The feasibility of using an autologous tissue-engineered cultivated nasal mucosal epithelial cell sheet (CNMES) for ocular surface reconstruction was investigated using a novel technique for the culture of nasal mucosal epithelial cells expanded ex vivo from biopsy-derived human nasal mucosal tissues. The CNMESs were transplanted onto the ocular surfaces of rabbits and the survival of this tissue, including the goblet cells, was confirmed up to 2 weeks.
Keywords: Nasal mucosa, Ocular surface, Dry eye, Goblet cell, Mucin
Abstract
Severe ocular surface diseases (OSDs) with severe dry eye can be devastating and are currently some of the most challenging eye disorders to treat. To investigate the feasibility of using an autologous tissue-engineered cultivated nasal mucosal epithelial cell sheet (CNMES) for ocular surface reconstruction, we developed a novel technique for the culture of nasal mucosal epithelial cells expanded ex vivo from biopsy-derived human nasal mucosal tissues. After the protocol, the CNMESs had 4–5 layers of stratified, well-differentiated cells, and we successfully generated cultured epithelial sheets, including numerous goblet cells. Immunohistochemistry confirmed the presence of keratins 3, 4, and 13; mucins 1, 16, and 5AC; cell junction and basement membrane assembly proteins; and stem/progenitor cell marker p75 in the CNMESs. We then transplanted the CNMESs onto the ocular surfaces of rabbits and confirmed the survival of this tissue, including the goblet cells, up to 2 weeks. The present report describes an attempt to overcome the problems of treating severe OSDs with the most severe dry eye by treating them using tissue-engineered CNMESs to supply functional goblet cells and to stabilize and reconstruct the ocular surface. The present study is a first step toward assessing the use of tissue-engineered goblet-cell transplantation of nonocular surface origin for ocular surface reconstruction.
Introduction
In cases of severe ocular surface disease (OSD), such as Stevens-Johnson syndrome (SJS) and ocular cicatricial pemphigoid (OCP), corneal epithelial stem cells are destroyed and the neighboring conjunctival epithelial cells (ECs) invade onto the corneal surface, resulting in chronic inflammation, stromal scarring, and neovascularization that ultimately leads to pathologic keratinization [1, 2]. In most such cases, a definite observable loss occurs of mucin-producing goblet cells from the ocular surface, severely affecting ocular-surface homeostasis and leading to severe dry eye [1–4].
To date, many studies have focused on the establishment of a surgical treatment of severe OSDs [5–10]. For such patients, cultivated corneal, conjunctival, and oral epithelial stem cell transplantation has been developed to improve the postoperative outcomes of ocular surface reconstruction [11–15]. When these surgical modalities are used, a reasonable amount of tear film is required to maintain the transplanted graft; thus, the patients with the most severe form of dry eye do not meet the surgical indication, and no therapeutic methods are available to reconstruct the ocular surface. To date, no reports have been published regarding the surgical procedures from the aspect of supplying tears or goblet cells to the ocular surface. Therefore, potential new treatments are needed for patients with severe OSDs (e.g., SJS and OCP) and the most severe form of dry eye.
Human nasal mucosal epithelium contains enriched goblet cells that secrete the necessary amount of mucin required for tear stabilization. Several groups have previously reported that whole nasal mucosal tissue transplantation (both epithelial and subepithelial tissues) is, in part, effective for treating severe conjunctival mucin deficiency syndrome and eyelid and ocular surface reconstruction [16, 17]. Because of the limited nasal donor site, we believe that the ex vivo expansion of goblet cells using a novel cell culture technique is a useful and promising procedure for developing the next generation of ocular-surface reconstruction surgeries. However, currently, no reports have been published regarding the development of tissue-engineered goblet cell transplantation using nasal mucosal epithelium.
The purpose of the present study was to investigate the use of a tissue-engineered cultivated nasal mucosal epithelial cell sheet (CNMES) to supply functional goblet cells and stabilize and reconstruct the ocular surface in an attempt to ultimately overcome the difficulties associated with treating patients with severe OSDs with the most severe form of dry eye (supplemental online Fig. 1). In the present study, we cultured nasal cells on amniotic membrane (AM) expanded ex vivo from biopsy-derived human nasal mucosal tissues and successfully generated CNMESs that included a high density of goblet cells. The generated CNMESs were then transplanted onto the ocular surfaces of rabbits, and the survival of the cell sheets was subsequently evaluated. The present study is a first step toward assessing the use of tissue-engineered goblet cell transplantation of nonocular surface origin for ocular surface reconstruction.
Materials and Methods
Nasal Mucosal Epithelial Cell Culture
The Institutional Review Board for Human Studies of Kyoto Prefectural University of Medicine, Kyoto, Japan approved all the surgical procedures performed in the present study (approval no. RBMR-C-978), and all patients and enrolled volunteer subjects had provided informed consent in accordance with the tenets set forth in the Declaration of Helsinki for research involving human subjects.
Human AM was obtained at elective cesarean section from volunteers who were seronegative for human immunodeficiency virus, human hepatitis B and C, and syphilis. The AM was then deprived of amniotic epithelial cells by incubation with 0.02% EDTA (Nacalai Tesqu Inc., Kyoto, Japan, http://www.nacalai.co.jp/en) at 37°C for 2 hours.
The nasal mucosal tissues used in the present study were obtained during nasal disease surgery performed in the Department of Otolaryngology-Head and Neck Surgery, Kyoto Prefectural University of Medicine. Nasal mucosal epithelial cells were cultured via the use of our newly developed culture system and were cocultured with human mesenchymal stem cells (MSCs) (Lonza Co., Basel, Switzerland, http://www.lonza.com). To culture the cells, the MSCs were first plated onto 6-well, collagen-coated dishes (Iwaki Glass Co., Ltd., Chiba, Japan, https://www.igc.co.jp/en/) at a density of 3 × 104 cells per cm2. The denuded AM was then spread, epithelial basement membrane side up, on the bottom of a 0.4-μm pore-size culture insert (Corning Inc., Corning, NY, http://www.corning.com), and these culture inserts were then placed in dishes containing MSCs. In addition, a previously reported air-lifting technique was used to promote goblet cell differentiation and epithelial barrier functions [13, 14, 18].
In brief, the blood and submucosal connective tissues were first removed to the extent possible using microscissors. The tissues were then cut into small pieces and incubated overnight at 4°C with 1% pronase (Roche Applied Science, Penzberg, Germany, https://www.roche-applied-science.com). Next, the tissues were carefully removed from the dishes and then put into a 0.05% trypsin-EDTA solution for 8 minutes at room temperature (RT) to separate the cells. Enzyme activity was stopped using culture medium containing Dulbecco’s modified Eagle’s medium/F12 (Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com), defined keratinocyte-serum-free medium (Life Technologies) (1:5 mixture ratio) with 3% fetal bovine serum (HyClone Laboratories, Tauranga, New Zealand, http://www.hyclone.com), insulin (1 μl/ml), hydrocortisone (1 μl/ml), GA1000 (1 μl/ml), retinoic acid (1 μl/ml), transferrin (1 μl/ml), triiodothyronine (1 μl/ml), and epinephrine (1 μl/ml) (all from Lonza Co.). The cell suspension was filtered through a cell strainer (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) and then centrifuged 2 times for 5 minutes at 1,000 rpm. The resultant cell pellet was then resuspended in culture medium containing keratinocyte growth factor (KGF) (20 ng/ml) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and ρ-associated protein kinase (ROCK) inhibitor Y-27632 (1 μl/ml) (Abcam, Cambridge, MA, http://www.abcam.com). The nasal mucosal cells (3 × 105 cells per milliliter) were then seeded onto denuded AM spread on the bottom of culture inserts and cocultured with human MSCs. From 1 day after plating, culture medium without ROCK inhibitor Y-27632 was used. The cultured cells were then submerged in medium for 1 week at 37°C in 5% carbon dioxide and exposed to air by lowering the medium level (air lifting) for 1 additional week. The culture medium was changed every other day during the submerged condition and daily during the air-lifting process.
Corneal, Conjunctival, and Oral Mucosal Epithelial Cell Culture
We cultured human corneal, conjunctival, and oral mucosal epithelial cells using a previously reported system [19]. In brief, for the corneal or conjunctival epithelial cell culture, the donor corneal rims (SightLife, Seattle, WA, http://www.sightlife.org) were first incubated at 37°C for 1 hour with 1.2 IU of dispase to separate the epithelial cells. Cells from the limbal and conjunctival regions were carefully separated from the underlying tissue. The resultant corneal or conjunctival epithelial cells were then seeded onto denuded AM spread on the bottom of culture inserts and cocultured with mitomycin C (MMC)-inactivated 3T3 fibroblasts.
For the oral mucosal epithelial cell culture, a small oral mucosal biopsy was performed with the patient under local anesthesia. The oral epithelium was then incubated at 4°C for 5 hours with 1.2 IU of dispase, followed by treatment with 0.05% trypsin-EDTA solution for 10 minutes to separate the cells. The resultant oral epithelial cells were then seeded onto denuded AM spread on the bottom of culture inserts and cocultured with MMC-inactivated 3T3 fibroblasts.
Electron Microscopy
Native human nasal mucosal tissue and CNMESs were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) [19, 20]. The specimens were fixed in 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS), washed 3 times for 15 minutes in PBS, and postfixed for 2 hours in 2% aqueous osmium tetroxide. They were then washed 3 more times in PBS before being passed through a graded ethanol series (i.e., 50%, 70%, 80%, 90%, 95%, and 100%). For SEM examination, the specimens were transferred to hexamethyldisilazane (TAAB Laboratories Equipment Ltd., Aldermaston, U.K., http://www.taab.co.uk) for 10 minutes and then allowed to air dry. Once dry, the specimens were mounted on aluminum stubs and sputter-coated with gold before undergoing examination using a digital SEM (JSM 5600; JEOL U.K., Ltd., Welwyn Garden City, U.K., http://www.jeoluk.com). For the TEM examination, the specimens were embedded in epoxy resin (Agar 100-epoxy resin; Agar Scientific Ltd., Stansted, U.K., http://www.agarscientific.com). Ultrathin (70-nm) sections were collected on copper grids and stained for 30 minutes with 1% vanadyl sulfate and 1% phosphotungstic acid and then for 20 minutes with Reynold’s lead citrate before examination using TEM (JEM 1010; Jeol U.K.).
Antibodies and Reagents
The following antibodies were used in the present study: mouse monoclonal antibodies anti-keratin 1 (dilution ×20) / 4 (×200) / 10 (×50) / 13 (×200) (Novocastra Ltd., Newcastle Upon Tyne, U.K., http://www.leicabiosystems.com), anti-keratin 3 (×50) (Progen Biotechnik GmbH, Heidelberg, Germany, http://www.progen.de), anti-ZO1 (×25) (Zymed Laboratories, South San Francisco, CA, http://www.invitrogen.com), anti-desmoplakin (×1) (Progen Biotechnik), anti-laminin 5 (×100) (Chemicon International, Inc., Temecula, CA, http://www.chemicon.com), anti-collagen 7 (×100) (Chemicon), anti-MUC1 (×20) (Progen Biotechnik), anti-MUC16 (×400) (Abcam), anti-mucin 5AC (MUC5AC) (×100) (Thermo Fisher Scientific, Inc., Waltham, MA, , http://www.thermofisher.com), anti-p75 (×200) (Abcam), and anti-Ki-67 (×200) (BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com). Rabbit polyclonal antibodies anti-ZO1 (×25) (Zymed Laboratories) and anti-keratin 12 (×200) (TransGenic Inc., Kumamoto, Japan, http://www.transgenic.co.jp). Goat polyclonal antibody anti-keratin 12 (×100) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, http://www.scbt.com). Rat polyclonal antibody anti-galectin 3 (×100) (Santa Cruz Biotechnology). Secondary antibodies included Alexa 488 goat anti-mouse or rabbit IgG (×1,500) (Molecular Probes, Inc., Eugene, OR, http://probes.invitrogen.com), and fluorescein-conjugated donkey anti-mouse, -goat, or -rat IgG (×100) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, http://www.jacksonimmuno.com). The sections were coverslipped using antifading mounting medium containing propidium iodide (Vectorshield; Vector Laboratories, Inc., Burlingame, CA, http://www.vectorlabs.com).
Immunohistochemistry
Immunohistochemical studies were performed according to our previously described method [19, 20]. In brief, 8-μm-thin frozen sections were placed on silane-coated slides, air dried, and fixed in 100% acetone at 4°C for 15 minutes. After washing in PBS containing 0.15% Triton X-100 surfactant (Dow Chemical Co., Midland, MI, http://www.dow.com) at RT for 15 minutes, the sections were incubated with 1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) at RT for 30 minutes to block nonspecific binding. The sections were then incubated with primary antibody at RT for 1 hour and washed 3 times in PBS containing 0.15% Triton X-100 for 15 minutes. Control incubations were conducted with the appropriate normal mouse, rat, goat, and rabbit IgG at the same concentration as the primary antibody, and the primary antibody for the respective specimen was omitted. The sections were then incubated with the appropriate secondary antibodies at RT for 1 hour. After being washed 3 times with PBS, the sections were coverslipped using glycerol containing propidium iodide (Nacalai Tesque, Inc., Kyoto, Japan, http://www.nacalai.co.jp), and examined under a confocal microscope (FluoView; Olympus Corporation, Tokyo, Japan, http://www.olympus-global.com).
Enzyme-Linked Immunosorbent Assay
Quantification of the mucin (MUC) 5AC (MUC5AC) expression level of the cultivated EC sheets (nasal, corneal, conjunctival, and oral ECs) was performed using enzyme-linked immunosorbent assay (ELISA). Each sheet was incubated with Hanks’ balanced salt solution (1 ml per well, 24-well plates) for 48 hours at 37°C. The resultant supernatant solution of each cultivated EC sheet was incubated overnight at 40°C. The wells were washed 3 times with 0.05% Tris-buffered saline with Tween-20 (TBST) and then incubated with 1% BSA for 1 hour at RT. Next, the wells were incubated with anti-MUC5AC antibody for 1 hour at RT. The wells were then washed 3 times with TBST and incubated with secondary antibody (sheep anti-mouse IgG-horseradish peroxidase; GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com) for 1 hour at RT. Finally, the wells were incubated with tetramethylbenzidine (Sigma-Aldrich) for 30 minutes at RT. The reaction was stopped by adding 0.5 M sulfuric acid solution (Nacalai Tesque). The absorbance was read at 450 nm using a microplate reader.
Human CNMES Transplantation
The human CNMESs were transplanted onto albino rabbits (2–2.5 kg) according to our previous protocol [21]. Anesthesia was induced in all rabbits by intramuscular injection of xylazine hydrochloride (5 mg/ml) and ketamine hydrochloride (50 mg/ml). All the rabbits were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the experimental procedure was approved by the Committee for Animal Research at Kyoto Prefectural University of Medicine.
First, the rabbit conjunctival tissues (approximately 10 mm × 5 mm in size) were removed. Next, the CNMESs were placed over the excised area and secured in place with 10-0 nylon sutures. After surgery, the rabbits were given FK506 (300 μm/day) (Astellas Pharma Inc., Tokyo, Japan, http://www.astellas.com) intramuscularly during the observation periods and treated with antibiotic ointment (Santen Pharmaceutical Co., Ltd, Osaka, Japan, http://www.santen.com) and steroid ointment (Shionogi Co., Ltd., Osaka, Japan, http://www.shionogi.com) every day [22].
Results
Histological Examination of CNMESs
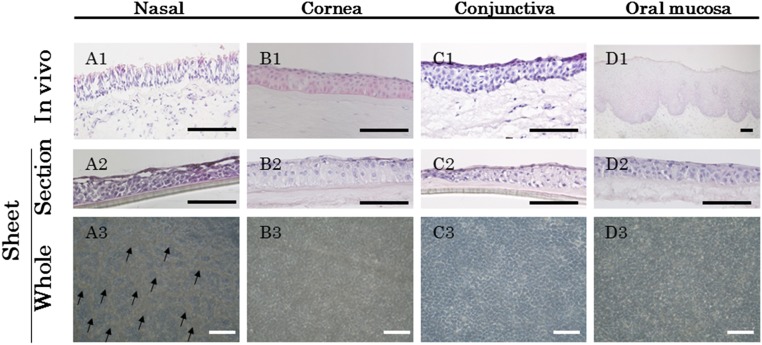
We first examined the histological characteristics of the CNMESs using hematoxylin and eosin staining (Fig. 1A). For comparison purposes, we also examined the cultivated corneal, conjunctival, and oral mucosa EC sheets using our previously reported technique (Fig. 1B–1D) [13, 14, 19]. Native nasal mucosal epithelium, the stratified ciliated columnar epithelium in the nasal mucosa, lies on the basement membrane, and ciliated cells and goblet cells were seen in the epithelial layer (Fig. 1A1). In contrast, the corneal, conjunctival, and oral mucosal epithelium all consisted of nonkeratinized, stratified squamous EC layers (Fig. 1B1, 1C1, 1D1).
Figure 1.
Histological examination of the cultivated nasal mucosal epithelial cell sheet. Light micrographs showing cross-sections of nasal (A1, A2), corneal (B1, B2), conjunctival (C1, C2), and oral (D1, D2) epithelial cells (ECs), both native tissue and in the cultured sheet stained with hematoxylin and eosin. Phase contrast images showing a confluent primary culture of nasal (A3), corneal (B3), conjunctival (C3), and oral (D3) ECs after 2 weeks in culture. Arrows indicate cells with a goblet-like appearance (A3). Scale bars = 100 μm.
After 2 weeks of culture, not only the cultivated corneal, conjunctival, and oral mucosal EC sheets, but also the CNMESs, showed 4–5 layers of stratified well-differentiated cells (Fig. 1A2, 1B2, 1C2, 1D2). Phase-contrast microscopy photographs indicated that a confluent primary culture of ECs had been established that covered the entire AM (Fig. 1A3, 1B3, 1C3, 1D3) and that goblet-like cells were only present in the CNMESs (Fig. 1A3). Thus, tissue-engineered CNMESs on AM were successfully generated.
Ultrastructural Examination of CNMESs
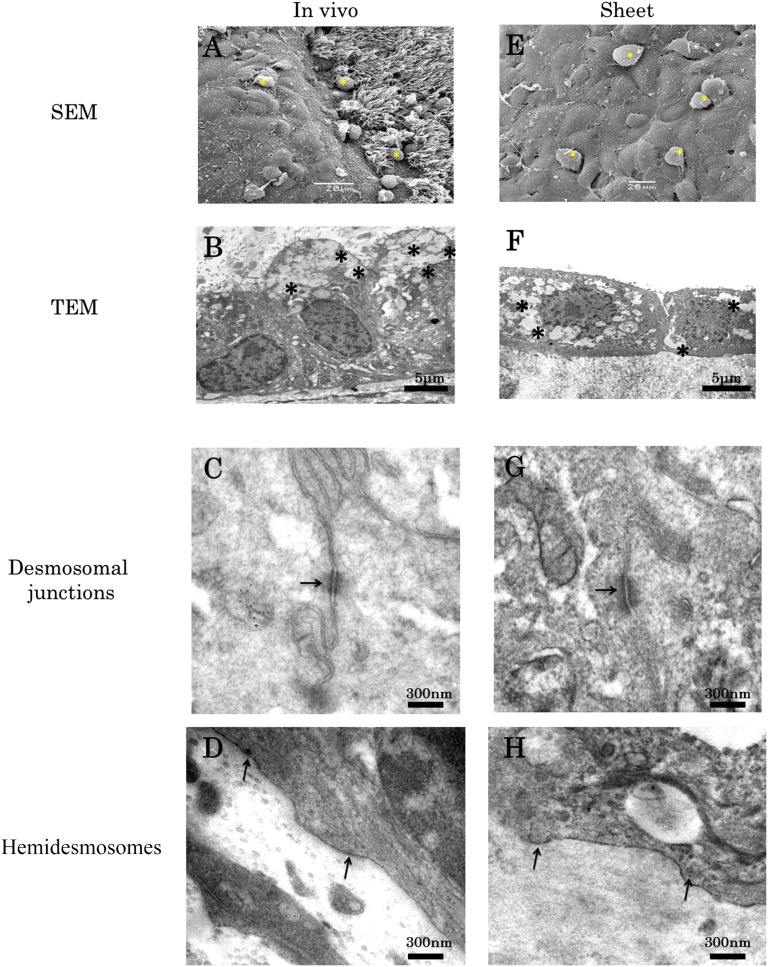
SEM examination of native nasal mucosa revealed ciliated and nonciliated regions with numerous blobs of mucus (Fig. 2A). In contrast, SEM examination of the CNMESs revealed that the surface consisted of nonciliated cells with microvilli that were covered by strands and blobs of mucus (Fig. 2E). TEM examination of the native nasal mucosa revealed a region of goblet cells that contained numerous mucus-filled vesicles (Fig. 2B). TEM examination of the CNMESs revealed multiple layers of goblet cells containing numerous mucus-filled vesicles (Fig. 2F), with some mucus observed on the apical cell surface or trapped between the base of the cells and the AM. Both native nasal mucosa and CNMESs were found to have desmosomal junctions (Fig. 2C, 2G) and hemidesmosomes (Fig. 2D, 2H), respectively, indicating that tissue-engineered CNMESs with numerous goblet cells were successfully generated.
Figure 2.
Ultrastructural examination of the cultivated nasal mucosal epithelial cell sheet (CNMES). Scanning electron microscopy examination of native nasal mucosa (A) and the CNMES (E). Yellow asterisks indicate blobs of mucus. Transmission electron microscopy examination of native nasal mucosa (B, C, D) and the CNMES (F, G, H). Asterisks indicate mucus-filled vesicles (B, F). Arrows indicate desmosomes (C, G) and hemidesmosomes (D, H). Abbreviations: SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Cell Biological Characteristics of CNMESs
Keratin Expression Patterns
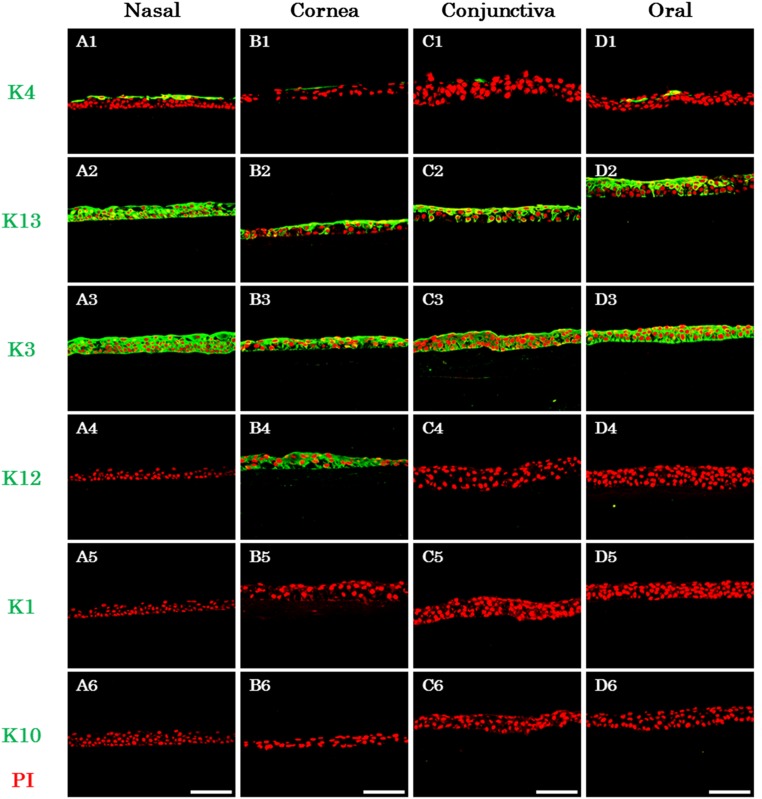
The expression patterns of several tissue-specific keratins in the native tissue (supplemental online Fig. 2) and in the CNMESs (Fig. 3) were investigated by immunohistochemistry. In the normal nasal mucous, expression of stratified keratin 4 was shown in the superficial layer and keratin 13 in all epithelial layers (supplemental online Fig. 2A1, 2A2). Cornea-specific keratin 3 was expressed in all epithelial layers (supplemental online Fig. 2A3). In contrast, expression of cornea-specific keratin 12 and keratinization-related keratins 1 and 10 were not observed (supplemental online Fig. 2A4–2A6). In addition, expression of keratins 4, 3, and 12 was confirmed in the corneal epithelium; however, no expression was found for keratins 1, 10, and 13 (supplemental online Fig. 2B1–2B6). The keratin expression patterns in the conjunctival and oral mucosal epithelium were nearly identical, except for the expression of keratin 3 (supplemental online Fig. 2C1–2C6, 2D1–2D6).
Figure 3.
Keratin expression patterns in the cultivated nasal mucosal epithelial cell sheet. Images show the immunofluorescence of keratin 4 (A1, B1, C1, D1), 13 (A2, B2, C2, D2), 3 (A3, B3, C3, D3), 12 (A4, B4, C4, D4), 1 (A5, B5, C5, D5), and 10 (A6, B6, C6, D6) in the nasal (A1–A6), corneal (B1–B6), conjunctival (C1–C6), and oral (D1–D6) cultured sheets. Scale bars = 100 μm. Abbreviations: K, keratin; PI, propidium iodide.
Immunohistochemical examination of the CNMESs revealed the expression of keratins 3, 4, and 13 (Fig. 3A1–3A3) but no expression of keratins 1, 10, and 12 (Fig. 3A4–3A6). Examination of the cultivated corneal epithelium revealed the expression of keratins 4, 3, 12, and 13 but no expression of keratins 1 and 10 (Fig. 3B1–3B6). The keratin expression patterns of the cultivated conjunctival and oral mucosal epithelium were nearly identical (Fig. 3C1–3C6, 3D1–3D6). These findings show that using our newly developed culture conditions, the cell biological characteristics of the keratin expression patterns of native nasal mucosal epithelium in the CNMESs were maintained.
Cell Junction and Basement Membrane Assembly Protein Expression
The immunohistochemistry findings revealed cell-junction and basement membrane assembly protein expression (supplemental online Fig. 3). ZO-1, a tight-junction-related component, was expressed in apical cells in the normal nasal, corneal, conjunctival, and oral mucosal epithelium (supplemental online Fig. 3A1, 3B1, 3C1, 3D1). Desmoplakin, a cell-to-cell junction component, was expressed in the cell membrane of epithelial cells in all tissues (supplemental online Fig. 3A2, 3B2, 3C2, 3D2). The basement membrane assembly proteins laminin 5 and collagen 7 were expressed in the basement membrane in all tissues (supplemental online Fig. 3A3–3A4, 3B3–3B4, 3C3–3C4, 3D3–3D4). The CNMESs were found to express all the markers examined in the present study (supplemental online Fig. 3A5–3A8). These expression patterns were also observed in the other cultivated sheets (supplemental online Fig. 3B5–3B8, 3C5–3C8, 3D5–3D8). Thus, the CNMESs exhibited the proper cell junction and basement membrane assembly protein, which is thought to be essential for adaptation to clinical applications.
Membrane-Type Mucin Expression Patterns
MUC1 and MUC16, membrane-bound mucins [23], were expressed in the superficial layer of the normal nasal, corneal, and conjunctival mucosal epithelium (supplemental online Fig. 4A1, 4A2, 4B1, 4B2, 4C1, 4C2). In contrast, they were not expressed in the normal oral mucosal epithelium (supplemental online Fig. 4D1, 4D2). Galectin 3 was expressed in all epithelium (supplemental online Fig. 4A3, 4B3, 4C3, 4D3).
MUC1, MUC16, and galectin 3 were expressed mainly in the superficial layer of the CNMESs (supplemental online Fig. 4A4–4A6). These expression patterns were similar to those seen in the native nasal mucosa. The membrane-type mucin expression patterns of the other cultured sheets were nearly identical to those of native epithelium (supplemental online Fig. 4B4–4B6, 4C4–4C6, 4D4–4D6).
Secretory-Type Mucin Expression (MUC5AC)
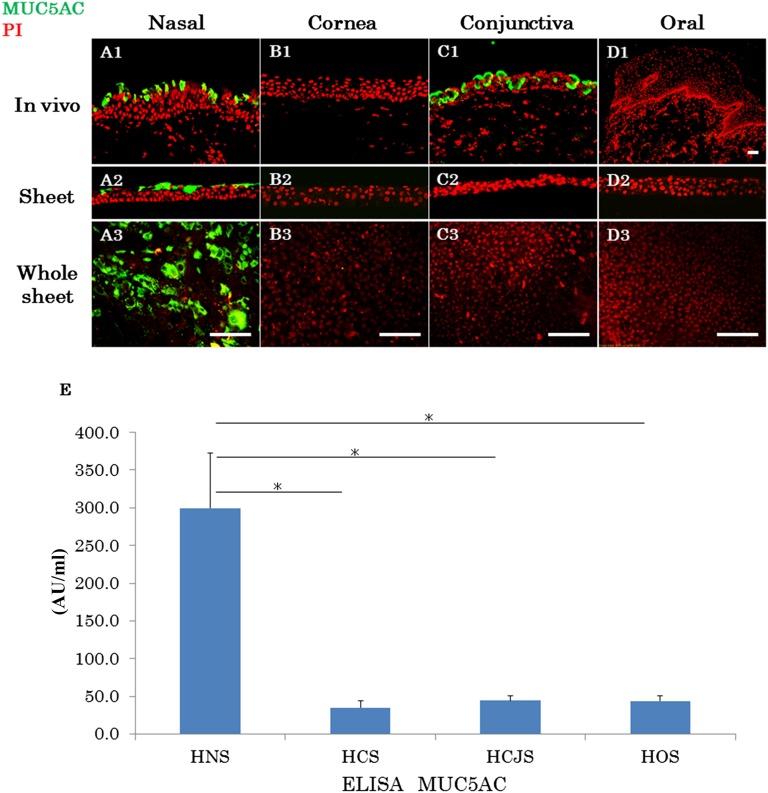
Immunohistochemical examination revealed the presence of MUC5AC, a marker of goblet cells, in the normal nasal mucosal and conjunctival epithelial cells (Fig. 4A1, 4C1). In contrast, no expression of MUC5AC was observed in the normal corneal and oral mucosal epithelium (Fig. 4B1, 4D1). MUC5AC was only expressed in the superficial cells of the CNMESs (Fig. 4A2, 4A3). The protein levels of MUC5AC in the cultured sheet were examined by ELISA (Fig. 4E). The expression level of MUC5AC in the CNMESs was higher than that in the other cultivated sheets (p < .02). These findings illustrate that tissue-engineered CNMESs with numerous MUC5AC-positive goblet cells were successfully produced.
Figure 4.
Expression patterns of mucin 5AC (MUC5AC). Immunofluorescence of MUC5AC in native nasal (A1), corneal (B1), conjunctival (C1), and oral (D1) mucosal epithelium. Immunofluorescence of MUC5AC in the cultured nasal (A2, A3), corneal (B2, B3), conjunctival (C2, C3), and oral (D2, D3) epithelial cell sheets. Scale bars = 100 μm. (E): The protein levels of MUC5AC in the cultured epithelial sheets were examined by ELISA. Mean ± SEM. ∗, p < .02; n = 8. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HCS, human corneal epithelial sheet; HCJS, human conjunctival epithelial sheet; HNS, human nasal mucosal epithelial sheet; HOS, human oral mucosal epithelial sheet; PI, propidium iodide.
Possible Epithelial Stem/Progenitor Cell Marker p75
A possible stem/progenitor cell marker [24], p75, was sporadically expressed in the basal cells of the human nasal, conjunctival, and oral mucosal epithelium; however, its expression was not observed in the normal corneal epithelium (supplemental online Fig. 5A–5D). Expectedly, p75 expression was observed in the basal cell layer of the CNMESs and other cultivated sheets (supplemental online Figure 5E–5H), suggesting that stem/progenitor cells might be included in the CNMESs that were generated. However, no reliable markers exist for nasal mucosal epithelial stem cells, and our findings only showed the expression of p75 in the nasal mucosal epithelium. Therefore, much more information is needed to clearly demonstrate these subjects.
Xenotransplantation of Human CNMESs
Clinical Findings
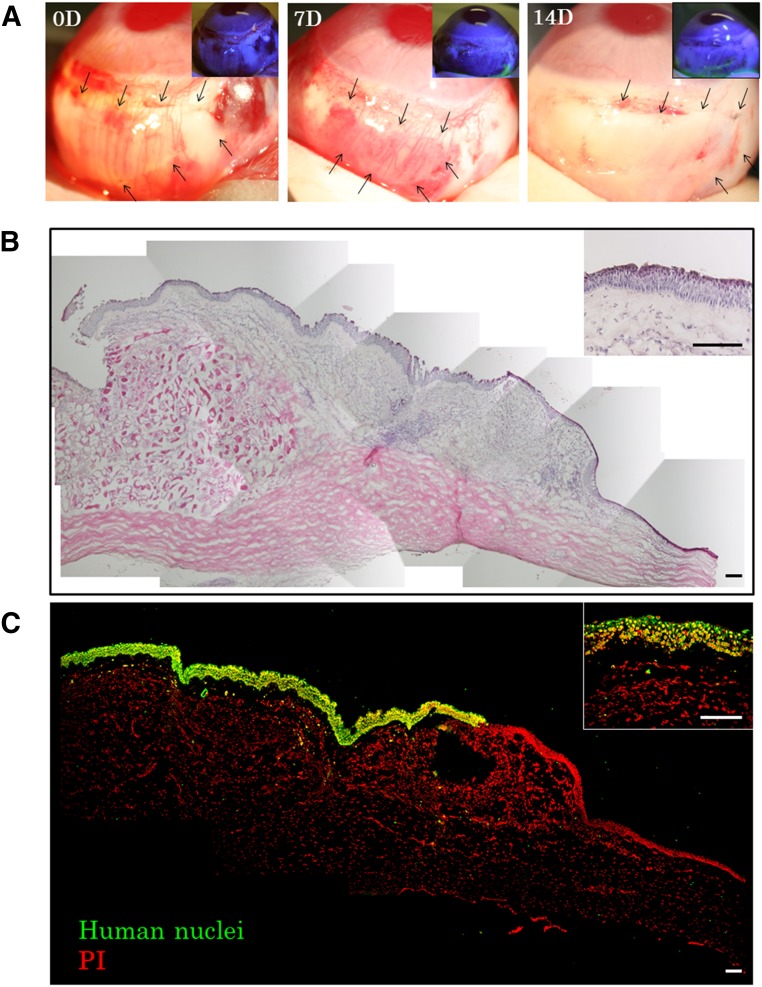
Human CNMESs were transplanted onto the conjunctival surface of the rabbit eyes and fixed with 10-0 nylon sutures (Fig. 5A). At both 7 days and 2 weeks after transplantation, the transplanted conjunctiva surfaces in all treated eyes were confirmed to be clear and smooth and without any extensive postoperative inflammation. In addition, fluorescein staining confirmed that the entire conjunctiva surface was covered by xeno-CNMESs (Fig. 5A). The slit-lamp examination findings were the same in all 3 rabbits.
Figure 5.
Xenotransplantation of a human cultivated nasal mucosal epithelial cell sheet. (A): Representative slit-lamp photographs of a rabbit taken immediately after transplantation, 7 days after transplantation, and 14 days after transplantation, with and without fluorescein. Arrows indicate the transplanted cell sheet. (B, C): Hematoxylin and eosin staining (B) and immunofluorescence (C) of anti-human nuclei at the transplanted conjunctival area. Scale bars = 100 μm. Abbreviations: D, day(s); PI, propidium iodide.
Cell Biological Characteristics of Transplanted CNMESs
Histological examination of the transplanted CNMESs at 14 days postoperatively revealed that they were well adhered to the host tissues, with evidence of subepithelial cell infiltration (Fig. 5B). Hematoxylin-eosin staining showed that the transplanted CNMESs contained well-stratified differentiated cells (Fig. 5B). In order to confirm the presence of transplanted human CNMES cells on the rabbit conjunctival surface, we examined their expression of anti-human nuclei and found positive expression of it in the transplanted areas (Fig. 5C).
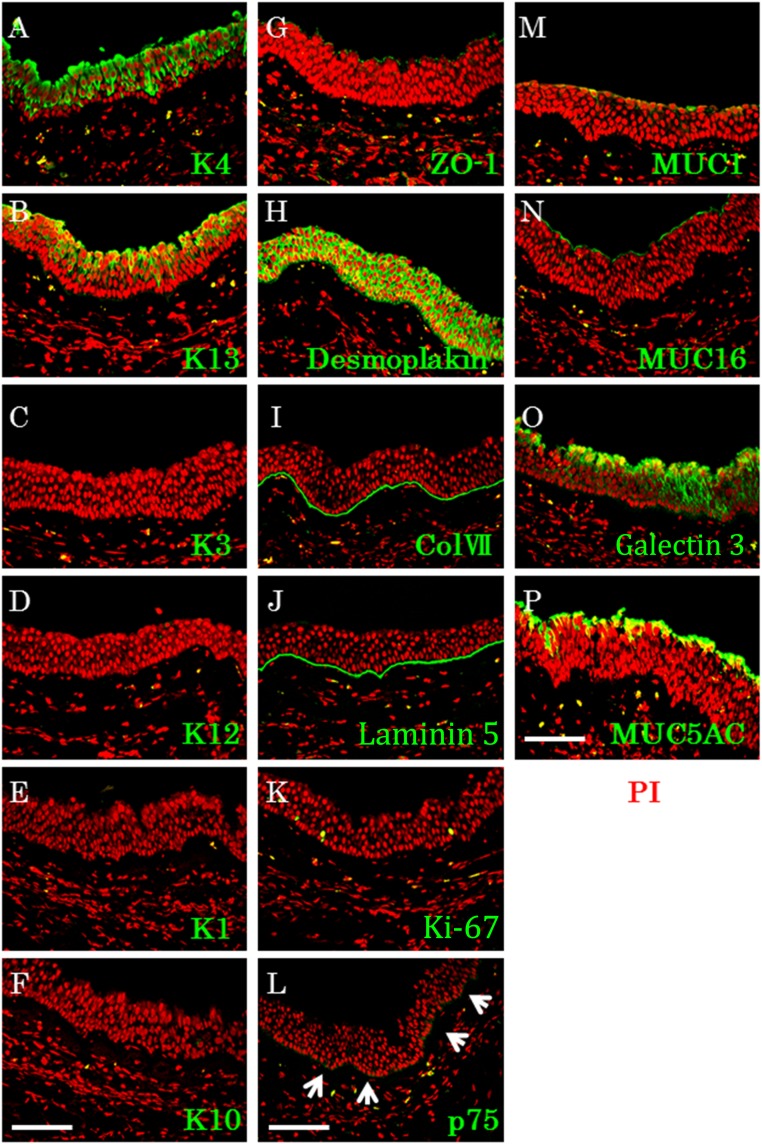
Next, the expression pattern of several cell biological markers in the transplanted CNMESs was examined (Fig. 6). Keratins 4 and 13 were found to be expressed in the superficial and intermediate layers, with no discernible immunostaining in the basal cell layers (Fig. 6A, 6B). Keratins 1, 3, 10, and 12 were not expressed in any layer (Fig. 6C–6F). ZO-1 was expressed in the apical cells in the transplanted CNMES, and desmoplakin was expressed in the cell membrane of the transplanted CNMES cells (Fig. 6G, 6H). The basement membrane assembly proteins collagen 7 and laminin 5 were also expressed in the transplanted CNMESs (Fig. 6I, 6J). Sporadic expression of Ki67 and p75 was found in the basal layer of the transplanted CNMESs (Fig. 6K, 6L). MUC1 and MUC16 were expressed in the superficial layer in the transplanted CNMESs, and galectin 3 was expressed in all CNMES layers (Fig. 6M–6O). Immunohistochemistry confirmed the presence of MUC5AC in the transplanted CNMESs (Fig. 6P). These findings indicate that the generated CNMESs are well-adapted to native situations, with good postoperative function.
Figure 6.
Cell biological characteristics of the transplanted cultivated nasal mucosal epithelial cell sheet (CNMES). Immunofluorescence of keratin 4 (A), 13 (B), 3 (C), 12 (D), 1 (E), and 10 (F), and ZO-1 (G), desmoplakin (H), collagen 7 (I), laminin 5 (J), Ki-67 (K), p75 (L), MUC1 (arrows) (M), MUC16 (N), galectin 3 (O), and MUC5AC (P) in the transplanted CNMES. Scale bars = 100 μm. Abbreviations: ColVII, collagen 7; K, keratin; MUC, mucin; PI, propidium iodide.
Discussion
Severe OSDs are some of the most challenging clinical entities facing ophthalmologists worldwide. Although much attention is focused each year on the development of regenerative cell therapy using corneal, conjunctival, and oral mucosal epithelial stem cells, no reports have been published regarding a comparative examination of the cell biological characterization of the corneal, conjunctival, and oral epithelial sheets. Moreover, severe OSDs with severe dry eye remain some of the most difficult disorders to treat using the currently available treatment methods. The ocular surfaces of those patients exhibited a complete loss of conjunctival goblet cells, resulting in the clinical change of the mucosal epithelium from wet to pathologically keratinized. Subsequently, the ocular surfaces in those cases become severely compromised. However, these patients are presently considered to not meet the surgical indications. As a first step in the development of a novel treatment method for these patients, the transplantation of functional goblet cells onto the ocular surface might offer one possible approach, because goblet cells with secreted mucin are an essential component for maintaining ocular surface homeostasis. Although it is quite difficult to maintain goblet cells in in vitro culture conditions, we successfully generated confluent cultures of nasal mucosal ECs with numerous goblet cells expanded ex vivo from biopsy-derived nasal mucosal tissues and compared them with other types of cultured epithelial sheets. The present study is a first step toward assessing the efficacy of a novel tissue-engineered autologous transplantation of functional goblet cells of nonocular surface origin.
In 1990, Naumann et al. introduced autologous nasal mucosal tissue transplantation in severe mucus deficiency syndrome [16]. Most recently, Kim et al. reported that autologous nasal mucosal tissue transplantation is a feasible treatment of patients with cicatricial OSD [17]. The ocular surface is composed of two different types of ECs (i.e., corneal and conjunctival), and goblet cells are diffusely located throughout the entire conjunctival area. In these reports, the nasal graft size was around 2 cm2, and because of the limited nasal donor site, it is impossible to supply the goblet cells to the entire ocular surface using this procedure. Hence, the condition is far from the physiological ocular surface. To reconstruct the ocular surface as close as possible to its normal physiological state, we believe that the ex vivo expansion of goblet cells using our newly discovered cell culture technique is a useful and promising procedure for developing the next generation of ocular surface reconstruction methods.
The culture of goblet cells has long been a challenging and difficult subject for scientists worldwide. In the present study, we successfully developed a novel culture system to generate CNMESs that include numerous goblet cells. Our new culture system is unique in several important aspects, because it includes the use of human MSCs to assist EC growth, the use of ROCK inhibitor Y-27632 to assist in the initial cell attachment, and the use of KGF to induce goblet cell differentiation.
Worldwide, the most popular cells used in the EC culture process are mouse-derived 3T3 feeder cells, because they are widely recognized for their effectiveness [25]. However, the use of those cells requires the use of xenobiotic materials in the culture system. Therefore, the use of human MSCs as an alternative to 3T3 feeder cells is significantly advantageous, because it eliminates the need for xenobiotic-free material in the culture process. Although we have not compared the effectiveness of 3T3 feeder cells with MSCs, the use of human MSCs is of clinical importance in the development of autologous tissue-engineered cell sheets for clinical transplantation.
Watanabe et al. reported that the application of ROCK inhibitor Y-27632 promotes the survival of dissociated human embryonic stem cells [26], and our group recently reported that Y-27632 enhances the adhesion of corneal endothelial cells to a substrate [27]. In our experimental observation under the phase-contrast microscope, we found that the application of Y-27632 to our culture system enhanced the initial attachment of human nasal ECs onto the AM. That finding encouraged us to explore the use of Y-27632 in our attempt to develop a better protocol of tissue-engineered nasal cell culture techniques.
Epidermal growth factor (EGF) is widely used for the culture of nasal ECs [18]. However, when using this method, we found it was difficult to generate cultured cell sheets that included a sufficient number of goblet cells, although the cell growth was comparatively fast. Thus, we developed our culture protocol and discovered that although the cell growth with KGF was slower than that with EGF, KGF effectively promotes sufficient goblet cell differentiation in human nasal ECs. It has been reported that KGF and its receptor are expressed in the nasal mucosa [28] and that KGF stimulates the proliferation of ECs without impairing differentiation [29]. KGF also reportedly promotes goblet cell differentiation through the regulation of a goblet cell silencer inhibitor [30]. In view of these findings, KGF is one of the key factors to generate the goblet cell-rich cultured EC sheets.
Reportedly, cytokeratins play an important physiological and biological role in maintaining the integrity of ECs [31–34]. In those studies, defined subsets of individual cytokeratin pairs were characteristically expressed, depending on the EC type and level of differentiation. We have demonstrated that the keratin 1 and 10 pair, an epidermal keratinization marker, is not expressed in any layers in native nasal epithelium or in the CNMESs. We also confirmed that the keratin 4 and 13 pair, a nonkeratinized, mucosal epithelial marker, is expressed in both of them. These results led us to believe that, similar to other epithelial sheets, the CNMESs have the characteristics of nonkeratinized mucosal epithelium. Moreover, immunohistochemical examination revealed no expression of cornea-specific keratin 12 in any layers of the native nasal epithelium or the CNMESs, although cornea-specific keratin 3 was expressed in all epithelial layers in both. Reportedly, keratin 3 is a marker for corneal differentiation and shows positive for corneal, oral, and nasal mucosal ECs [35], and that was confirmed by our results. Although the CNMESs we produced were not identical to corneal and conjunctival epithelium, we posit that they have the potential to become a substitute for ocular-surface ECs. In addition, it should be noted that we have yet to elucidate the molecular pathway of the differentiation of CNMESs. Thus, additional investigation is needed to clarify this point.
An important key point regarding the feasibility and efficacy of using the CNMESs to produce a successful transplantation is to elucidate how the cultured cells attach to the surrounding cells or substrate (i.e., cell-to-cell, cell-to-substrate). The results of our immunohistochemical examination revealed that the cell junction and basement membrane assembly proteins were well expressed in the CNMESs. Adjacent cells in the CNMESs were found to be joined with numerous desmosomal junctions (desmoplakin), and tight junctions (ZO-1) were evident between the most superficial cell layers. These findings encouraged us to perform the transplantation of the CNMESs onto a native ocular surface.
The ocular surface is normally covered with tear film, which has a variety of components, including the mucus layer on the epithelial surface [36–39]. Mucin is a major component of tear film, which is essential for good vision. Thus, the mucin expression involvement in the CNMESs produced in the present study is of particular interest. Similar to that with the other cultured cell sheets, membrane-associated mucins (MUC1 and MUC16) were expressed in the CNMESs. However, unlike the other cell sheet, which did not express secretory mucin MUC5AC, the CNMESs we produced involved abundant MUC5AC expression and might be a main advantage for tear film stabilization and ocular surface reconstruction.
After the successful culture of human CNMESs, we transplanted it onto the ocular surfaces to investigate the viability of using the CNMESs as a substitute for functional ocular surface ECs. Two weeks after xenotransplantation of the human CNMESs, the transplanted sites were free of epithelial defects, indicating the complete survival of the transplanted CNMESs. Importantly, we confirmed the presence of junction-related proteins, the proliferation marker, and the stem/progenitor cell marker in the transplanted CNMESs. Most importantly, although we could not completely rule out the contamination of MUC5AC derived from rabbit conjunctiva, we observed that the secretory-type mucin marker MUC5AC was intensely expressed in the transplanted CNMESs, suggesting that the CNMESs have the potential ability to treat severe OSDs with goblet cell deficiency. In addition, we noted a decreased expression of keratin 3 in the transplanted CNMESs, suggesting that transplanted CNMESs might change their biological character in adjustment to the surrounding microenvironment.
Conclusion
To the best of our knowledge, our study is the first to demonstrate the survival of tissue-engineered cultured epithelial cells derived from nasal biopsies. In the present study, we successfully generated functional CNMESs with numerous goblet cells. In addition, we successfully performed xenotransplantation of these cells onto a rabbit ocular surface. We believe that this approach to treating severe OSD eyes with severe dry eye has great potential. However, it is important to note that this technique is still in its early stages and that many questions have yet to be addressed, including questions about the longevity and mobility of the autologously transplanted nasal ECs on the host eye. Thus, the long-term postoperative outcomes of CNMES transplantation remain unclear, and additional investigation is needed to elucidate the feasibility and efficacy of using CNMESs in the clinical setting.
Supplementary Material
Acknowledgments
We thank John Bush for reviewing our report. This study was supported in part by Grants-in-Aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Kiban C), Biotechnology and Biological Sciences Research Council, and Wellcome Trust, United Kingdom.
Author Contributions
M.K.: provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing; T.N.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.Y., Y. Hata, and Y. Hisa: provision of study material or patients; S.O., M.I., M.N., and N.J.F.: collection and/or assembly of data; N.K. and S.K.: financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 2.Chiou AG, Florakis GJ, Kazim M. Management of conjunctival cicatrizing diseases and severe ocular surface dysfunction. Surv Ophthalmol. 1998;43:19–46. doi: 10.1016/s0039-6257(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita S, Adachi W, Sotozono C, et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001;20:639–673. doi: 10.1016/s1350-9462(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 4.Tseng SC, Hirst LW, Maumenee AE, et al. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. 1984;91:545–552. doi: 10.1016/s0161-6420(84)34251-8. [DOI] [PubMed] [Google Scholar]
- 5.Thoft RA. Conjunctival transplantation. Arch Ophthalmol. 1977;95:1425–1427. doi: 10.1001/archopht.1977.04450080135017. [DOI] [PubMed] [Google Scholar]
- 6.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1–6. doi: 10.1016/0002-9394(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 7.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 8.Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Inatomi T, Sotozono C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 16.Naumann GO, Lang GK, Rummelt V, et al. Autologous nasal mucosa transplantation in severe bilateral conjunctival mucus deficiency syndrome. Ophthalmology. 1990;97:1011–1017. doi: 10.1016/s0161-6420(90)32471-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Chun YS, Lee SH, et al. Ocular surface reconstruction with autologous nasal mucosa in cicatricial ocular surface disease. Am J Ophthalmol. 2010;149:45–53. doi: 10.1016/j.ajo.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Huang TW, Young YH, Cheng PW, et al. Culture of nasal epithelial cells using chitosan-based membranes. Laryngoscope. 2009;119:2066–2070. doi: 10.1002/lary.20609. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–116. doi: 10.1167/iovs.02-0195. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Hamuro J, Takaishi M, et al. LRIG1 inhibits STAT3-dependent inflammation to maintain corneal homeostasis. J Clin Invest. 2014;124:385–397. doi: 10.1172/JCI71488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaoka M, Nakamura T, Sugai H, et al. Sutureless amniotic membrane transplantation for ocular surface reconstruction with a chemically defined bioadhesive. Biomaterials. 2008;29:2923–2931. doi: 10.1016/j.biomaterials.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Tanioka H, Kawasaki S, Yamasaki K, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 2006;47:3820–3827. doi: 10.1167/iovs.06-0293. [DOI] [PubMed] [Google Scholar]
- 23.Argüeso P, Guzman-Aranguez A, Mantelli F, et al. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Endo K, Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- 25.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 27.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi T, Tanaka T, Nibu K, et al. Keratinocyte growth factor and its receptor messenger RNA expression in nasal mucosa and nasal polyps. Ann Otol Rhinol Laryngol. 1998;107:885–890. doi: 10.1177/000348949810701013. [DOI] [PubMed] [Google Scholar]
- 29.Miyashita H, Yokoo S, Yoshida S, et al. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Translational Medicine. 2013;2:758–765. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwakiri D, Podolsky DK. Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology. 2001;120:1372–1380. doi: 10.1053/gast.2001.24029. [DOI] [PubMed] [Google Scholar]
- 31.Franke WW, Schiller DL, Moll R, et al. Diversity of cytokeratins: Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981;153:933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- 32.Tseng SC, Jarvinen MJ, Nelson WG, et al. Correlation of specific keratins with different types of epithelial differentiation: Monoclonal antibody studies. Cell. 1982;30:361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- 33.Sun TT, Eichner R, Nelson WG, et al. Keratin classes: Molecular markers for different types of epithelial differentiation. J Invest Dermatol. 1983;81(suppl):109s–115s. doi: 10.1111/1523-1747.ep12540831. [DOI] [PubMed] [Google Scholar]
- 34.Eichner R, Bonitz P, Sun TT. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984;98:1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols BA, Chiappino ML, Dawson CR. Demonstration of the mucous layer of the tear film by electron microscopy. Invest Ophthalmol Vis Sci. 1985;26:464–473. [PubMed] [Google Scholar]
- 37.Inatomi T, Spurr-Michaud S, Tisdale AS, et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–1692. [PubMed] [Google Scholar]
- 38.Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzman-Aranguez A, Argüeso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf. 2010;8:8–17. doi: 10.1016/s1542-0124(12)70213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.