Abstract
Type 1 diabetes (T1D) is a chronic disease caused by autoimmune destruction of insulin-producing pancreatic β-cells. T1D is typically diagnosed in children, but information regarding immune cell subsets in juveniles with T1D is scarce. Therefore, we studied various lymphocytic populations found in the peripheral blood of juveniles with T1D compared to age-matched controls (ages 2–17). One population of interest is the CD28− CD8+ T cell subset, which are late-differentiated cells also described as suppressors. These cells are altered in a number of disease states and have been shown to be reduced in adults with T1D. We found that the proportion of CD28− cells within the CD8+ T cell population is significantly reduced in juvenile type 1 diabetics. Furthermore, this reduction is not correlated with age in T1D juveniles, although a significant negative correlation between proportion CD28− CD8+ T cells and age was observed in the healthy controls. Finally, correlation analysis revealed a significant and negative correlation between the proportion of CD28− CD8+ T cells and T1D disease duration. These findings show that the CD28− CD8+ T cell population is perturbed following onset of disease and may prove to be a valuable marker for monitoring the progression of T1D.
Keywords: Juvenile type 1 diabetes, CD28− CD8+ T cells, T suppressor cells
1. Introduction
Type 1 diabetes (T1D) is caused by both environmental and genetic factors and, for unknown reasons, the prevalence of this disease has been rising by 2–5% per year in recent years [1, 2]. Approximately 1.4 million people in the United States have been diagnosed with T1D and roughly 40% of newly diagnosed patients are under the age of 20 [1–4]. T1D is a chronic disease that can cause many health complications, including hypertension, heart and kidney disease, stroke, and blindness [5].
T1D is considered an autoimmune disease, generally characterized by lymphocytic destruction of insulin-producing pancreatic β-cells. Although metabolic stabilization after diagnosis can temporarily increase insulin production, β-cell mass continues to decline and thus necessitates the use of exogenous insulin [6]. The first indication of immune involvement was through the discovery of islet cell autoantibodies [7], including those specific for insulin, glutamic acid decarboxylase (GAD), zinc transporter 8 (ZnT8), islet cell antibody 512 (ICA512), and insulinoma antigen 2 (IA2), among others [8]. Measurement of these markers is useful for diagnosis and also to identify those at risk of developing the disease. Since the initial discovery of autoantibodies, CD4+ and CD8+ T cells, B cells, and macrophages have been observed in infiltrated human islets [9, 10]. Furthermore, increased numbers of B and CD8+ T cells in islets is associated with increased β-cell death [10].
T-cell specificities change over time in T1D progression and, interestingly, changes in the immunodominance of CD8+ T cell responses occur more quickly than changes in autoantibody titers in humans [11]. One CD8+ T cell population of interest in T1D is the CD28− CD8+ T cell subset. This population, which is altered in a number of disease states, including rheumatoid arthritis, multiple sclerosis (MS), multiple myeloma, and following cytomegalovirus (CMV) and human immunodeficiency virus (HIV) infection [12–14], is significantly reduced in adult T1D patients [15].
CD28 is a co-stimulatory receptor required for T cell signaling [16]. Interaction between CD28 and its ligands, B7-1/CD80 and B7-2/CD86, has been shown to be critical for T-cell proliferation and differentiation, maintenance of the regulatory T (Treg) cell population, interaction with B cells, and production of IL-2 [17]. Nearly all CD8+ T cells are CD28+ at birth, but CD28 expression is lost over time such that healthy human adults amass CD28− CD8+ T cells that typically express CD57 [18–21]. This population, which expands in response to changes in Hsp27 and the Fas pathway, is senescent, lacks proliferative capacity, and has shortened telomeres compared to CD28+ cells [13, 14, 22–24]. Indeed, CD28− CD8+ T cells have undergone more rounds of replication than their CD28+ counterparts due to chronic antigen stimulation [12, 24, 25].
CD28− CD8+ T cells are also reportedly T suppressor (TS) cells, now commonly known as Treg cells, which impair the responsiveness of other immune cells [26, 27]. TS cells are typically induced after multiple rounds of stimulation [26]. This subset of T cells can modulate function of antigen-presenting cells (APCs) by enhancing expression of inhibitory receptors or down-regulating expression of co-stimulatory factors on these cells [28, 29]. Furthermore, CD28− CD8+ TS cells can suppress CD4+ T cell proliferation and memory CD4+ T cell responses [28, 30]. In a study of CD28− CD56+ CD8+ T cells from the synovial tissues of rheumatoid arthritis patients, Davila and colleagues found that these cells inhibited production of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and various chemokines, resulting in a robust anti-inflammatory response [30]. CD8+ TS cells have also been shown to dampen the immune response via secretion of interleukin (IL)-6 and IL-10 [31, 32].
As mentioned, CD28− CD8+ T cell expression has been reported to be significantly reduced in adults with T1D [15], suggesting that this population may be involved in the dysregulated autoimmunity seen in these patients. We extended this work by studying various lymphocytic populations found in the peripheral blood of juveniles with T1D compared to age-matched controls (ages 2–17). Similar to what was found in the adult population, children with T1D exhibit significantly reduced CD28− CD8+ T cells when compared to healthy controls. Interestingly, this subset of cells is negatively correlated with age in normal controls but not in type 1 diabetics. However, within the T1D group, the proportion of CD28− CD8+ T cells was negatively associated with disease progression, such that those individuals that had T1D for a greater duration of time had significantly fewer CD28− CD8+ T cells. Overall, our results show that this CD28− CD8+ T cell population is perturbed after T1D onset and thus may prove to be a valuable marker for monitoring the progression of this disease.
2. Materials and Methods
2.1. Patient population and sample processing
Peripheral venous blood was obtained from type 1 diabetics (presence of ≥1 autoantibody), ages 2–16, and healthy, age-matched controls with no history of autoimmune disorders (Table 1). Blood was held overnight (<18hours) in EDTA-coated BD vacutainers. Red blood cells were then lysed using ammonium chloride lysis buffer and white blood cells per milliliter (mL) were calculated using a haemocytometer. Prior to flow cytometric antibody staining, dead cells were labeled using LIVE/DEAD® Fixable Dead Cell Stain (Life Technologies Corporation) per manufacturer’s protocol. Cells were then washed twice with phosphate buffered saline (PBS) and resuspended in a flow cytometry staining buffer (FCSB; PBS, 0.75% bovine serum albumin, 0.05% sodium azide, and 1 mM ethylenediaminetetraacetic acid). Fc receptors were then blocked using irrelevant unlabeled human IgG, and cells were subsequently incubated with antibody cocktails for 30 minutes at 4°C in the dark (for a list of all antibodies used, see Supplementary Table S1). Cells were then washed twice with FCSB and incubated with streptavidin-conjugated fluorochromes in the dark for 20 minutes at 4°C. Following two more washes, cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature in the dark, washed once with FCSB, and finally resuspended in FCSB and analyzed on an LSR II flow cytometer (BD Biosciences) within 24 hours.
Table 1.
Juvenile patient population.
| Control | T1D | NT1D | LT1D | |
|---|---|---|---|---|
| n (n female) | 35 (19) | 31 (12) | 18 (7) | 13 (5) |
| Mean age, years (range) | 10.2 (2–17) | 10.0(2–16) | 9.3 (2–16) | 11.0 (4–16) |
| Mean T1D duration, months (range) | n/a | 14.4 (0–57) | 4.5 (0–11) | 28.1 (14–57) |
| Mean HbA1c at draw (range) | n/a | 8.2 (5.55–15) | 8.7 (5.5–15) | 7.4 (6–9.4) |
2.2. Data analysis and T cell gating
Scatterplots were generated and flow cytometry data was analyzed using FlowJo X (Tree Star, Inc.). In this study, CD8 T cells are defined as mononuclear singlets that are LIVE/DEAD−, CD45+, CD3+, CD4− and CD8+. First, singlets were selected using forward scatter area (FSC-A) versus forward scatter height (FSC-H) parameters. Dead cells were then removed by gating on LIVE/DEAD− events. CD45, a pan-leukocyte marker, was then used to select total leukocytes. We subsequently gated on lymphocytes and monocytes based upon FSC and side scatter (SSC) properties, and identified T cells through expression of the pan-T cell marker CD3. Finally, CD8 T cells were identified as the CD8+, CD4− subset of total T cells.
2.3. Statistics
The percent of parent, percent of CD8 T cells, percent of total T cells, and percent of total leukocytes were calculated using FlowJo X. The number of circulating cells per milliliter was calculated by dividing the percent of total leukocytes by 100, then multiplying this value by the number of total white blood cells per mL as calculated above. The Mann-Whitney U Test was used to test for statistical significance of proportional and numerical differences between subsets for all cohorts. For correlation analysis, immune subset data was first transformed to logarithmic values, and Pearson product-moment correlation coefficient (Pearson’s r) analysis was then performed to test for strength of correlations. Finally, linear regression analysis was performed on our correlations to generate trend lines and to compare correlations between groups. For all statistical tests, results were considered significant when p < 0.05. Statistical tests were performed using SPSS v21 and GraphPad Prism v6 (GraphPad Software), and figures were created using GraphPad Prism v6.
3. Results
3.1. The proportion of CD28− CD8+ T cells within the total CD8+ T cell population is reduced in juvenile type 1 diabetics
Type 1 diabetes mellitus (T1D) is a disease caused by autoimmune-mediated destruction of pancreatic beta cells. CD4, CD8 and natural killer (NK) T cells have all been implicated in the pathogenesis of T1D [33–35], but little is known about these cell populations in juveniles. To this end, we collected peripheral venous blood from 31 juvenile T1D patients (ages 2–16) and 35 healthy, age-matched controls (ages 2–17) (Table 1). We subsequently analyzed peripheral blood mononuclear cells (PBMCs) from these subjects using flow cytometry to characterize differences in leukocyte subsets between the T1D and control populations.
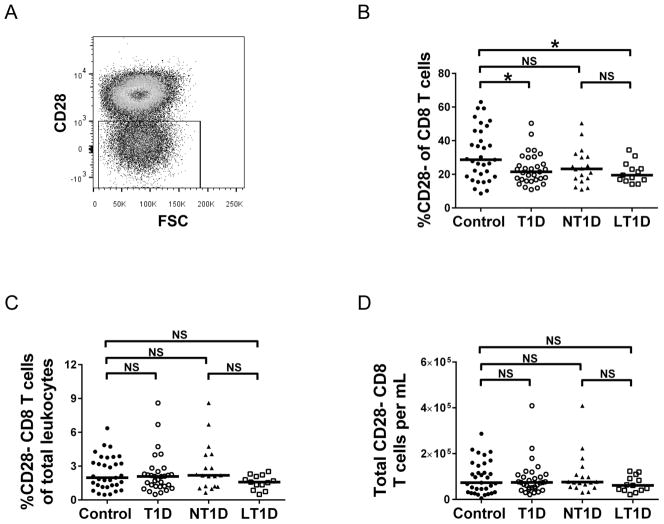
CD28− CD8+ T cells have been reported to be significantly reduced in adult T1D patients [15]. Therefore, we analyzed this CD28− CD8+ T cell population in the PBMCs harvested from juveniles. CD28− cells were gated from the CD8+ T cell population as shown in Fig. 1A. Similar to Mikulkova’s report in adults with T1D [15], a significant decrease (p<0.05 as determined by Mann-Whitney U test) in the percentage of CD28− CD8+ T cells is seen in juvenile patients with T1D compared to healthy controls (Fig. 1B). We further subdivided the T1D population into new-onset type 1 diabetics (NT1D; T1D <1 year) and long-standing type 1 diabetics (LT1D; T1D >1 year – 5 years). Interestingly, a significant decrease in percent CD28− cells of CD8+ T cells was seen in LT1D compared to controls, but not in NT1D compared to controls (Fig. 1B). Furthermore, no significant differences in proportion of CD28− CD8+ T cells of total leukocytes or of CD28− CD8+ T cells per milliliter was found when comparing control to T1D, NT1D and LT1D (Fig. 1C, D). These results indicate that, while the CD28− CD8+ T cell subset is decreased in proportion of CD8+ T cells, other leukocyte populations must also be altered in T1D patients because no difference is seen when looking at the proportion of this population in total leukocytes and in total cells/ml.
Figure 1. CD28− CD8+ T cells are significantly reduced in proportion of CD8 T cells among diabetics.
(A) Representative scatter plot depicting gating of CD28− CD8+ T cells. CD8+ T cells were first selected as described in the Methods section. (B) Percentage CD28− T cells of CD8+ T cells. (C) Percent CD28− CD8+ T cells of total leukocytes. (D). Total CD28− CD8+ T cells per ml. Statistical analysis was performed using the Mann-Whitney U-test; *p<0.05; NS=not significant. T1D=Type 1 Diabetes. NT1D=new-onset T1D. LT1D=long-standing T1D.
3.2. Proportion of CD28− cells of CD8+ T cells is negatively correlated with age among controls but not type 1 diabetics
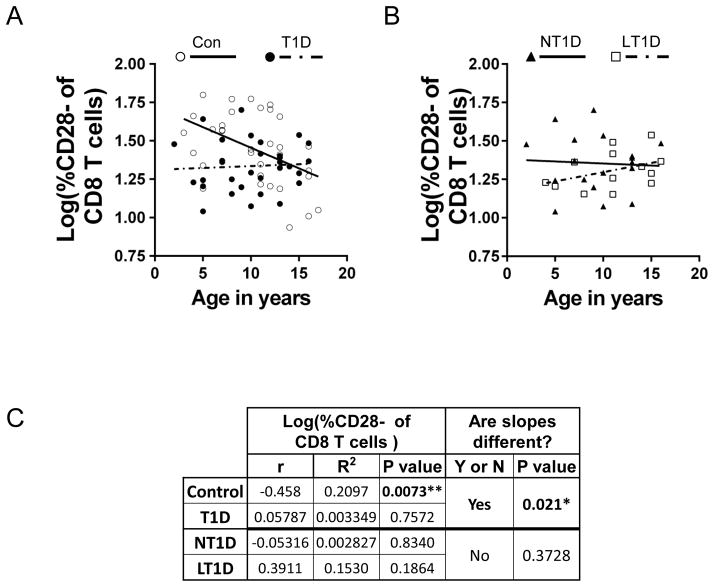
Since a significant reduction in CD28− CD8+ T cells was seen in juvenile T1D patients as a whole and in LT1D subject, but not in NT1D subjects, we next wanted to determine if the proportion of CD28− cells of CD8+ T cells was correlated with age in type 1 diabetics as well as controls. Therefore, linear regression analysis was performed (Fig. 2A) and it was determined that the percent CD28− cells of CD8+ T cells is not correlated with age in juvenile T1D patients (p=0.7572 determined by Pearson’s r analysis, as shown in Fig. 2C). Accordingly, age and proportion CD28− cells of CD8+ cells are also not correlated in NT1D (p=0.8340) and LT1D (p=0.1864) subgroups (Fig. 2B; statistical analysis shown in Fig. 2C). Also, in total T1D, NT1D and LT1D groups, no correlation was established between age and percent CD28− CD8+ T cells of total T cells (Supplementary Fig. S1) or between age and percent CD28− CD8+ T cells of total leukocytes (Supplementary Fig. S2). Interestingly, however, a significant negative correlation between age and percent CD28− cells of CD8+ T cells is seen in the control group (p=0.0073; Fig. 2A, C). The significant correlation between age and percent CD28− CD8+ T cells found in healthy individuals but not in juvenile type 1 diabetics points to a dysfunctional immune system present in these young patients.
Figure 2. Proportion CD28− cells of CD8+ T cells is significantly and negatively correlated with age among controls but not among type 1 diabetics.
(A) Regression line showing correlation between % CD28− of CD8+ T cells and age in controls (Con; open circle, solid line) but not in type 1 diabetics (T1D; filled circle, dashed line). (B) Regression line showing no correlation between % CD28− of CD8+ T cells and age in new-onset type 1 diabetics (NT1D; filled triangle, solid line) or in long-standing type 1 diabetics (LT1D; open square, dashed line). (C) Statistical analysis of regressions shown in A and B using Pearson’s r analysis.
3.3. Proportion of CD28− CD8+ T cells is negatively correlated with T1D disease duration
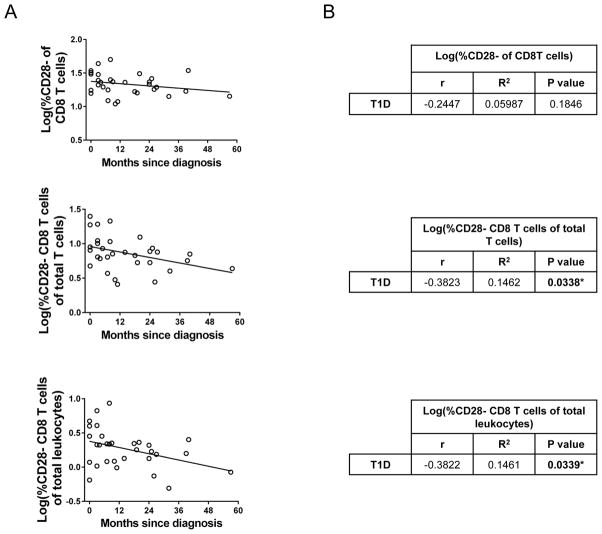
Although there is no correlation between age and proportion of CD28− CD8+ T cells in juveniles with T1D (Fig. 2), there is a significant reduction in this cell population in the LT1D group but not the NT1D group (Fig. 1B). Based on this information, we next performed correlation analyses to determine if the proportion of CD28− CD8+ T cells in T1D patients is related to disease duration. As shown in Fig. 3, there is no correlation between the percentage of CD28− cells of CD8+ T cells and months since diagnosis (p=0.1846). Significantly, however, the percent CD28− CD8+ T cells of total T cells and of total leukocytes is negatively correlated with disease duration (p=0.0338 and 0.0339, respectively; Fig. 3). In other words, the longer the juvenile has had T1D, the fewer CD28− CD8+ T cells that patient will have.
Figure 3. Proportion of CD28− CD8+ T cells is negatively correlated with T1D disease duration.
(A) Regression lines and (B) statistical analysis using Pearson’s r analysis, showing a significant negative correlation between % CD28− CD8+ T cells of total T cells and of total leukocytes and months since diagnosis. *p<0.05.
4. Discussion
T1D is an autoimmune disorder characterized by alterations in immune cell subsets that ultimately leads to the destruction of insulin-producing pancreatic β-cells. Although various immune subsets have been shown to be important for T1D progression, the exact mechanism for the pathogenesis of disease has not been determined in humans, and only few studies have been performed in juveniles. We therefore analyzed peripheral blood lymphocytic cells collected from juveniles with T1D and age-matched controls. Analysis of these cells revealed a perturbation in the CD28− CD8+ T cell subset in the juveniles with T1D, suggesting that this population may be involved in the dysregulated autoimmunity seen in these patients.
In this study, we show that the proportion of CD28− CD8+ T cells is significantly reduced in juvenile T1D patients compared to healthy controls. Similar results have also been reported in adults with T1D [15], as well as in adults with multiple sclerosis, another autoimmune disease [36]. The CD28− CD8+ T cell population is oligoclonal and senescent, having undergone many rounds of replication [13, 23, 24]. These results may seem counterintuitive, then, when considering T1D as an autoimmune disease characterized by chronic antigen stimulation. Indeed, loss of CD28 expression on CD8+ T cells has been observed in other clinical settings where chronic antigen stimulation may occur, such as in rheumatoid arthritis, HIV and CMV infection, and after transplantation [12, 14]. In the case of T1D, however, it is possible that CD8+ T cells are less chronically stimulated in comparison to these other situations. It is also possible that the CD28− CD8+ T cell population may be undergoing apoptosis at a faster rate in type 1 diabetics compared to healthy individuals. In support of this proposition, Glisic-Milosavljevic and colleagues reported a CD4+ Treg population that had significantly elevated levels of apoptosis in T1D patients compared to controls [37]. Other potential explanations are that juveniles with T1D have dysregulated T cell maturation or are responding to disease-specific homeostatic disruption.
Importantly, CD28− CD8+ T cells also have suppressor or regulatory activity (referred to as TS or Treg cells), allowing these cells to repress the function and responsiveness of other immune cells [26–32]. In this regard, it is possible that appropriate levels of this TS cell population are necessary to keep the immune system in check. Other Treg populations may also play a role, and decreased function of CD4+ Treg cells has been reported in pediatric T1D cases [37–39]. Of note, CD28− CD8+ TS cells can be further classified based on surface expression of other markers. CD28− FoxP3+ CD8+ T cells and CD28− CD56+ CD8+ T cells, for example, suppress immune activity through direct cell-to-cell contact [40, 41], whereas CD28− CD45RA+ CCR7− FoxP3− CD8+ T cells mediate their suppressive effects through secretion of soluble factors [31, 42]. A more in-depth study is required to further characterize the CD28− CD8+ T cell population described in this study.
Although there was a significant reduction in the proportion of CD28− cells within the CD8+ T cell subset in type 1 diabetics compared to controls (Fig. 1B), no difference was observed in the proportion of CD28− CD8+ T cells of total leukocytes (Fig. 1C) or in total CD28− CD8+ T cells per ml (Fig. 1D). These results indicate that other subsets of cells within the total leukocyte population of juvenile type 1 diabetics are altered in abundance as well. We are currently analyzing a wide array of CD4+ and CD8+ immune cell subpopulations to provide a clearer picture of what is occurring in the immune system as a whole in juveniles with T1D.
We determined that the proportion of CD28− cells of CD8+ T cells was not correlated with age in juveniles with T1D (Fig. 2A), and this lack of correlation held even when the T1D population was further divided into new-onset T1D (NT1D; T1D<1year) and long-standing T1D (LT1D; T1D>1 year-5 years) (Fig. 2B). Unexpectedly, however, we did find a significant negative correlation between the proportion of CD28− cells of CD8+ T cells and age in the healthy control population. This subset of CD8+ T cells significantly decreases from ages 2 to 17 (Fig. 2C). Others have reported that expansion of CD28− CD8+ T cells is a consequence of aging [18, 20, 43]. Fagnoni and colleagues, for example, reported a significant positive correlation between this T cell population and age in healthy adults ranging from 20 to 105 years of age [18]. The decrease in CD28− CD8+ cells seen when healthy juveniles age compared to the expansion of CD28− CD8+ cells as adults age is intriguing and highlights the fact that scientific observations in adults do not always extend to children. Studies of the CD28− CD8+ T cell population in children have shown that this cell population is not affected by age [44] or that this cell population increases with age [45, 46]. The discrepancy between these different studies and between our current work may perhaps be explained by subtle differences in experimental design, or because of a difference in the population sampled, which may have consisted of different ethnicities or CMV infection rates, for example.
Based on the linear regression analysis to determine if CD28− CD8+ T cell proportions correlate with age (Fig. 2A), the regression lines suggest that young children with T1D (<5 years old) have a lower percentage of CD28− CD8+ T cells to start with, when compared to healthy controls. If very young T1D patients do indeed have a lower percentage of CD28− CD8+ T cells, the data would suggest that these young patients, and perhaps type 1 diabetics in general, have a perturbed immune system from very early on, possibly at birth. These patients may have been under-exposed to normally-encountered environmental pathogens, or have inherent, genetically- or metabolically-derived abnormalities in antigen processing and presentation resulting in insufficient T cell activation. This differential homeostasis may not allow for certain individuals to thwart an autoimmune response, resulting in the pathogenesis of T1D. A larger study in newborns and infants would be required, however, to determine if this theory holds true.
Although no correlation with age and percentage of CD28− CD8+ T cells was observed, the proportion of these cells in type 1 diabetics is negatively correlated with disease duration (Fig. 3). In other words, the percentage of CD28− CD8+ T cells decreases as T1D progresses. It is therefore possible that the proportion of CD28− CD8+ T cells also correlates with C-peptide levels, and future studies will determine if this is indeed the case. Our data show that the proportion of CD28− CD8+ T cells in new-onset T1D juveniles is similar to healthy controls, but significantly decreased in long-standing type 1 diabetics. These results suggest that a reduction in the proportion of CD28− CD8+ T cells may contribute to the progression of T1D after diagnosis. As discussed above, since CD28− CD8+ T cells are late-differentiated, this negative correlation does not seem to support the theory that these T1D patients are undergoing chronic antigen stimulation. However, these results do suggest that since CD28− CD8+ cells have suppressor activity, the longer a juvenile has T1D, the less able that patient’s immune system may be to inhibit any autoimmune response. In the early stages following T1D diagnosis, individuals typically undergo a “honeymoon period,” characterized by a decrease in exogenous insulin requirements due to metabolic stabilization [47]. This transient period is followed by further destruction of pancreatic β-cells and a resultant increased reliance on exogenous insulin. Finding a way to enhance the CD28− CD8+ T cell population in juvenile type 1 diabetics at the early stages of disease, or to preserve this population as the disease progresses, may dampen the autoimmune response and thus extend the honeymoon period or lessen the downstream complications associated with this disease.
In summary, our study shows that the CD28− CD8+ T cell population is significantly reduced in juvenile T1D patients compared to healthy controls, and that this population is negatively correlated with disease duration. These results provide further insight into the dysregulated immune system of juveniles with T1D, and suggest that the CD28− CD8+ T cell subset may be important for regulating the autoimmune response in humans.
Supplementary Material
Acknowledgments
This work was supported by funding to NES from the National Institute of Diabetes and Digestive and Kidney Diseases (5 U01 AI102012-02) and the J.W. Kieckhefer Foundation.
Abbreviations
- T1D
type 1 diabetes
- NT1D
new-onset type 1 diabetes
- LT1D
long-standing type 1 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diamond Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Nokoff NJ, Rewers M, Cree Green M. The interplay of autoimmunity and insulin resistance in type 1 diabetes. Discov Med. 2012;13:115–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Malik FS, Taplin CE. Insulin therapy in children and adolescents with type 1 diabetes. Paediatr Drugs. 2014;16:141–50. doi: 10.1007/s40272-014-0064-6. [DOI] [PubMed] [Google Scholar]
- 4.Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1–15. doi: 10.1111/nyas.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, US Department of Health and Human Services. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. 2011 NIH Publication No. 11–3892. [Google Scholar]
- 6.Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–56. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–83. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 8.Watkins RA, Evans-Molina C, Blum JS, Dimeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014 doi: 10.1016/j.trsl.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013;34:583–91. doi: 10.1016/j.it.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, et al. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes. 2008;57:1312–20. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- 12.Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28− and CD8+CD28− T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–7. [PubMed] [Google Scholar]
- 13.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood KL, Twigg HL, 3rd, Doseff AI. Dysregulation of CD8+ lymphocyte apoptosis, chronic disease, and immune regulation. Front Biosci (Landmark Ed) 2009;14:3771–81. doi: 10.2741/3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, et al. Numerical defects in CD8+CD28− T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell Immunol. 2010;262:75–9. doi: 10.1016/j.cellimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Hoyne GF. Mechanisms that regulate peripheral immune responses to control organ-specific autoimmunity. Clin Dev Immunol. 2011;2011:294968. doi: 10.1155/2011/294968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, et al. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–7. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labalette M, Leteurtre E, Thumerelle C, Grutzmacher C, Tourvieille B, Dessaint JP. Peripheral human CD8(+)CD28(+)T lymphocytes give rise to CD28(−)progeny, but IL-4 prevents loss of CD28 expression. Int Immunol. 1999;11:1327–36. doi: 10.1093/intimm/11.8.1327. [DOI] [PubMed] [Google Scholar]
- 20.Lelic A, Verschoor CP, Ventresca M, Parsons R, Evelegh C, Bowdish D, et al. The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog. 2012;8:e1003076. doi: 10.1371/journal.ppat.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML. Progressive decrease of CD8high+ CD28+ CD57− cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batliwalla F, Monteiro J, Serrano D, Gregersen PK. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68–76. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 23.Batliwalla FM, Rufer N, Lansdorp PM, Gregersen PK. Oligoclonal expansions in the CD8(+)CD28(−) T cells largely explain the shorter telomeres detected in this subset: analysis by flow FISH. Hum Immunol. 2000;61:951–8. doi: 10.1016/s0198-8859(00)00157-9. [DOI] [PubMed] [Google Scholar]
- 24.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–90. [PubMed] [Google Scholar]
- 25.Scheuring UJ, Sabzevari H, Theofilopoulos AN. Proliferative arrest and cell cycle regulation in CD8(+)CD28(−) versus CD8(+)CD28(+) T cells. Hum Immunol. 2002;63:1000–9. doi: 10.1016/s0198-8859(02)00683-3. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–9. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai S, Clemente-Casares X, Santamaria P. CD8(+) Tregs in autoimmunity: learning “self”-control from experience. Cell Mol Life Sci. 2011;68:3781–95. doi: 10.1007/s00018-011-0738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28− T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 30.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, et al. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174:7292–301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 31.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452–7. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 32.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 34.Chatenoud L. Immune therapy for type 1 diabetes mellitus-what is unique about anti-CD3 antibodies? Nat Rev Endocrinol. 2010;6:149–57. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 35.Faustman DL, Davis M. The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J Mol Med (Berl) 2009;87:1173–8. doi: 10.1007/s00109-009-0516-6. [DOI] [PubMed] [Google Scholar]
- 36.Crucian B, Dunne P, Friedman H, Ragsdale R, Pross S, Widen R. Alterations in levels of CD28−/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2:249–52. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glisic-Milosavljevic S, Waukau J, Jailwala P, Jana S, Khoo HJ, Albertz H, et al. At-risk and recent-onset type 1 diabetic subjects have increased apoptosis in the CD4+CD25+ T-cell fraction. PLoS One. 2007;2:e146. doi: 10.1371/journal.pone.0000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–14. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 39.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 40.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999;162:4293–9. [PubMed] [Google Scholar]
- 41.Scotto L, Naiyer AJ, Galluzzo S, Rossi P, Manavalan JS, Kim-Schulze S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− T suppressor cells. Hum Immunol. 2004;65:1297–306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142–56. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Wedderburn LR, Patel A, Varsani H, Woo P. The developing human immune system: T-cell receptor repertoire of children and young adults shows a wide discrepancy in the frequency of persistent oligoclonal T-cell expansions. Immunology. 2001;102:301–9. doi: 10.1046/j.1365-2567.2001.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prelog M, Schwarzenbrunner N, Sailer-Hoeck M, Kern H, Koppelstaetter C, Wurzner R, et al. Indications for a disturbed peripheral T-cell homeostasis in juvenile idiopathic arthritis (JIA): absent expansion of CD28 T-cells and no decrease of naive T-cells in cytomegalovirus-positive patients with JIA. J Rheumatol. 2008;35:520–7. [PubMed] [Google Scholar]
- 45.McCloskey TW, Cavaliere T, Bakshi S, Harper R, Fagin J, Kohn N, et al. Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children, and adults. Clin Immunol Immunopathol. 1997;84:46–55. doi: 10.1006/clin.1997.4370. [DOI] [PubMed] [Google Scholar]
- 46.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28− CD8+ T-cell population. Immunology. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101–7. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.