Abstract
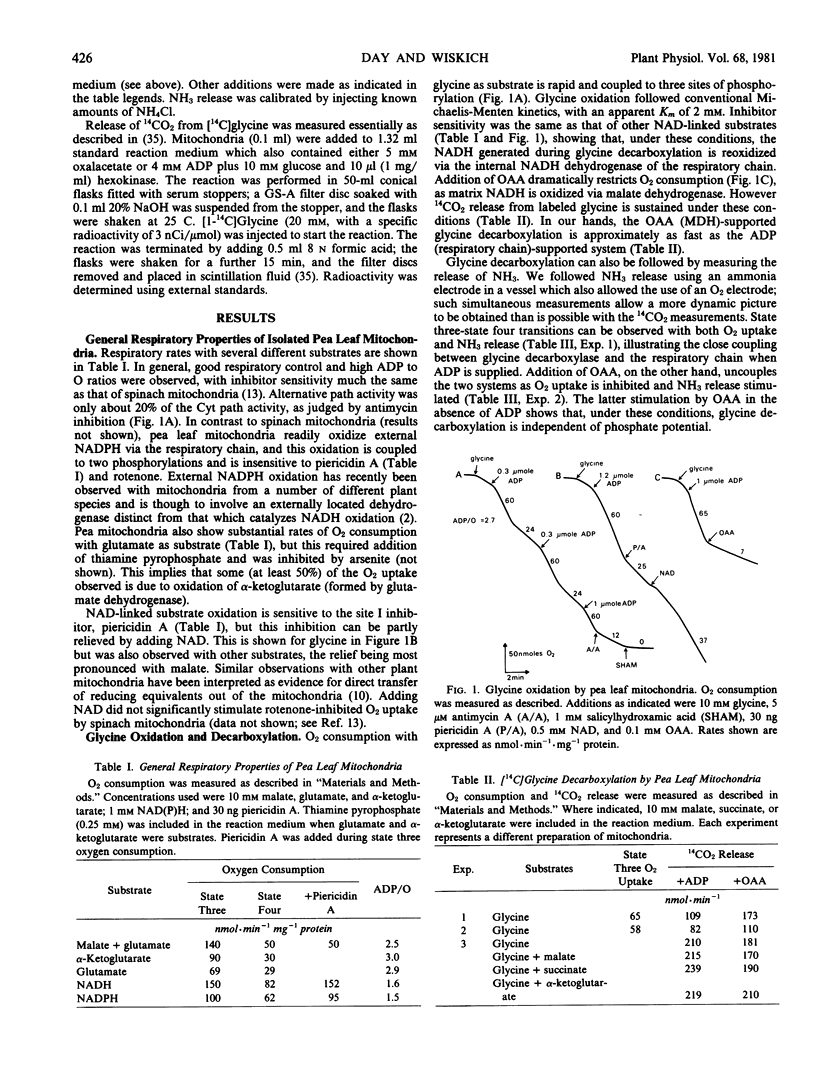
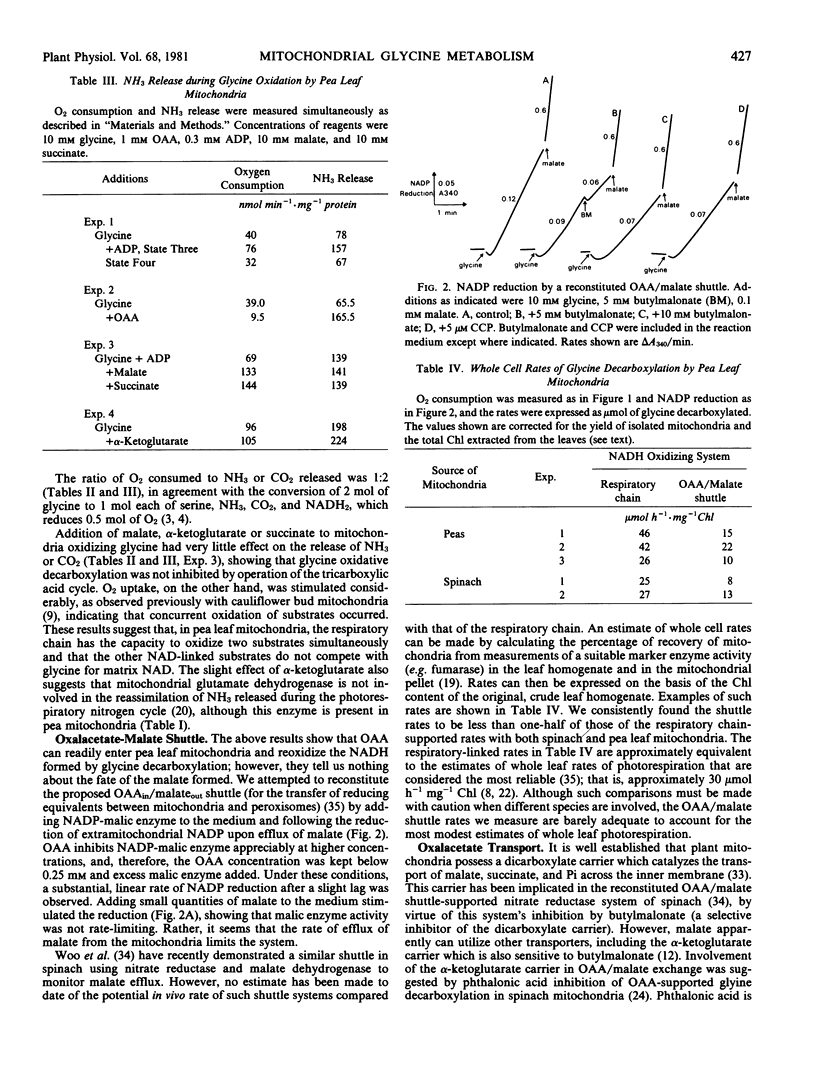
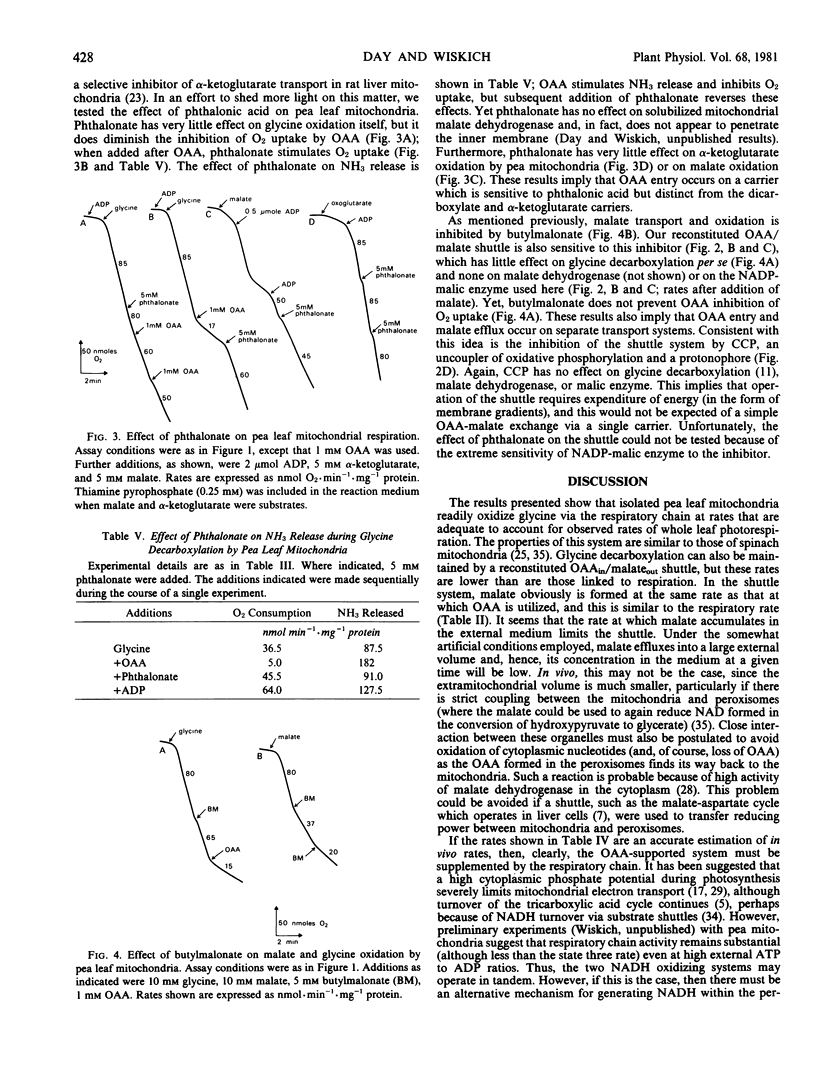
Isolated pea leaf mitochondria oxidatively decarboxylate added glycine. This decarboxylation could be linked to the respiratory chain (in which case it was coupled to three phosphorylations) or to mitochondrial malate dehydrogenase when oxalacetate was supplied. Decarboxylation rates measured as O2 uptake, or CO2 and NH3 release were adequate to account for whole leaf photorespiration. Oxalacetate-supported glycine decarboxylation, measured by linking malate efflux to added malic enzyme, yielded rates considerably less than the electron transport rates. Butylmalonate inhibited malate efflux but not oxalacetate entry; phthalonate inhibited oxalacetate entry but had little effect on malate or α-ketoglutarate oxidation. It is suggested that oxalacetate and malate transport are catalyzed by separate carrier systems of the mitochondrial membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron G. P., Edwards G. E. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by plant mitochondria. Can J Biochem. 1979 Dec;57(12):1392–1399. doi: 10.1139/o79-185. [DOI] [PubMed] [Google Scholar]
- Arron G. P., Spalding M. H., Edwards G. E. Stoichiometry of carbon dioxide release and oxygen uptake during glycine oxidation in mitochondria isolated from spinach (Spinacia oleracea) leaves. Biochem J. 1979 Nov 15;184(2):457–460. doi: 10.1042/bj1840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The Effect of Light on the Tricarboxylic Acid Cycle in Green Leaves: II. Intermediary Metabolism and the Location of Control Points. Plant Physiol. 1974 Jun;53(6):886–892. doi: 10.1104/pp.53.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. S., Hatch M. D. Regulation of mitochondrial NAD-malic enzyme involved in C4 pathway photosynthesis. Arch Biochem Biophys. 1977 Nov;184(1):298–306. doi: 10.1016/0003-9861(77)90354-x. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Pyridine nucleotide interactions with isolated plant mitochondria. Biochim Biophys Acta. 1978 Mar 13;501(3):396–404. doi: 10.1016/0005-2728(78)90107-x. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L., Kagawa T. Properties of leaf NAD malic enzyme from plants with C4 pathway photosynthesis. Arch Biochem Biophys. 1974 Nov;165(1):188–200. doi: 10.1016/0003-9861(74)90155-6. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Mitochondria as a site of C4 acid decarboxylation in C4-pathway photosynthesis. Arch Biochem Biophys. 1975 Apr;167(2):687–696. doi: 10.1016/0003-9861(75)90513-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., von Woerkom G. M., Eggelte T. A. Phthalonic acid, an inhibitor of alpha-oxoglutarate transport in mitochondria. Biochim Biophys Acta. 1976 Apr 9;430(1):53–61. doi: 10.1016/0005-2728(76)90221-8. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Oliver D. J. Mechanism of decarboxylation of glycine and glycolate by isolated soybean cells. Plant Physiol. 1979 Dec;64(6):1048–1052. doi: 10.1104/pp.64.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Malate dehydrogenases of leaf tissue from Spinacia oleracea: properties of three isoenzymes. Arch Biochem Biophys. 1971 Nov;147(1):114–122. doi: 10.1016/0003-9861(71)90316-x. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Jokinen M., Canvin D. T. Reduction of Nitrate via a Dicarboxylate Shuttle in a Reconstituted System of Supernatant and Mitochondria from Spinach Leaves. Plant Physiol. 1980 Mar;65(3):433–436. doi: 10.1104/pp.65.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



