Abstract
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a well-known immunotoxic compound affecting the expression of inflammatory genes. We found that TCDD induces the expression of several chemokines including B-cell activating factor of the tumor necrosis factor family (BAFF), B-lymphocyte chemoattractant (BLC), CC-chemokine ligand 1 (CCL1), and the transcription factor interferon γ responsive factor (IFR3) in U937 macrophages in an aryl hydrocarbon receptor- (AhR) and RelB-dependent manner. The induction was associated with increased binding activity of an AhR/RelB complex without participation of ARNT to a NF-κB element that is recognized by the NF-κB subunit RelB and localized on promoters of the chemokine genes BAFF, BLC, CCL1, and the transcription factor IRF3. The interaction of AhR with RelB binding on a novel type of NF-κB binding site of promoters of chemokines represents a new regulatory function of the AhR.
Keywords: AhR, BAFF, IRF3, chemokines, inflammation, macrophages, TCDD
Introduction
Exposure to AhR activating compounds like the environmental pollutant TCDD has been found to elicit chronic active inflammation in different organs and sites of the body [1,2]. Several reports including our own studies have shown that TCDD induces multiple inflammatory genes including chemokines such as Interleukin 8 (IL-8), monocyte attracting protein (MCP)-1, and CC-chemokine ligand 1 (CCL1) in an AhR-dependent manner [3-5]. The AhR is a cytoplasmic receptor protein and has been described as a ligand-activated transcription factor that mediates induction of xenobiotic metabolizing enzymes. Upon ligand binding the AhR translocates into the nucleus and dimerizes with the AhR nuclear translocator (ARNT). The ligand-activated AhR/ARNT complex binds specific gene promoter elements (xenobiotic response elements, XRE) identified on certain drug metabolizing enzymes like cytochrome P450 1A1 (CYP1A1) [6]. Numerous exogenous compounds with various binding affinities have been described to activate the AhR but the physiological role of the AhR remained unclear.
Recent reports started elucidating the physiological role of the AhR which is functioning in absence of any exogenous ligand [7]. For instance, the AhR has been shown to have a critical role in development: AhR null mice show deficiencies in liver development and decreased accumulation of lymphocytes in the spleen and lymph nodes [8]. Dejardin et al. [9] and others [10] have shown that the development of lymphocytes and secondary lymphoid organs require the activation of organogenic chemokines. The activation of organogenic chemokines is regulated through the alternative NF-κB pathway. IκB Kinase (IKK)α is required for activation of these genes in response to engagement of the lymphotoxin β receptor (LTβR), B-cell activating factor (BAFF)-receptor, CD40 ligand or receptor activator of NF-κB (RANK) ligand to process p100 and to generate active p52/RelB dimers. A recent study identified a novel type of NF-κB-binding site on promoters of several organogenic chemokine genes which is recognized by RelB [10].
There are several reports on the effect of NF-κB activation through TCDD-induced expression of AhR target genes including the possible interaction of the AhR with the NF-κB subunit RelA [11]. The current study focuses on the effect of TCDD and interaction of the AhR with recently identified NF-κB-RelB-binding sites located on the promoters of chemokine genes such as BAFF, BLC, CCL1, and the transcription factor interferon γ responsive factor (IRF)3.
Materials and methods
Reagents and Antibodies
Dimethylsulfoxide (Me2SO) and Phorbol-12-myristate-13-acetate (TPA) were obtained from SIGMA (St. Louis, MO). [y-32P] ATP (6000 Ci/mmol) was purchased from ICN (Costa Mesa, CA). TCDD (>99% purity) was originally obtained from Dow Chemicals Co (Midland, MI). Other molecular biological reagents were purchased from Qiagen (Valencia, CA) and Roche (Indianapolis, IN). Monoclonal ARNT, polyclonal RelA (Santa Cruz Biotechnology, Santa Cruz, CA), NF-κB member RelB, c-Rel, p50 (Active Motif, Carlsbad, CA) and polyclonal AhR (Novus Biologicals, Littleton, CO) antibodies were used for supershift in EMSA.
Cell Culture and siRNA Transfection Experiments
Human U937 monocytic cells were obtained from A.T.C.C. (Manassas, VA) and maintained in RPMI 1640 medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA). For transient transfection of U937 macrophages, cells were plated in RPMI with 10% FBS containing 0.5 μg/ml TPA to promote differentiation into macrophages after 2 days. Transfection of short interfering RNA (siRNA) or expression plasmids into U937 macrophages was performed via Nucleofector technology. U937 macrophages were resuspended in 100 μl Nucleofector Solution V (Amaxa GmbH, Köln, Germany) and nucleofected with 1.5 μg of the corresponding siRNA using the Nucleofector device (Amaxa GmbH) according the manufacturer's instructions. In case of siRNA transfection, the reduction of the target protein was detected by Western blot as shown in Fig. 1B. siRNA to target human AhR was designed and synthesized by Dharmacon (Lafayette, CO). siRNA to target human RelB and ARNT and a negative control siRNA were synthesized by Qiagen (Valencia, CA).
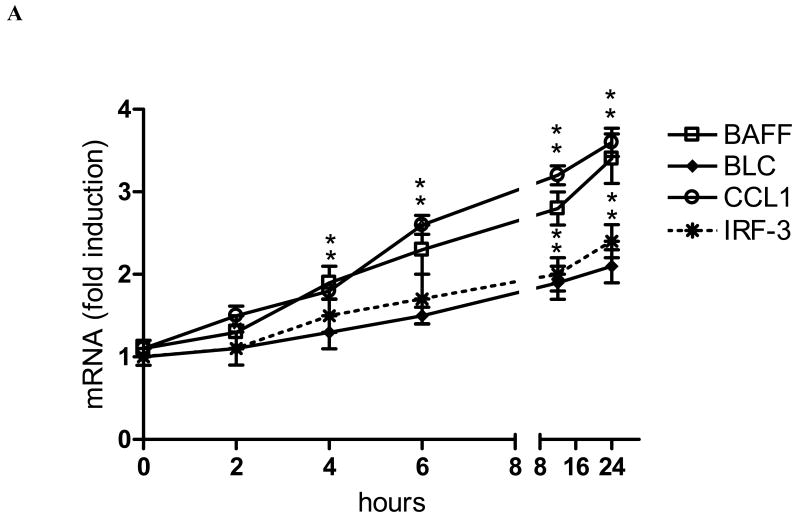
Fig. 1.
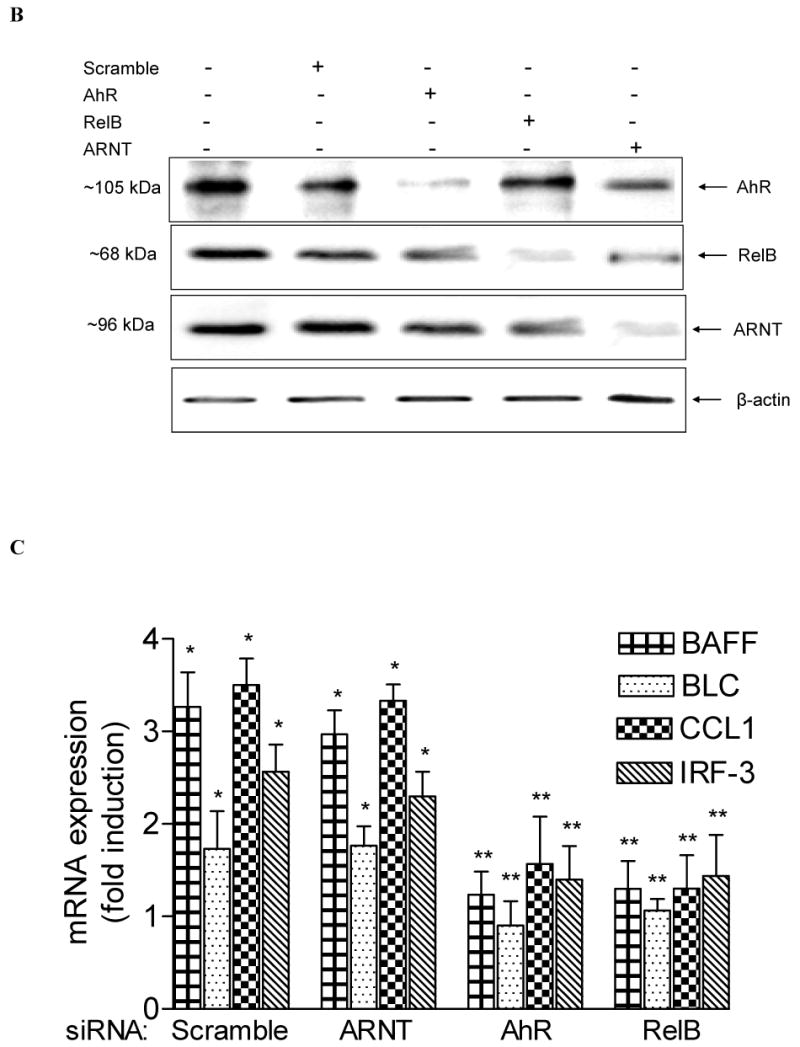
Induction of BAFF, BLC, CCL1, and IRF3 by TCDD is RelB- and AhR-dependent. (A) Time-course study of mRNA expression in U937 macrophages. Cells were treated for 1 to 24h with 10 nM TCDD or 0.1% Me2SO (Control). Quantitative detection of mRNA was performed using real time RT-PCR. Values for mRNA expression are normalized to the expression of β-actin. (B) Western blot analysis of AHR, RelB, and ARNT protein levels 48h posttransfection with the indicated siRNAs. (C) Quantitative mRNA expression analyses after treatment with TCDD for 24 h. Total RNA was prepared 72 h post-transfection with either a scrambled siRNA or a specific siRNA targeted against ARNT, AhR, or RelB. *, significantly different from control (p<0.01); **, significantly lower than cells transfected with scrambled or ARNT siRNA.
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from U937 macrophages using a high pure RNA isolation kit and cDNA synthesis was carried out as previously described [12]. Quantitative detection of mRNA level was performed with a LightCycler Instrument (Roche Diagnostics, Mannheim, Germany) using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The primers for each gene were designed using OLIGO primer analysis software, provided by Steve Rosen and Whitehead Institute/MIT Center for Genome Research. The primer sequences for human β-actin BAFF, BLC, CCL1, and IRF3 are available upon request. All PCR assays were performed in triplicate. The intra-assay variability was <7%.
Electromobility Shift Assay (EMSA)
Nuclear extracts were isolated from U937 cells as described previously [12]. Protein concentrations of the nuclear protein extracts were determined by the method of Bradford. Sequences for double-stranded oligonucleotides used in EMSA are shown in the legend of figure 3. DNA-protein binding reactions were carried out in a total volume of 15 μl containing 10 μg nuclear protein, 80,000 cpm of DNA oligonucleotide, 25 mM Tris buffer (pH 7.5), 50 mM NaCl, 1 mM EDTA, 0.5 mM dithiothreitol, 5% glycerol, and 1 μg poly (dI-dC). The samples were incubated at room temperature for 20 min. Supershift analysis were performed by adding 2 μg of the corresponding antibodies to the reaction mixtures. Competition experiments were performed in the presence of a 100-fold molar excess of unlabeled DNA fragments. Protein-DNA complexes were resolved on a 4% nondenaturating polyacrylamide gel and visualized by exposure of the dehydrated gels to X-ray films. For quantitative analysis, respective bands were quantified using a ChemiImager™4400 (Alpha Innotech Corporation, San Leandro, CA).
Fig. 3.
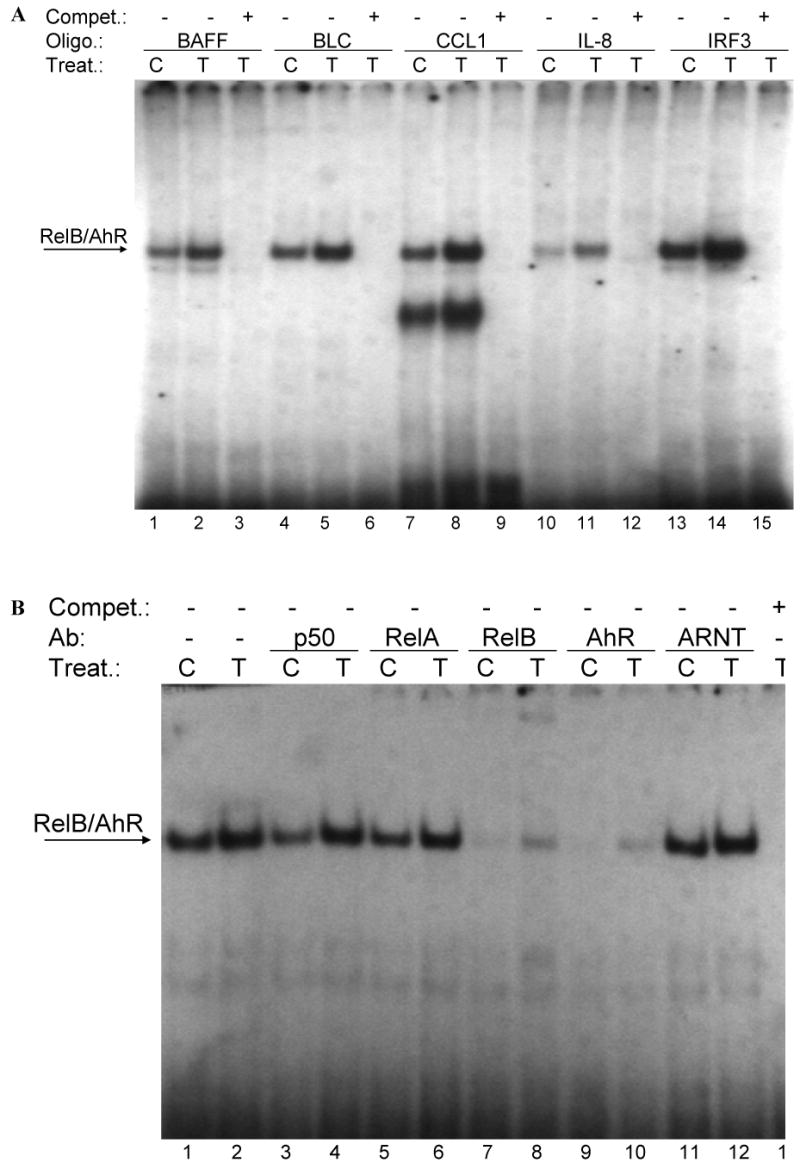
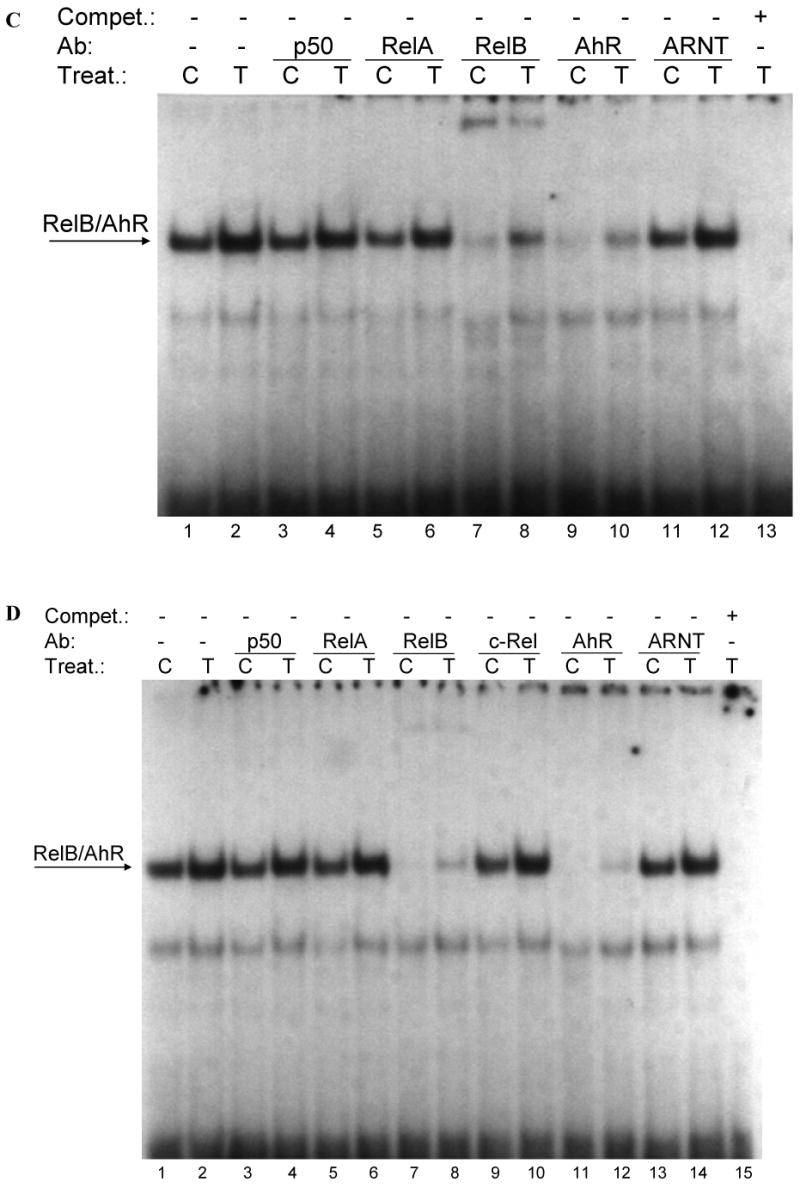
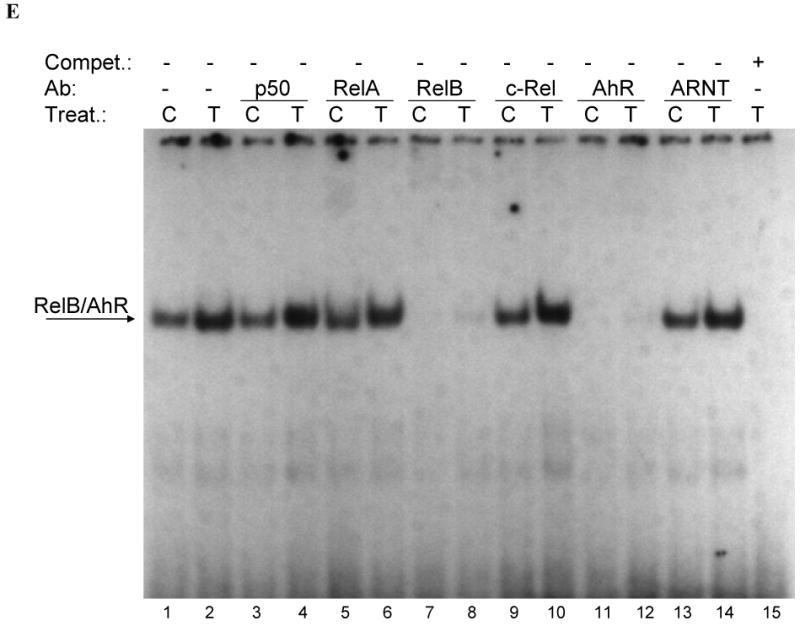
(A) Effect of TCDD on binding activity of RelB/AhR response elements located on promoters of BAFF, BLC, CCL1, IL-8, and IRF3. Nuclear protein extracts of control (C) or TCDD- (T) stimulated U937 macrophages were incubated with oligonucleotides containing the NF-κB-RelB-binding site identified on promoters of the corresponding chemokines. (B-E) Supershift analysis of nuclear protein extracts from control or macrophages treated with 10 nM TCDD for 2h. A possible binding of AhR, ARNT, and NF-κB subunits p50, RelA, RelB, or c-Rel was identified by supershift analyses in control cells and TCDD-treated cells using specific antibodies for the corresponding protein. The binding activity of RelB/AhR response elements located on promoters of (B) BAFF (5′-ACAGTTCTGTGAGATTTGACAAGTGCA-3′), (C) BLC (5′-GATGAGGGAGATTTGGTTCTCTAG-3′), (D) CCL1 (5′-ATCTTGAAAAAGTGCGTGGGAACTGTCCTG-3′), and (E) IRF3 (5′-AGAGCTCTGGGAGATGTAACATATCCT-3′) was analyzed. To confirm specificity a 100-fold excess of the unlabeled oligonucleotides was added.
Statistical analysis
All data were obtained from at least three independent experiments performed in duplicate, and the results are given as the mean ± the standard error of the mean. To demonstrate statistical significance, the variables were examined for one-sided Student's t test. The level of significance was p<0.05.
Results
Time-dependent induction of BAFF, BLC, CCL1, and IRF3 by TCDD
In the present study we found that activation of the AhR by TCDD leads to a time-dependent induction of the chemokines BAFF, BLC, CCL1, and IRF3 in human U937 macrophages (Fig. 1A). First significant increase of BAFF and CCL1 was observed 4h after treatment with 10 nM TCDD which was further increased over time period tested in this study and reached an about 3-fold increased level compared to control cells at 24h. The mRNA levels of BLC and IRF3 were 1.5-fold increased at 6h and 1.8- and 2-fold elevated above control level 24h after treatment with TCDD.
TCDD-mediated induction of BAFF, BLC, CCL1, and IRF3 is AhR- and RelB-dependent
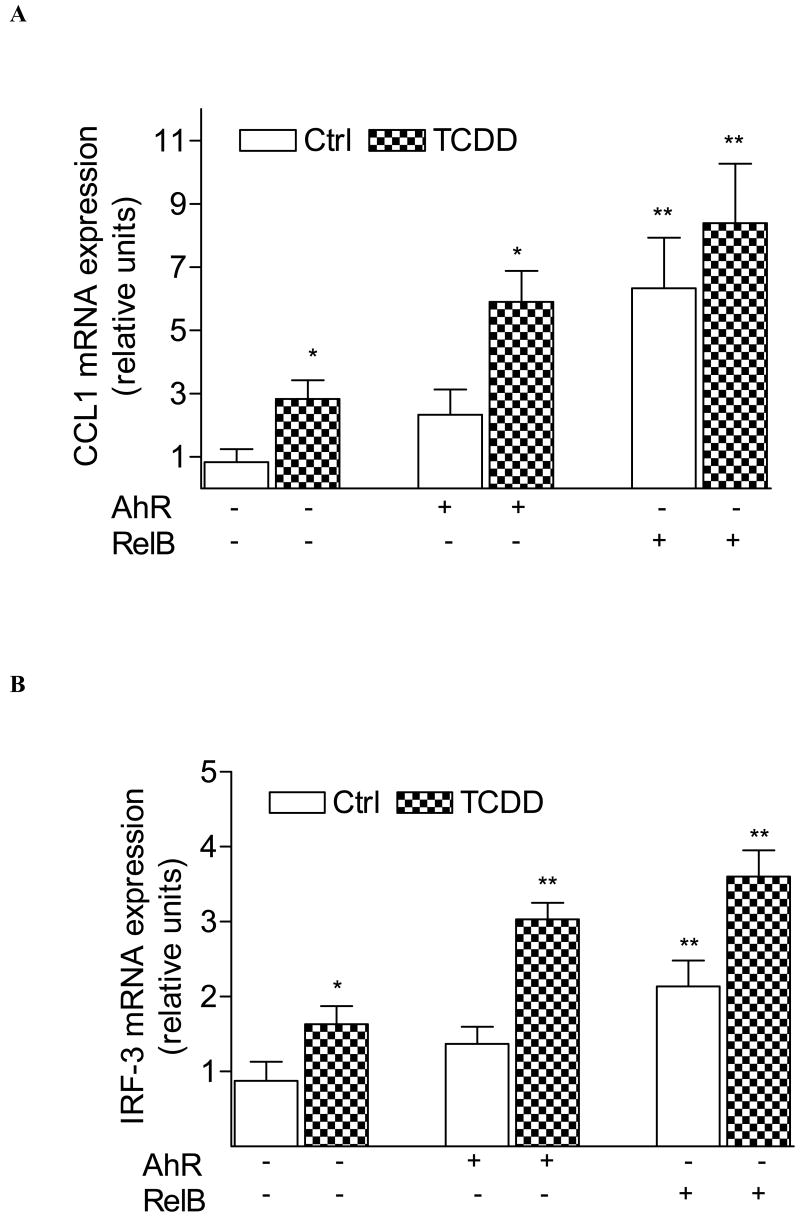
Transfection studies with siRNA to target AhR and RelB suggest that TCDD activates BAFF, BLC, CCL1 and IRF3 through an AhR- and RelB-dependent mechanism (Fig. 1B and C). Results with the AhR antagonist MNF confirmed the critical role of AhR (data not shown). In contrast, downregulation of ARNT by siARNT did not significantly change the TCDD-induced level of BAFF, BLC, CCL1, or IRF3 mRNA (Fig. 1C). These results are supported by over-expression of RelB and AhR which further increased the level of CCL1 and IRF3 in control and TCDD-stimulated U937 macrophages (Fig. 2A and B).
Fig. 2.
Induction of (A) CCL1 and (B) IRF3 is increased in AhR and RelB overexpressing cells. Cells were transiently transfected with 200 ng/ml of AhR or RelB expression plasmid. Control cells were transfected with an empty control vector. After 48 h cells were treated with 10 nM TCDD for 24 h. Expression of CCL1 and IRF3 mRNA was analyzed by real-time PCR as described above. *, significantly different from control cells (p<0.01); **, significantly higher than non-transfected cells (p<0.01)
Enhanced binding activity of RelB/AhR complexes to NF-κB RelB binding sites of the BAFF, BLC, CCL-1 and IRF3 promoter
To address whether a recently identified κB-binding site (5′-GGGAGATTTG-3′) located on the promoter of chemokines like BAFF, BLC, or IRF3 that is preferentially recognized by RelB/p52 dimers is also a target for AhR/RelB-containing dimers we performed EMSA with the specific κB-binding site located on promoters of BAFF, BCL, CCL1 and IRF3 at position -71 bp, -115 bp, -326 bp, and -450 bp, respectively. All probes exhibited strong binding activity to nuclear extracts of U937 macrophages which was further increased using nuclear extracts of cells stimulated with 10 nM TCDD for 2h (Fig. 3A). In case of CCL1, we observed a second faster migrating complex which obviously does not contain AhR or RelB (Fig. 3A and D).
Supershift analysis revealed clear binding activity of AhR in complex with RelB. The presence of ARNT, p50, or c-Rel subunits dimerized with RelB or AhR could not be detected in control or TCDD-stimulated cells (Fig. 3B-E). Migration and binding activity of the RelB/p52 probes were comparable for all probes tested. In all cases, the detected protein-DNA complexes were specific, as indicated by competition experiments with excess of unlabeld oligonucleotide.
Discussion
The AhR has been shown to mediate various immunotoxic responses induced by environmental pollutants like TCDD or benzo[a]pyrene (B[a]P) [13,14]. The physiological function of the AhR is also likely to play an important role in developmental processes of the immune system such as accumulation of lymphocytes and the development of lymphoid organs [8,15]. The present study focused on the regulation of genes involved in the development of lymphocytes such as BAFF, BLC, CCL1 and IRF3 after treatment with TCDD in human U937 macrophages.
Results from transfection studies with siRNA suggest that the increased expression of these chemokines mediated by TCDD requires the AhR. This is in line with a previous study showing the AhR-dependent induction of CCL1 by the environmental contaminant B[a]P [3]. Interestingly, our results show, that the TCDD-induced expression of BAFF, BLC, IRF3, as well as CCL1 requires not only AhR, but also RelB. Furthermore, we could show that the induction of the chemokines by TCDD does not involve the classical AhR dimerization partner ARNT. In a recent study Bonizzi et al. [10] found that the promoters of chemokine genes including BAFF, BLC or the transcription factor IRF3 are recognized by RelB/p52 dimers and not by RelA/p50 dimers, the target of the classical NF-κB signaling pathway. They identified a novel NF-κB-RelB-binding site that is preferentially recognized by RelB dimers on promoters of BAFF, BLC, IRF3 and other chemokine genes.
To address whether the novel NF-κB-RelB-binding site is targeted by the AhR we performed supershift analyses with the corresponding probes of the chemokines. The results revealed clear binding activity of the AhR in complex with RelB on the common regulatory NF-κB-RelB element of BAFF, BLC, and IRF3 which was further increased using nuclear extracts of macrophages treated with TCDD for 2h. A previous study identified an AhR-related XRE consensus element on the CCL1 promoter [16] located at -312 bp. Although, we found that this XRE-like sequence binds the AhR which is in line with a previous report [3], the AhR is obviously not complexed with ARNT. Moreover, the XRE-like sequence of CCL1 binds AhR associated with the NF-κB subunit RelB as in the case of BAFF, BLC, and IRF3. The activation of AhR by TCDD leads to an increased binding activity of an AhR/RelB complex on the probes tested which was associated with an increased mRNA level of the chemokines. Overexpression of AhR and RelB led to an increased level of CCL1 and IRF3 in control as well as TCDD-stimulated cells supporting the role of RelB and AhR for the transcriptional regulation of the chemokines. Together, these data establish an example of how an activated AhR pathway connects to the NF-κB subunit RelB in order to cooperatively regulate chemokine gene expression.
Acknowledgments
We thank Pat Wong and Wen Li for excellent technical assistance. This work was supported by research grant R01-ES005233 and core center grant, P30-ES05707 from the National Institute of Environmental Health Sciences.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARNT
AhR nuclear translocator
- BAFF
B-cell activating factor
- CCL1
CC-chemokine ligand 1
- IKK
IκB Kinase
- IRF3
interferon γ responsive factor3
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XRE
xenobiotic response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pande K, Moran SM, Bradfield CA. Aspects of dioxin toxicity are mediated by interleukin 1-like cytokines. Mol Pharmacol. 2005;67:1393–1398. doi: 10.1124/mol.105.010983. [DOI] [PubMed] [Google Scholar]
- 2.Nyska A, Jokinen MP, Brix AE, Sells DM, Wyde ME, Orzech D, Haseman JK, Flake G, Walker NJ. Exocrine pancreatic pathology in female Harlan Sprague-Dawley rats after chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like compounds. Environ Health Perspect. 2004;112:903–909. doi: 10.1289/ehp.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel CF, Nishimura N, Sciullo E, Wong P, Li W, Matsumura F. Modulation of the chemokines KC and MCP-1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Arch Biochem Biophys. 2007;461:169–175. doi: 10.1016/j.abb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.N'Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, Monteiro P, Rauch C, Galibert MD, Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine CCL1 by the environmental contaminant benzo[a]pyrene. J Biol Chem. 2006;281:19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 7.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007 doi: 10.1016/j.febslet.2007.03.046. In press. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 9.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;4:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 10.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;21:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 12.Vogel CF, Sciullo E, Park S, Liedtke C, Trautwein C, Matsumura F. Dioxin increases C/EBPbeta transcription by activating cAMP/protein kinase A. J Biol Chem. 2004;279:8886–8894. doi: 10.1074/jbc.M310190200. [DOI] [PubMed] [Google Scholar]
- 13.Lecureur V, Ferrec EL, N'Diaye M, Vee ML, Gardyn C, Gilot D, Fardel O. ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005;579:1904–1910. doi: 10.1016/j.febslet.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 14.Silkworth JB, Lipinskas T, Stoner CR. Immunosuppressive potential of several polycyclic aromatic hydrocarbons (PAHs) found at a Superfund site: new model used to evaluate additive interactions between benzo[a]pyrene and TCDD. Toxicology. 1995;105:375–386. doi: 10.1016/0300-483x(95)03235-8. [DOI] [PubMed] [Google Scholar]
- 15.Thurmond TS, Staples JE, Silverstone AE, Gasiewicz TA. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2000;165:227–236. doi: 10.1006/taap.2000.8942. [DOI] [PubMed] [Google Scholar]
- 16.Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004;32:4512–4523. doi: 10.1093/nar/gkh782. [DOI] [PMC free article] [PubMed] [Google Scholar]