a). The addition of superoxide dismutase (SOD) further decreased luminescence to the basal level (Fig. 3a). Next we added SOD prior to adding CAT to incubations containing L-012, HRP, and HX/XO and SOD almost completely inhibited the luminescence intensity (Fig. 3b and d). The order of addition of XO and HRP is different in Figure 3b and d, with the results indicating the requirement of both enzymes to obtain the luminescence signal. Inclusion of SOD at the beginning of incubation completely suppressed the luminescence from L-012, HRP, and HX/X system. These results suggest that O2·− is also critical for HRP/HX/XO/L-012-derived luminescence (Fig. 3d), with a small signal attributable to H2O2-dependent, and O2·−-independent pathway.
Figure 3.
The dynamics of generation of luminescence from L-012 probe. (a–d) L-012 (100 μM) was incubated under aerobic conditions in phosphate buffer (50 mM, pH 7.4) containing dtpa (100 μM) and HX (250 μM) at 37°C. Where indicated, XO (1 mU/ml), HRP (0.1 U/ml), SOD (50 μg/ml) or CAT (3 kU/ml) were added during reaction monitoring.
Peroxidase activity mediated L-012 chemiluminescence: The effect of molecular oxygen
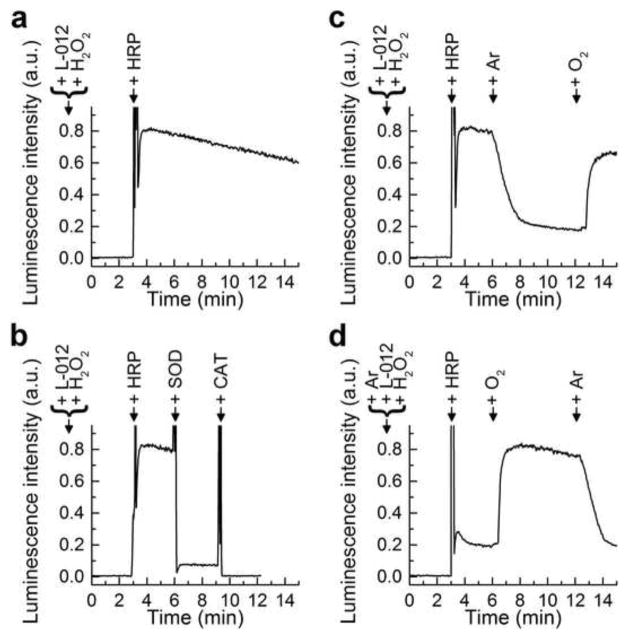
To determine whether O2·− is involved in HRP/H2O2/L-012-derived luminescence, we investigated the effects of SOD and deoxygenation/oxygenation on luminescence signal intensity. The kinetics of luminescence formed under aerobic conditions from the sequential additions of L-012, H2O2, and HRP is shown in Figure 4a. This signal was drastically inhibited following SOD addition, with the remaining signal inhibitable by catalase (Fig. 4b). This implicates O2·− formation during oxidation of L-012 by HRP and H2O2. To test this hypothesis, we added HRP to aerobic and argon-purged incubations containing L-012 and H2O2 (Fig. 4c and d). The luminescence derived from L-012/HRP/H2O2 system in argon was greatly inhibited indicating that activation of molecular oxygen to O2·− possibly occurs during oxidation of L-012 by HRP and H2O2. We then investigated the effect of deoxygenation and oxygenation by switching between argon to oxygen and vice versa in a gas-tight cuvette (Fig. 4c and d). Deoxygenation by passing argon gas through the solution rapidly decreased luminescence, and reoxygenation by switching to oxygen gas increased the luminescence signal intensity. This increase in signal intensity was again inhibited by SOD and/or CAT (not shown).
Figure 4.
The dynamics of generation of luminescence from L-012 probe during deoxygenation and reoxygenation. (a–d) L-012 (100 μM) was incubated in aqueous solution of phosphate buffer (50 mM, pH 7.4) containing dtpa (100 μM) and H2O2 (50 μM) at 37°C. Where indicated, HRP (0.1 U/ml), SOD (50 μg/ml) or CAT (3 kU/ml) were added during reaction monitoring. In (d), sample was deoxygenated by passing argon gas through the solution before and during the reaction. In (c) and (d), Ar and O2 denote time points at which purging of argon gas and oxygen gas was started.
Discussion
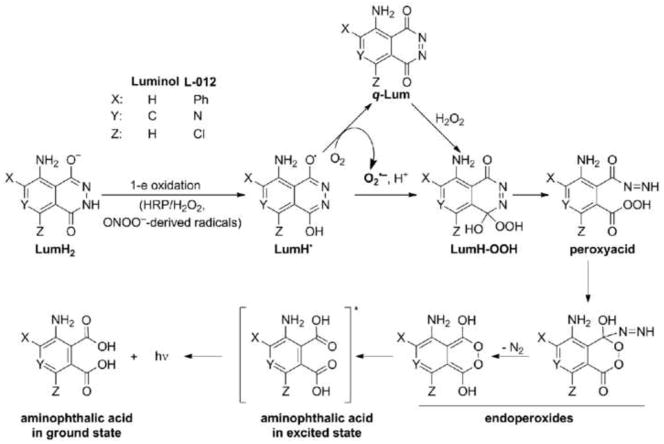
L-012, a chemical analog of luminol, gives rise to significantly higher luminescence yield and increased sensitivity as compared to other CL probes, lucigenin and MCLA (1,2). Based on the present results, we have modified the previous mechanism proposed for luminol (13,14). The modified mechanism for L-012-derived chemiluminescence in the presence of reactive oxygen and nitrogen species and peroxidase is shown in Figure 5. Key modifications included in the mechanism are as follows: (i) L-012 (LumH2) does not appreciably react with O2·− but needs to be oxidized via a one-electron oxidation to the corresponding L-012-derived radical (LumH·); (ii) The LumH· radical reacts with O2·− to generate O2·− and L-012 quinone (q-Lum); (iii) O2·− reacts with the LumH· radical leading to the formation of an endoperoxide that decomposes to form an excited state intermediate that emits luminescence. In the presence of SOD, luminescence is inhibited due to a rapid dismutation of O2·− and to the quenching of the reaction between O2·− and LumH· radical; and (iv) a small fraction of the luminescent signal that is SOD-independent likely originates from H2O2 reaction with q-Lum. This can be eliminated by using a higher amount of CAT during oxidation of L-012 by H2O2 and HRP. Addition of ethyl boronic acid (a scavenger of hydroperoxides and peroxynitrite) (15,16) significantly inhibited L-012 chemiluminescence (not shown). As boronic acids are unlikely to efficiently scavenge endoperoxides, this is tentatively attributed to the rapid reaction between the L-012 peracid intermediate (Fig. 5) and ethyl boronate.
Figure 5.
A modified mechanism proposed for luminescence formation during oxidation of luminol-based probes.
Based on the modified mechanistic scheme for L-012-derived chemiluminescence, it is evident that peroxidase substrates (e.g., apocynin, diapocynin, phenothiazines) will significantly inhibit luminescence by decreasing formation of L-012-derived peroxide and endoperoxide intermediates, and ultimately attenuating luminescence (17). Published results suggest that L-012 is not a very efficient peroxidase substrate, as typically much higher concentrations of L-012 have been used in previous studies (1–6). Thus, as indicated earlier the use of L-012 probe in HTS assay for determination of Nox inhibitors fails to identify true “hits”, generating a high rate of false positives via the competitive inhibition of peroxidase mechanism (18). In conclusion, the present results indicate that O2·− produced from molecular oxygen during oxidation of L-012 by H2O2/HRP is responsible for the majority of the luminescence signal. The inhibition of L-012-derived luminescence signal by SOD may lead to a false conclusion on the identity of the species responsible for the probe oxidation. The L-012/HRP-based HTS assay is not optimal for identifying Nox inhibitors because of the involvement of the peroxidatic mechanism in L-012-derived luminescence and the lack of a direct reaction between the probe and O2·−.
Highlights.
Peroxidase and H2O2 induces O2-dependent, SOD-inhibitable luminescence from L-012
In the absence of peroxidase, O2·− alone does not cause L-012 luminescence
Luminol/HRP based HTS assay are not optimal for identifying Nox inhibitors
Acknowledgments
This work was supported by NIH grant R01 HL063119 (to B.K.). A support from a grant coordinated by JCET, No. POIG.01.01.02-00-069/09 (supported by the European Union from the resources of the European Regional Development Fund under the Innovative Economy Programme) is acknowledged. The authors thank Mrs. Monika Zielonka for performing the plate reader-based experiments.
Abbreviations
- CAT
catalase
- CL
chemiluminescence
- dtpa
diethylenetriaminepentaacetate
- H2O2
hydrogen peroxide
- HE
hydroethidine
- HRP
horseradish peroxidase
- HX
hypoxanthine
- L-012
8-amino-5-chloro-7-phenyl-pyrido[3,4-d]pyridazine-1,4(2H,3H)dione
- Nox
NADPH oxidases
- O2·−
superoxide radical anion
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- XO
xanthine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun. 1993;193:554–549. doi: 10.1006/bbrc.1993.1659. [DOI] [PubMed] [Google Scholar]
- 2.Sohn HY, Gloe T, Keller M, Schoenafinger K, Pohl U. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J Vasc Res. 1999;36:456–464. doi: 10.1159/000025688. [DOI] [PubMed] [Google Scholar]
- 3.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 5.Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, Kleschyov AL, Munzel T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004;38:259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- 6.Ichibangase T, Ohba Y, Kishikawa N, Nakashima K, Kuroda N. Evaluation of lophine derivatives as L-012 (luminol analog)-dependent chemiluminescence enhancers for measuring horseradish peroxidase and H2O2. Luminescence. 2013 Apr 30; doi: 10.1002/bio.2513. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Kielland A, Blom T, Nandakumar KS, Holmdahl R, Blomhoff R, Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47:760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Han W, Li H, Segal BH, Blackwell TS. Bioluminescence imaging of NADPH oxidase activity in different animal models. J Vis Exp. 2012;(68) doi: 10.3791/3925. pii: 3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielonka J, Zielonka M, Sikora A, Adamus J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J Biol Chem. 2011;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 12.Zielonka J, Kalyanaraman B. Hydroethidine- and Mito-SOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merényi G, Lind J, Eriksen TE. Luminol chemiluminescence: chemistry, excitation, emitter. J Biolumin Chemilumin. 1990;5:53–56. doi: 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2 - Free Radic Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 15.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol. 2011;24:687–697. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 18.Gianni D, Nicolas N, Zhang H, Der Mardirossian C, Kister J, Martinez L, Ferguson J, Roush WR, Brown SJ, Bokoch GM, Hodder P, Rosen H. Probe Reports from the NIH Molecular Libraries Program [Internet] Vol. 2010. Bethesda (MD): National Center for Biotechnology Information (US); 2010. Oct 13, Optimization and Characterization of an Inhibitor for NADPH Oxidase 1 (NOX-1) updated 2011 Dec 12. [PubMed] [Google Scholar]